Abstract
Objective
To study the longitudinal changes of cartilage and relaxation time measurements in hip-OA patients.
Methods
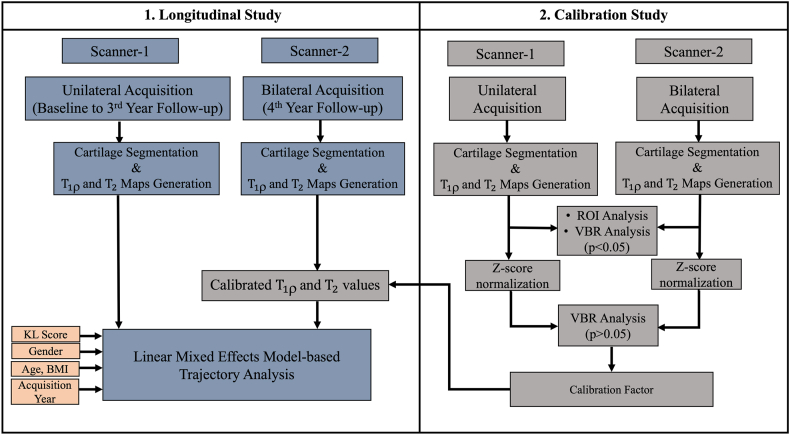
A calibration study compared two scanner data, Scanner-1 (GE Discovery MR750 3.0T) with unilateral acquisition protocol and Scanner-2 (GE Signa Premier 3.0T) with bilateral acquisition protocol, using nine subjects(average age = 40.33 ± 13.53 years, 5 females), including one hip-OA subject. Quantified parameters from the Scanner-2 were adjusted using voxel-based relaxometry(VBR) and Z-score normalization to reduce the inter-scanner variability. Eighteen hip-OA Subjects (age = 53.11 ± 14.96 years, 12 females) were recruited to the longitudinal variability study from 2016, comprising five assessments at 1-year intervals. Baseline to 3rd-year data used unilateral acquisition with Scanner-1, while 4th-year data used bilateral acquisition with Scanner-2. A linear mixed-effects model(LME) assessed trajectory analyses, with acquisition year, age, sex, body mass index(BMI), and Kellgren-Lawrence(KL) score as predictor variables and cartilage mean and values as outcomes.
Results
VBR analysis after Z-score normalization showed that only a few of the whole cartilage voxels had significant differences in femur-2.36 % and acetabular-3.23 %) and (femur-2.30 % and acetabular-2.94 %) values between the scanners. The LME analysis showed that the BMI predictor variable was significantly correlated with the femur (p < 0.0001) and (p < 0.0001) and acetabular (p < 0.0001) and (p < 0.0001) cartilage region.
Conclusion
The calibration study demonstrated the effectiveness of VBR and Z-score normalization in reducing inter-scanner variability. The longitudinal study revealed a significant correlation between and values of the cartilage and BMI; also the and values increased over time in some of the cartilage subregions.
Keywords: Articular cartilage, Hip osteoarthritis, VBR analysis, Longitudinal study
1. Introduction
Hip osteoarthritis(OA) is the second most affected joint disease and causes pain and disability among the elderly [1]. The articular cartilage degeneration in the weight-bearing joints is the onset of OA [2]. Traditionally, conventional radiographs have been employed in the diagnosis and treatment planning of hip-OA based on the assessment of joint space narrowing and the presence of osteophytes [3,4]. The severity of the disease is often characterized using the well-established Kellgren-Lawrence(KL-score) clinical score [5]. However, this radiograph-based analysis lacks soft tissue information, leading to a low sensitivity to the changes that result from OA progression [2]. Incorporating imaging biomarkers to identify the early and subtle changes in soft tissue is essential for improving OA diagnosis and treatment planning.
Conventional magnetic resonance imaging(MRI) techniques are widely used to analyze morphological changes in articular cartilage due to its soft tissue contrast [6,7]. However, early biochemical changes of articular cartilage before structural damage can be evaluated with the help of compositional imaging techniques such as and relaxation time measurements of cartilage [8,9]. Multiple cross-sectional and longitudinal studies reported increased and values in hip cartilage are associated with disease progression [[10], [11], [12], [13]]. Recently developed automatic voxel-based relaxometry(VBR) [14,15] employs a method centered around aligning all subjects onto a unified reference space and is validated as a tool to provide robust and reliable quantification of hip cartilage. It helps to assess local patterns of quantification maps in the cartilage region and the differences between different subject groups [11].
A few longitudinal studies have shown the association of baseline and relaxation times in hip cartilage lesion progression [11,14]. One recent study reported a significant increase of baseline hip femoral cartilage and values in subjects who experienced femoral cartilage lesion progression using traditional ROI analysis and VBR approach [11]. VBR was reported to be more sensitive to local patterns of and increase in the cartilage region. In this study, the results indicated age, body mass index(BMI), presence of baseline hip-OA and female sex showed a positive trend for OA progression. However, this study was limited to baseline and 18-month follow-up periods. Hence, conducting a longitudinal study with an extended follow-up period is essential to establish a more robust correlation between values and the hip-OA progression.
Major hardware or software changes with MRI scanners can occur during longitudinal studies and variability might affect the resultant analysis [16]. An appropriate correction method is required to achieve unbiased and measurements. Recent studies showed the Z-score-based normalization technique is more effective for correcting the scaling factor to the and measurements due to the scanner or coil updates [17,18]. A recent study showed a significant improvement in intra-subject comparability of (ICC of 0.11 vs. 0.78) and (ICC of 0.35 vs. 0.83) when using Z-scores across three scanners [20]. Therefore, such normalization methods can be useful for eliminating the bias due to the scanner coil upgrade or multicenter acquisition.
The aims of the current study were to 1) Reduce the inter-scanner variability of and quantification between MR scanners and 2) Evaluate the changes in femoral and acetabular cartilage and relaxation time measurements for patients with early to moderate hip-OA over 5-years.
2. Materials and methods
Two separate studies were included: (i) Calibration study (ii) Longitudinal variability study.
2.1. Calibration study
A calibration study was performed to reduce the inter-scanner variability between the two scanners involved in the longitudinal variability study.
Study Population and Data Selection: Nine subjects were involved in the calibration study(age = 40.33 ± 13.53 years, 5 females), including one OA subject with KL-score 1 for both hips. The inclusion criteria of the subject selection include(1) Subjects over 18 years(2) Subjects with no history of hip or knee surgery and no contraindications for MRI.
MRI Acquisition: The images were acquired using unilateral acquisition protocol on a GE Discovery MR750 3.0T scanner (GE Healthcare, Waukesha, WI) named as Scanner-1 with a 32-channel phased-array cardiac coil(Invivo Corp., Amsterdam, Netherlands) positioned over the left and right hip sequentially. The scanning started with the left side, followed by the right hip and the participant arrived 30 min before the scan. The same subjects were imaged using simultaneous bilateral hip imaging protocol on a GE Signa Premier 3.0T MR Scanner (GE Healthcare, Waukesha, WI) named as Scanner-2 with a 30-channel adaptive image receive(AIR) anterior array coil and a 60-channel spine posterior-array coil embedded into the table(GE Healthcare, Waukesha, WI). The subjects were imaged using Scanner-1 and Scanner-2 in 2022 with a maximum gap of one month between the acquisition. The subjects were positioned in a supine posture and feet-first inside the scanner. Magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots(MAPSS) sequence was acquired for cartilage and assessments [19]. The detailed scanning protocol for both unilateral and bilateral acquisition is summarized in Table 1.
Table 1.
MRI acquisition parameters and scanner details.
| Parameters | Scanner-1 (Unilateral) | Scanner-2 (Bilateral) |
|---|---|---|
| Scanner used | GE Discovery MR750 3.0T scanner (GE Healthcare, Waukesha, WI) | GE Signa Premier 3.0T MR scanner (GE Healthcare, Waukesha, WI) |
| Coils used | 32-Channel phased-array cardiac coil (Invivo Corp., Amsterdam, Netherlands) | 30-Channel adaptive image receive (AIR) anterior array coil and a 60-channel spine posterior-array coil (GE Healthcare, Waukesha, WI) |
| Sequence name | Hip MAPSS sagittal | Hip MAPSS sagittal |
| Acquisition time | 11 min | 16 min 30 s |
| Acquisition Matrix | 256 128 | 256 128 |
| TR (per view) | 5.2 | 5.2 |
| TSLs (ms) | 0, 15, 30, 45 | 0, 15, 30, 45 |
| TEs (ms) | 0, 10.4, 20.8, 41.6 | 0, 10.4, 20.8, 41.6 |
| FOV (cm × cm) | 14 14 | 14 14 |
| Slice thickness (mm) | 4 | 4 |
| ARC Acceleration factor | 2 1 (ky x kz) | 2 2 (ky x kz) |
| Y-FOV Oversampling factor | 100 % | 40 % |
| Number of slices | 20 | 60 |
Image Processing: All post-processing techniques were performed using an in-house program developed in MATLAB integrated with the elastix toolbox for the non-rigid image registration(version R2019a, The MathWorks Inc., Natick, MA, USA).
Cartilage Segmentation andQuantification: From the acquired bilateral MAPSS images(sagittal view) from Scanner-2, the left and right hip images were automatically divided into left and right image stacks. In the case of Scanner-1 unilateral acquisition images, the left and right-side hip images were acquired separately. The following post-processing steps were common for all the images acquired using both scanners [20]. The acetabular and femoral hip cartilage were automatically segmented using a previously validated atlas-based approach [14]. Briefly, the first echo MAPSS images were registered to a previously defined reference atlas using a non-rigid elastix registration method. The registration transformation was applied to the remaining echo images. The reference cartilage region of interest(ROI) of four slices near the hip center was generated using a semi-automated segmentation algorithm based on Bezier splines and edge detection.
The mapping was obtained by fitting the images from multiple TSLs using Levenberg-Marquardt mono-exponential equation without considering the noise level [21]:
| (1) |
Similarly, the mapping was obtained by fitting the multiple TEs corresponding to the images by Levenberg-Marquardt mono-exponential equation without considering the noise level [21]:
| (2) |
While bi-exponential fitting can distinguish between tightly and loosely bound water in cartilage, monoexponential fitting still offers valuable insights. With only four data points for fitting T₁ρ and T₂, bi-exponential fitting is challenging. The fitted and maps were used for the quantitative evaluation after the segmentation. The reference atlas-based femur, acetabular cartilage masks, and subregion segmentations were superimposed on and maps of each patient data. The cartilage subregions(Fig. 2(B)) are: R2 as posterior, R3 as posterior-superior, R4 as superior, R5 as anterior-superior, R6 as anterior, and R7 as anterior-inferior cartilage regions [20]. In the eight subregions(-), 5 subregions were selected from the femur cartilage(-) and 4 subregions(-) from acetabular cartilage and excluded subregions with less than 50 pixels overall segmented slices( and for the femur and , and for the acetabulum cartilage) [11,22]. The upper threshold of 100 ms and 80 ms were used for and maps respectively to remove the effects by fluid or partial volume [20].
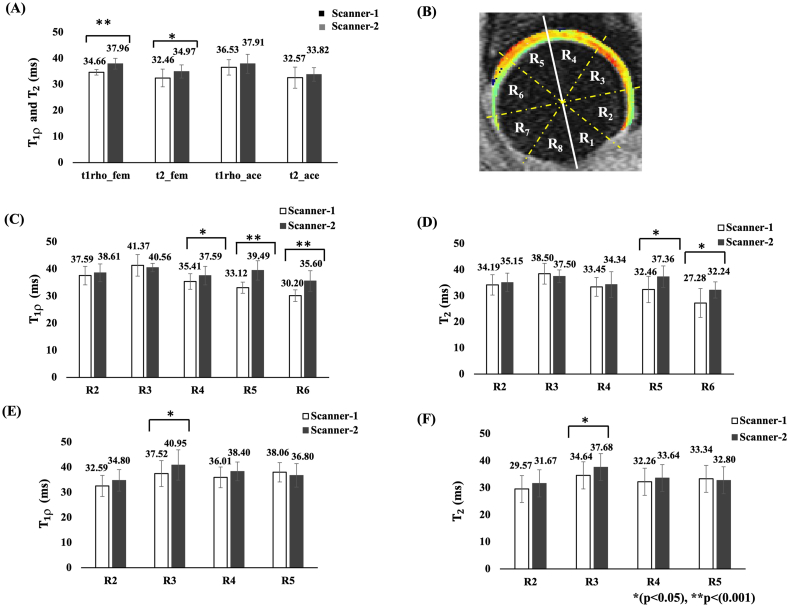
Fig. 2.
Image(A) shows the bar graph representation of mean and measurements of the femur and acetabular cartilage using ROI analysis(calibration study). Image(B) represents the divisions of the femur and acetabular cartilage subregions. The solid white line represents a reference line parallel with the femoral neck; the spherical shape is divided into eight equal subregions with 45° each represented by the yellow dashed line, and the regions are labeled as R1–R8. From this, R2–R7 are considered in the current study. R2:posterior, R3:posterior-superior, R4:superior, R5:anterior-superior, R6:anterior and R7:anterior-inferior cartilage regions. Image(C) and (D) shows the representation of mean and values of femur cartilage sub regions(-) whereas image(E) and (F) represent the mean and values of acetabular cartilage subregions(-). t1rho_fem: mean values of femoral cartilage, t2_fem: mean values of femoral cartilage, t1rho_ace: mean values of acetabular cartilage, t2_ace: mean values of acetabular cartilage.
ROI analysis: In the calibration study, T₁ρ and T₂ values in the hip cartilage of volunteers were compared between Scanner-1 and Scanner-2 using the mean ROI selection method, which averaged values across all pixels in the femoral and acetabular cartilage regions.
Voxel-based Relaxometry analysis: VBR technique was used to compare local patterns of and relaxation time measurements between Scanner-1 and Scanner-2 on a voxel-basis [14].
Z-score Normalization: The mean and standard deviation of and values obtained from VBR analysis were used for scanner specific(Scanner-1 and Scanner-2) Z-score generation using the formulas:
| (3) |
| (4) |
Where is the Z-score of voxel, is the value of voxel, represents the mean values of the Scanner-1 or Scanner-2 groups and represents the standard deviation of the values of the Scanner-1 or Scanner-2 groups.
All Z-score values beyond to +4 or −4, respectively [22]. The Z-score maps were generated and qualitatively analyzed the map patterns of cartilage values and quantitatively analyzed with the help of VBR. The calibration factor derived from(3) and(4) is:
| (5) |
The observed calibration factor from equation (5) was used to update the and values acquired bilaterally from Scanner-2 using the expression:
| (6) |
2.2. Longitudinal variability study
In the longitudinal variability study, subjects were imaged using unilateral acquisition protocol on Scanner-1 from baseline to 3rd-year follow-up. During the 4th-year follow-up, same subjects were images using bilateral acquisition protocol on Scanner-2.
Study Population and Data Selection: Eighteen hip-OA subjects(age = 53.11 ± 14.96 years, 12 females) were recruited from a previous study [22]. The inclusion criteria of the subject selection included:(1) Hip-OA subjects having KL-score less than or equal to 3(KL-score>3 considered advanced-OA). The KL-score was assessed from radiographs acquired at the baseline by an experienced musculoskeletal radiologist with 3-years of training. (2) Subjects age above 18-years. (3) Absence of intra-articular injection in the past 6 months of recruitment. (4) Subjects not having previous history of hip or knee surgery and no contraindications for using MRI.
MRI Acquisition: In the longitudinal study, MR images were acquired for all patients using unilateral acquisition protocol on Scanner-1 with a 32-channel phased-array cardiac coil(Invivo Corp., Amsterdam, Netherlands) positioned sequentially over the left and right hip. The images were acquired at baseline, 1st-year follow-up, 2nd-year follow-up and 3rd-year follow-up from a previous study and were imaged yearly from 2016 [22]. During the 4th-year follow-up, the same subjects were imaged using simultaneous bilateral hip imaging protocol on Scanner-2 with a 30-channel adaptive image receive(AIR) anterior array coil and a 60-channel spine posterior-array coil embedded into the table(GE Healthcare, Waukesha, WI). All the baseline to 3rd-year follow-up study, subjects were segmented and the mean and values were obtained using the post-processing steps explained in the section ‘Cartilage Segmentation and Quantification’ [14]. In the case of the 4th-year follow-up study, the obtained and values were modified using the scaling factor derived from equation (5). The overall processing pipeline of the study is illustrated in Fig. 1.
Fig. 1.
Flow diagram of the processing pipeline. Scanner-1 is a GE Discovery MR750 3.0T scanner and Scanner-2 is a GE Signa Premier 3.0T MR Scanner.
The mean and measurements of the hip femur and acetabular cartilage were evaluated at baseline, 1st-year, 2nd-year and 3rd-year follow-up. The 4th-year follow-up quantification was adjusted from the original and values using the scaling factor derived from the calibration study. For evaluating the changes in cartilage and measurements after calibration was obtained by subtracting the mean values at follow-up from the initial baseline values. The percentage change was calculated based on 3rd-year and 4th-year follow-up time as follows:
| (7) |
2.3. Statistical analysis
Paired Student's t-test was used in ROI and VBR analysis for evaluating the difference in cartilage mean and values between Scanner-1 and Scanner-2. A p-value of 0.05 was selected as the significant threshold level. VBR p-maps were generated and overlayed on the hip cartilage region to display the voxels with significant differences between the scanners.
The different time point trajectory analyses of hip-OA patients were assessed using a linear mixed effect (LME) model adopted from the R Development Core Team, v4.2.3 with the “lmertest”, and “lme4” packages. Initially, a null model was generated and compared with a full model where the acquisition year, sex, age, BMI and KL-score were selected as predictor variables in the mixed effect model. In these two models, hip femur and acetabular mean and , sub-region mean and values were considered outcome variables.
The fixed-effects formula was:
| (8) |
and random-effect formula was:
| random = ∼1 | Participant-ID | (9) |
3. Results
3.1. Calibration study
In the calibration study, one subject was excluded due to its poor image registration. The ROI analysis results show a significant difference in the femur mean (p < 0.001) and p = 0.024) values between Scanner-1 and Scanner-2. The subregion mean values of femoral cartilage region show a significant difference in p = 0.002), p < 0.0001) and p = 0.002) between scanners, whereas the mean value of acetabular cartilage region showed the significant difference in one subregion (p = 0.02). The subregion mean values of the femoral cartilage region showed a significant difference in p = 0.002) and p = 0.008), whereas the mean value of acetabular cartilage region showed a significant difference in one subregion p = 0.0372).
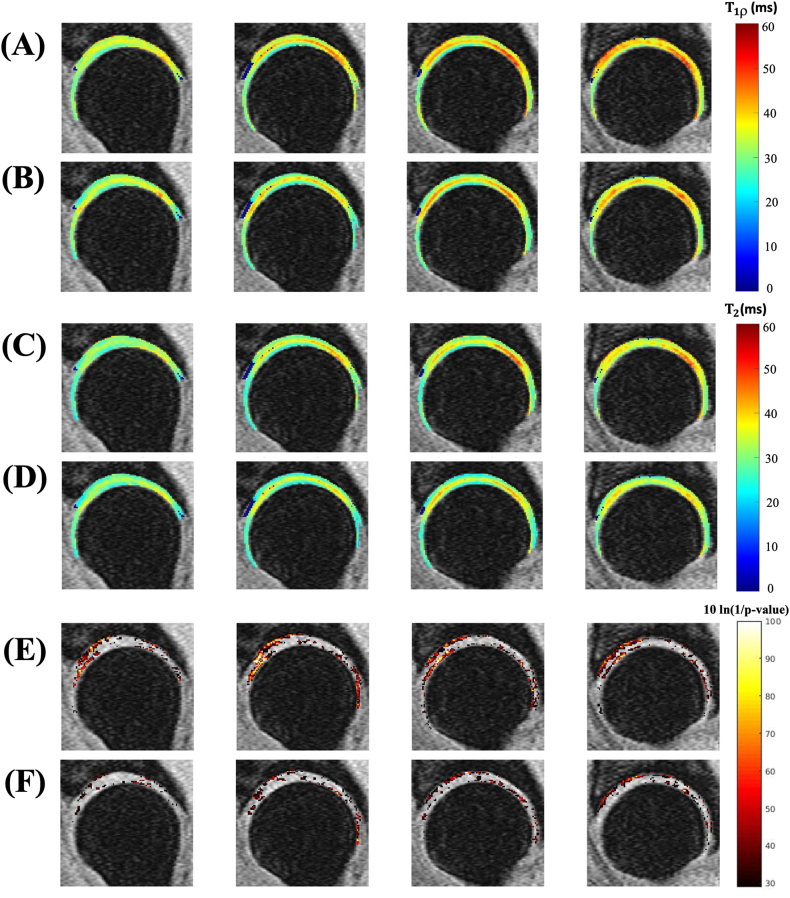
The VBR analysis based on eight subjects showed that, before Z-score normalization, 29.68 % of the femur cartilage and 23.9 % of the acetabular cartilage had significant differences in mean values between the scanners (Fig. 3(E)). Also, the VBR analysis showed that 17.65 % of the femur cartilage and 10.61 % of the acetabular cartilage voxels significantly differ in values between the scanners (Fig. 3(F)). The measurements showed a comparatively lesser number of voxels have statistically significant difference than values. The trend was like the corresponding ROI analysis results shown in Fig. 2. Hence, the ROI and VBR analysis results showed a statistically significant difference between Scanner-1 and Scanner-2.
Fig. 3.
Visualization of hip cartilage images with VBR results. Eight subjects(including 16 hips from both the left and right sides) hip images were included in the VBR analysis, utilizing four central slices from the sagittal view. Image(A) is the mean value observed from Scanner-1 and image(B) showed the mean value from Scanner-2. Image(C) and (D) showed the mean value observed from Scanner-1 and Scanner-2 respectively. Images(E) and (F) represent the p-value maps(logarithmic scale) corresponding to mean and values of both the scanners.
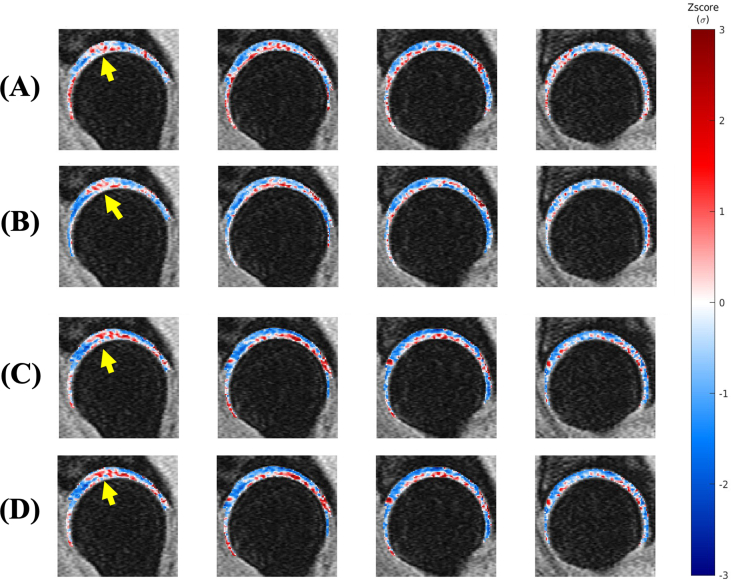
The generated Z-score map patterns were qualitatively analyzed after thresholding, based on the values. Example of one Z-score map from a healthy volunteer (age = 44years, side = right hip) were shown in Supplementary Fig. 1 based on the mean and standard deviation of values of Scanner-1 (Supplementary Fig. 1(A)) and Scanner-2 (Supplementary Fig. 1(B)) and the mean and standard deviation of values of Scanner-1 (Supplementary Fig. 1(C)) and Scanner-2 (Supplementary Fig. 1(D)). From these maps, the focal lesion presence(yellow arrow) was observed in Scanner-1 and Scanner-2 corresponding to values indicating the reduced inter-scanner variability bias between the scanners.
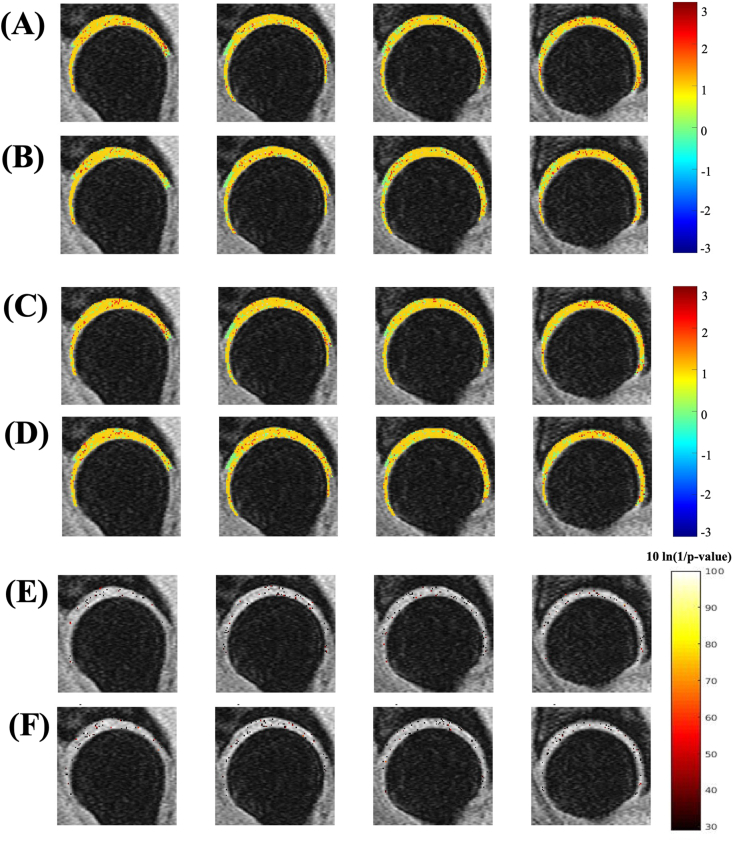
The VBR results after Z-score normalization are shown in Fig. 4. The VBR p-maps after Z-score normalization show that only 2.36 % and 3.23 % of the whole cartilage voxels have a significant difference in between the scanners for the femur and acetabular regions, respectively. Also, the VBR p-maps analysis after Z-score normalization shows that 2.30 % and 2.94 % of the whole cartilage voxels displayed significant differences in between the scanners for the femur and acetabular regions, respectively. That means the VBR analysis after Z-score normalization showed a very small number of voxels displayed a statistically significant difference between Scanner-1 and Scanner-2.
Fig. 4.
VBR results after calibration correction. Image(A) shows the Z-score maps of Scanner-1 and image(B) shows the Z-score maps of Scanner-2, which correspond to values. Images(C) and(D) show the Z-score maps of Scanner-1 and Scanner-2 correspond to values. Image(E) represents the p-value maps(logarithmic scale) corresponding to values and image(F) represents the p-value maps corresponding to values.
3.2. Longitudinal variability study
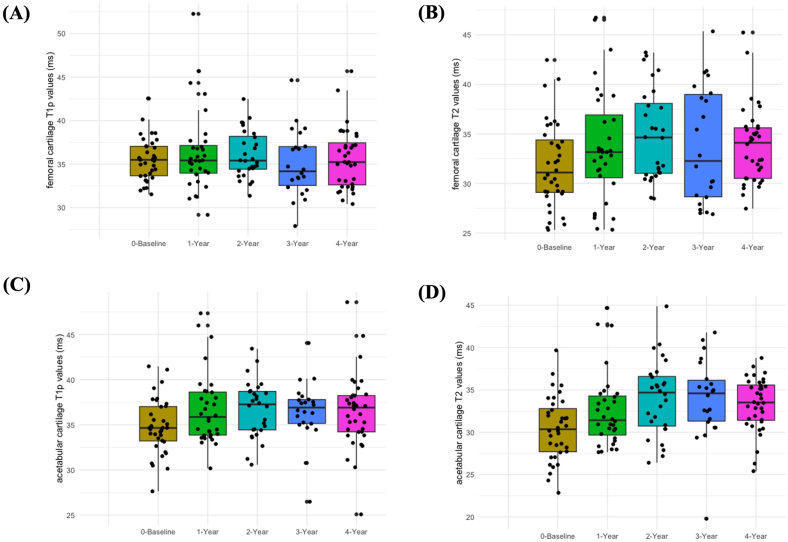
In the longitudinal variability study, all the eighteen subjects data were processed and evaluated successfully. Based on the KL-score assessment, 17 hips were in the KL-score 0 group, 15 hips were in the KL-score 1 group, 3 hips were in the KL-score 2 group, and 1 hip was in the KL-score 3 group. Fig. 5 represents the Box-and-Whisker plots of all the hip-OA patients mean and values of the femur and acetabular cartilage in the longitudinal study.
Fig. 5.
Box and Whisker plots illustrating the femur cartilage mean and values(A) and (B) and acetabular cartilage mean and values(C) and (D) at various time points(baseline, 1st-year, 2nd-year, 3rd-year and 4th-year follow-up).
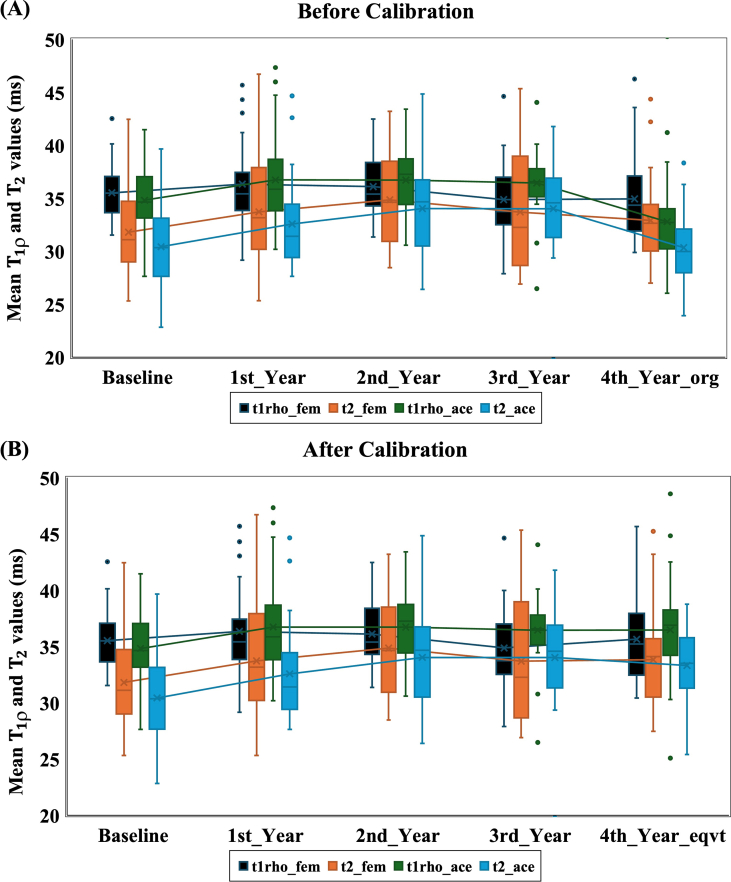
The Box-and-Whisker plots in the Supplementary Fig. 2 demonstrated an upward trajectory of and values after calibration (Supplementary Fig. 2(B)) during the 4th year follow-up. The PC analysis results showed that 52 % of subjects in the longitudinal variability study have a positive trend in the mean values of the femur cartilage after calibration correction, whereas 42 % showed the same trend without calibration. Similarly, 48 % of subjects showed increased femur cartilage after calibration, whereas 44 % showed the same trend without calibration. The results showed that 52 % of subjects have an increasing trend in the mean values of the acetabular cartilage after calibration, whereas only 11 % showed the same trend without calibration. 38 % of the subjects showed an increase in the acetabular cartilage after calibration, whereas 19 % showed the same trend without calibration. The percentage change analysis observed that the and values increased after calibration more on the acetabular cartilage.
LME model analysis revealed significant correlations between variables and cartilage regions. As a predictor variable, BMI significantly correlated with the femur and acetabular cartilage, as shown in Table 2. Acquisition time points exhibited positive correlations with mean values of femoral cartilage and mean and values of the acetabular cartilage. Female sex was significantly correlated with mean values of femoral cartilage.
Table 2.
LME results based on mean and values of the whole femur and acetabular cartilage region. Variables and cartilage regions showing significant correlations are presented in this table. BMI: body mass index, Sex: Male or Female, Acq_year: Longitudinal study time points.
| andvalues | Value | Std.Error | DF | t-value | p-value | Parameter |
|---|---|---|---|---|---|---|
| _Femur | 1.19 | 0.14 | 115 | 8.79 | <0.0001∗ | BMI |
| 4.76 | 1.60 | 35 | 2.98 | 0.0052∗ | Sex | |
| _Femur | 1.18 | 0.16 | 115 | 7.53 | <0.0001∗ | BMI |
| 2.56 | 0.90 | 115 | 2.85 | 0.0052∗ | Acq_year | |
| _Acet | 1.41 | 0.11 | 115 | 12.37 | <0.0001∗ | BMI |
| 2.03 | 0.88 | 115 | 2.30 | 0.0230∗ | Acq_year | |
| _Acet | 1.27 | 0.12 | 115 | 10.45 | <0.0001∗ | BMI |
| 4.04 | 0.97 | 115 | 4.18 | 0.0001∗ | Acq_year |
∗p-value <0.05.
In subregion analysis, BMI was significantly correlated with mean and values across various subregions, including - of the femur cartilage and - of the acetabular cartilage (Table 3). Female sex was significantly correlated with femoral mean values in , regions, while it correlated with femoral mean values in the region only. Similarly, the acetabular mean and mean values in the subregion showed significant correlations with female sex. mean values of femoral subregion were significantly correlated with the acquisition year time point, whereas mean values of femoral subregions and correlated with the acquisition year. In the acetabular region, mean values of were significantly correlated, while mean values of subregions showed significant correlations with the acquisition year. Additionally, the KL-score demonstrated a significant correlation with mean and values of the femoral subregion.
Table 3.
LME results based on and cartilage mean values of femur (-) and acetabular (-) cartilage subregions. Variables and cartilage regions showing significant correlations are presented in this table. BMI: body mass index, Sex: Male or Female, Acq_year: Longitudinal study time points, KL-score: Kellgren-Lawrence score.
| Subregions | Value (beta) | Std.Error | DF | t-value | p-value | Parameter | |
|---|---|---|---|---|---|---|---|
| MeanValues | Femur | 1.18 | 0.17 | 115 | 6.86 | <0.0001 | BMI |
| 7.17 | 1.86 | 35 | 3.86 | 0.0005 | Sex | ||
| 3.46 | 1.22 | 115 | 2.84 | 0.0053 | KL -score | ||
| Femur | 1.45 | 0.15 | 115 | 9.42 | <0.0001 | BMI | |
| 4.03 | 1.69 | 35 | 2.38 | 0.021 | Sex | ||
| 2.92 | 1.07 | 115 | 2.74 | 0.0071 | Acq_year | ||
| Femur | 1.22 | 0.16 | 115 | 7.85 | <0.0001 | BMI | |
| 4.25 | 1.76 | 35 | 2.41 | 0.0211 | Sex | ||
| Femur | 1.55 | 0.16 | 115 | 9.42 | <0.0001 | BMI | |
| Femur | 1.27 | 0.14 | 115 | 9.13 | <0.0001 | BMI | |
| Mean Values | Femur | 2.85 | 1.21 | 115 | 2.35 | 0.0204 | KL-score |
| 1.08 | 0.17 | 115 | 6.41 | <0.0001 | BMI | ||
| 6.31 | 1.81 | 35 | 3.49 | 0.0013 | Sex | ||
| 5.54 | 1.36 | 115 | 4.06 | 0.0001 | Acq_year | ||
| Femur | 0.74 | 0.20 | 115 | 3.80 | 0.0002 | BMI | |
| 3.19 | 1.08 | 115 | 2.96 | 0.0038 | Acq_year | ||
| Femur | 1.29 | 0.17 | 115 | 7.67 | <0.0001 | BMI | |
| Femur | 1.33 | 0.18 | 115 | 7.23 | <0.0001 | BMI | |
| Femur | 1.09 | 0.20 | 115 | 5.57 | <0.0001 | BMI |
| LME results of acetabular (-) cartilage subregions |
Parameter |
||||||
| Subregions | Value (beta) | Std.Error | DF | t-value | p-value | ||
| Meanvalues | Acetabular | 0.97 | 0.133 | 115 | 7.24 | <0.0001 | BMI |
| 4.57 | 1.43 | 35 | 3.19 | 0.0030 | Sex | ||
| Acetabular | 1.69 | 0.13 | 115 | 13.40 | <0.0001 | BMI | |
| Acetabular | 1.43 | 0.15 | 115 | 9.53 | <0.0001 | BMI | |
| Acetabular | 1.46 | 0.15 | 115 | 9.84 | <0.0001 | BMI | |
| 7.16 | 1.42 | 115 | 9.84 | <0.0001 | Acq_year | ||
| Meanvalues | Acetabular | 0.83 | 0.14 | 115 | 5.92 | <0.0001 | BMI |
| 3.33 | 1.49 | 35 | 2.24 | 0.0317 | Sex | ||
| 5.65 | 1.27 | 115 | 4.44 | <0.0001 | Acq_year | ||
| Acetabular | 1.38 | 0.13 | 115 | 10.37 | <0.0001 | BMI | |
| 4.61 | 1.18 | 115 | 3.90 | 0.0002 | Acq_year | ||
| Acetabular | 1.27 | 0.15 | 115 | 8.32 | <0.0001 | BMI | |
| 3.05 | 1.20 | 115 | 2.55 | 0.0121 | Acq_year | ||
| Acetabular | 1.37 | 0.15 | 115 | 9.40 | <0.0001 | BMI | |
| 4.87 | 1.25 | 115 | 3.89 | 0.0002 | Acq_year | ||
4. Discussion
In our 5-year follow-up study, the associations between cartilage and relaxation times and parameters such as BMI, sex, KL-score and acquisition time points were established. Our longitudinal variability study used a second scanner (Scanner-2) to acquire the 4th-year follow-up images. The ROI and VBR analyses showed a statistically significant difference in mean cartilage values between the scanners based on an extra calibration study. A Z-score normalization method incorporated with the VBR technique was implemented to address the inter-scanner variability inherent in a multi-center study with unilateral and bilateral protocols. With our patient study, the LME analysis showed a notable correlation between BMI and the and values of hip cartilage in both the femur and acetabular region. Furthermore, we observed a significant increase in acetabular and values, as well as femoral values over the time points. As a result, our research builds upon previous investigations in hip cartilage compositional analysis, reinforcing and values as valuable biomarkers for hip cartilage degradation, providing support for its use in multi-center studies through careful calibration and analytical approach [11].
The present longitudinal study conducted image acquisition unilaterally using Scanner-1 and 4th-year follow-up bilaterally using Scanner-2. Our group shows the preliminary results of comparing bilateral hip acquisition using Scanner-2 with unilateral acquisition using Scanner-1 [23]. The findings indicated that the simultaneous bilateral MAPSS acquisition technique reduced total imaging time by 28.41 % compared to two sequential unilateral hip acquisitions. Another reported study showed that the recently developed VBR analysis could be a more accurate tool for the local pattern evaluation of cartilage biochemical changes [14]. VBR aligns all subjects in a group to a single reference space, which serves as the basis for this technique. The findings from the VBR analysis align with the conventional ROI analysis. Also, studies showed that for reducing the inter-scanner variability, the scaling factor correction was done on quantified parameters using the Z-score based normalization technique [17,19]. In the calibration study, over 20 % of voxels exhibited a significant difference in values and more than 10 % of voxels showed significant differences in values. However, after applying the Z-score based scaling factor, the VBR results showed that only less than 3.3 % of the voxels showed a significant difference. Hence, in the current study, this normalization technique was effectively utilized to mitigate inter-scanner variability. Based on these results, we recommend that adopting the Z-score based normalization method in future longitudinal studies can have the ability to reduce the inter-scanner variability, potentially enhancing the interpretability and applicability of and mapping results in clinical practice and multicenter longitudinal research studies.
Based on our patient study, BMI was the prime characteristic correlated with the and values in the femur, acetabular cartilage regions and its subregions. The onset and progression of hip osteoarthritis can be attributed to irregular mechanical stress resulting from increased weight on weight-bearing joints [24]. Generally, the biochemical changes in hip cartilage were quantitatively assessed through measurements of and values. A prior study indicated that BMI was significantly increased in acetabular cartilage lesion progressors(p = 0.05) and showed a higher mean and values in the femoral cartilage of the lesion group at 18 months [11]. Another study reported that values of cartilage in meniscal tear patient data were significantly increased in an obese group compared to a normal BMI group(p = 0.008) [25]. So, the positive correlation between biochemical changes of cartilage and BMI from our study showed the use of quantitative MR imaging for finding the progression of hip-OA [11,25]
In addition to BMI, sex, acquisition year time point and KL-score also showed a positive correlation with some femur and acetabular cartilage subregions. This correlation was consistent with the previous longitudinal study result, which showed that age, BMI and female sex had a positive trend of progression [11]. Subburaj et al. reported regional differences in individuals with and without femoroacetabular impingement, particularly observing significant differences in the anterior-superior region of the hip joint cartilage between patients and healthy subjects [26]. Another study showed that the anterior-superior region of the acetabulum was associated with increased hip contact stress in a finite element analysis [27]. Wyatt et al. recently highlighted that elevated and values were detected in the anterior-superior and central regions of the acetabular cartilage in individuals with radiographic hip-OA [13]. Gallo et al. reported higher and in the superoposterior region of the femoral cartilage for subjects with cartilage lesions over an 18-month follow-up [11]. Consistent with these findings, our study revealed that the values of posterior-superior and values of posterior, posterior-superior subregions of femoral cartilage showed a statistically significant correlation with acquisition year. Also, the values of anterior-superior subregion and values of posterior, posterior-superior, superior and anterior-superior subregions of acetabular cartilage showed a statistically significant correlation with acquisition year. The and values are derived from different relaxation mechanisms; however, both parameters effectively examine the slow motion of water protons [28,29]. Therefore, the increasing trend of both and values in cartilage over time may indicate hip cartilage degradation, showing their potential as valuable biomarkers for hip cartilage health.
There are limitations to this study that need to be taken into consideration, including a relatively small study population. Subsequent studies involving larger cohorts and extended follow-up periods can provide further substantiation for the results obtained in this study. Second, the atlas-based segmentation and VBR analysis were only applied to the four center slices selected from each hip. The cartilage segmentation and post-processing tools need to be developed based on the newly developed deep learning methods to mitigate this limitation. Third, the 4 mm slice thickness of MAPSS sequence might cause the partial volume effect during the quantitative analysis. Finally, the current study performed a calibration study in a single vendor(GE) multi-site scanner. Additional studies are required to determine if these same techniques could be applied to scale data between vendor scanners.
In summary, the calibration approach showcased the effectiveness of VBR and Z-score normalization in mitigating inter-scanner variability. The LME analysis revealed a significant correlation between BMI and and values in the femur and acetabular cartilage over the 5-year follow-up study. Additionally, and values of the posterior-superior region of the femur and anterior-superior region of acetabular cartilage exhibited significant increases over time. These results affirm the significance of increasing trends in and relaxation times as biomarkers for assessing treatment efficacy in patients with hip-OA.
Author contributions
Rafeek Thahakoya: Conceptualization, Investigation, Data Acquisition, Methodology, Software, Validation, Formal analysis, Data curation, Writing - Original draft, review & editing.
Koren E. Roach, Rupsa Bhattacharjee: Conceptualization, Investigation, Methodology, Software, Writing - Review & editing.
Misung Han: Investigation, Data Acquisition, Methodology, Validation, Writing - Review & editing.
Fei Jiang: Software, Formal analysis, Writing - Review & editing.
Johanna Luitjens: Investigation, Validation, Writing - Review & editing.
Emma Bahroos: Investigation, Validation, Writing - Review & editing.
Valentina Pedoia, Richard B. Souza, Sharmila Majumdar: Conceptualization, Investigation, Methodology, Supervision, Funding acquisition, Writing - Review & editing.
All authors approved the final version of the article.
Role of the funding source
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers: R01AR069006, K24AR072133.
Declaration of competing interest
The authors have declared that no conflict of interest exists.
Acknowledgment
The authors would like to thank Dr. Michael Carl for his assistance in adapting MAPSS pulse sequence programs following the scanner software upgrade.
Handling Editor: Professor H Madry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2024.100538.
Contributor Information
Rafeek Thahakoya, Email: rafeek.thahakoya@ucsf.edu.
Koren E. Roach, Email: koren.roach@ucalgary.ca.
Misung Han, Email: misung.han@ucsf.edu.
Rupsa Bhattacharjee, Email: rupsa.bhattacharjee@ucsf.edu.
Fei Jiang, Email: Fei.Jiang@ucsf.edu.
Johanna Luitjens, Email: johanna.luitjens@gmail.com.
Emma Bahroos, Email: Emma.Bahroos@ucsf.edu.
Valentina Pedoia, Email: valentina.pedoia@ucsf.edu.
Richard B. Souza, Email: richard.souza@ucsf.edu.
Sharmila Majumdar, Email: sharmila.majumdar@ucsf.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Supplementary figure 1.
The Z-score maps based on the mean and standard deviation of (A) and C) values of Scanner-1 and Z-score maps based on the mean and standard deviation of (B) and D) values of Scanner-2. The yellow arrow shows the presence of the focal lesion was qualitatively observed in the Z-score maps of both scanners.
Supplementary figure. 2.
Box and Whisker plots show the upward changes in cartilage after calibration. Image(A) shows the mean and values of the femur and acetabular cartilage region from baseline to 4th year follow-up scans(without calibration). Image(B) shows the mean and values of the femur and acetabular cartilage after calibration.
References
- 1.Scott D. Osteoarthritis of the hip. Am. Fam. Physician. 2010;81(4):444–445. [PubMed] [Google Scholar]
- 2.Gold G.E., Cicuttini F., Crema M.D., Eckstein F., Guermazi A., Kijowski R., et al. OARSI Clinical Trials Recommendations: hip imaging in clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):716–731. doi: 10.1016/j.joca.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalian M., Li X., Guermazi A., Obuchowski N.A., Carrino J.A., Oei E.H., et al. The QIBA profile for MRI-based compositional imaging of knee cartilage. Radiology. 2021;301(2):423–432. doi: 10.1148/radiol.2021204587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abramson S.B., Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res. Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16(4):494. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koster I.M., Oei E.H.G., Hensen J.H.J., Boks S.S., Koes B.W., Vroegindeweij D., et al. Predictive factors for new onset or progression of knee osteoarthritis one year after trauma: MRI follow-up in general practice. Eur. Radiol. 2011;21(7):1509–1516. doi: 10.1007/s00330-011-2089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang M., Min Z., Rana N., Liu H. Accuracy of magnetic resonance imaging in grading knee chondral defects. Arthrosc. J. Arthrosc. Relat. Surg. 2013;29(2):349–356. doi: 10.1016/j.arthro.2012.04.138. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Pedoia V., Kumar D., Rivoire J., Wyatt C., Lansdown D., et al. Cartilage T1ρ and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage. 2015;23(12):2214–2223. doi: 10.1016/j.joca.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guermazi A., Alizai H., Crema M.D., Trattnig S., Regatte R.R., Roemer F.W. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthritis Cartilage. 2015;23(10):1639–1653. doi: 10.1016/j.joca.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Carballido-Gamio J., Link T.M., Li X., Han E.T., Krug R., Ries M.D., et al. Feasibility and reproducibility of relaxometry, morphometric, and geometrical measurements of the hip joint with magnetic resonance imaging at 3T. J. Magn. Reson. Imag. 2008;28(1):227–235. doi: 10.1002/jmri.21411. [DOI] [PubMed] [Google Scholar]
- 11.Gallo M.C., Wyatt C., Pedoia V., Kumar D., Lee S., Nardo L., et al. T1ρ and T2 relaxation times are associated with progression of hip osteoarthritis. Osteoarthritis Cartilage. 2016;24(8):1399–1407. doi: 10.1016/j.joca.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemeth A., Marco L.D., Boutitie F., Sdika M., Grenier D., Rabilloud M., et al. Reproducibility of in vivo magnetic resonance imaging T1 rho and T2 relaxation time measurements of hip cartilage at 3.0T in healthy volunteers. J Magn Reson imaging : JMRI. 2017;47(4):1022–1033. doi: 10.1002/jmri.25799. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt C., Kumar D., Subburaj K., Lee S., Nardo L., Narayanan D., et al. Cartilage T1ρ and T2 relaxation times in patients with mild-to-moderate radiographic hip osteoarthritis. Arthritis Rheumatol. 2015;67(6):1548–1556. doi: 10.1002/art.39074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedoia V., Gallo M.C., Souza R.B., Majumdar S. Longitudinal study using voxel-based relaxometry: association between cartilage T1ρ and T2 and patient reported outcome changes in hip osteoarthritis. J. Magn. Reson. Imag. 2017;45(5):1523–1533. doi: 10.1002/jmri.25458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaan M.A., Pedoia V., Zhang A.L., Gallo M.C., Link T.M., Souza R.B., et al. A novel mr-based method for detection of cartilage delamination in femoroacetabular impingement patients. J. Orthop. Res. : Off Publ Orthop Res Soc. 2017;36(3):971–978. doi: 10.1002/jor.23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medawar E., Thieleking R., Manuilova I., Paerisch M., Villringer A., Witte A.V., et al. Estimating the effect of a scanner upgrade on measures of grey matter structure for longitudinal designs. PLoS One. 2021;16(10) doi: 10.1371/journal.pone.0239021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razzaq S., Haririsanati L., Eyre K., Garg R., Chetrit M., Friedrich M.G. Inter-scanner comparability of Z-scores for native myocardial T1 and T2 mapping. J. Cardiovasc. Magn. Reson. 2024 doi: 10.1016/j.jocmr.2023.100004. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kranzusch R., Siepen F aus dem, Wiesemann S., Zange L., Jeuthe S., Silva TF da, et al. Z-score mapping for standardized analysis and reporting of cardiovascular magnetic resonance modified Look-Locker inversion recovery (MOLLI) T1 data: normal behavior and validation in patients with amyloidosis. J. Cardiovasc. Magn. Reson. 2020;22(1):6. doi: 10.1186/s12968-019-0595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Han E.T., Busse R.F., Majumdar S. In vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots(3D MAPSS) Magn. Reson. Med. 2008;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharjee R., Thahakoya R., Luitjens J., Han M., Roach K.E., Jiang F., et al. Effects of T1p characteristics of load-bearing hip cartilage on bilateral knee patellar cartilage subregions: subjects with none to moderate radiographic hip osteoarthritis. J. Magn. Reson. Imag. 2023 doi: 10.1002/jmri.29009. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koff M.F., Amrami K.K., Kaufman K.R. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15(2):198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Roach K.E., Souza R.B., Majumdar S., Pedoia V. Local patterns in 2-year T1ρ and T2 changes of hip cartilage are related to sex and functional data: a prospective evaluation on hip osteoarthritis participants. J. Magn. Reson. Imag. 2023;57(4):spcone. doi: 10.1002/jmri.28247. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach K.E., Han M., Link T.M., Souza R.B., Pedoia V., Majumdar S. Feasibility of simultaneous bilateral hip quantitative MRI. Proc Int Soc Magn Reson Med 2022. London, UK: International Society of Magnetic Resonance in Medicine. 2022:2309. [Google Scholar]
- 24.Jiang L., Rong J., Wang Y., Hu F., Bao C., Li X., et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Jt Bone Spine. 2011;78(2):150–155. doi: 10.1016/j.jbspin.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich K.M., Shepard T., Oliveira VS de, Wang L., Babb J.S., Schweitzer M., et al. T2 measurements of cartilage in osteoarthritis patients with meniscal tears. AJR Am. J. Roentgenol. 2009;193(5):W411–W415. doi: 10.2214/ajr.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subburaj K., Valentinitsch A., Dillon A.B., Joseph G.B., Li X., Link T.M., et al. Regional variations in MR relaxation of hip joint cartilage in subjects with and without femoralacetabular impingement. Magn. Reson. Imaging. 2013;31(7):1129–1136. doi: 10.1016/j.mri.2013.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris M.D., Anderson A.E., Henak C.R., Ellis B.J., Peters C.L., Weiss J.A. Finite element prediction of cartilage contact stresses in normal human hips. J. Orthop. Res. 2012;30(7):1133–1139. doi: 10.1002/jor.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regatte R.R., Akella S.V.S., Lonner J.H., Kneeland J.B., Reddy R. T1ρ relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1ρ with T2. J. Magn. Reson. Imag. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 29.Stahl R., Luke A., Li X., Carballido-Gamio J., Ma C.B., Majumdar S., et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients—a 3.0-Tesla MRI study. Eur. Radiol. 2009;19(1):132–143. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]