Abstract
Background
Extrapulmonary tuberculosis (TB) is a relatively rare form of tuberculosis infection, accounting for approximately 15% of all tuberculosis infections. Lymph nodes are the most commonly affected sites, while involvement of the parotid gland is extremely rare.
Case presentation
We present the case of a 65-year-old male patient with a one-month history of a left parotid mass. The patient has a history of diabetes and long-term smoking, and a chest X-ray revealed secondary fibrotic pulmonary tuberculosis, while sputum smears were culture-negative for Mycobacterium tuberculosis (Mtb). The parotid mass was surgically removed and subjected to routine HE staining, acid-fast staining, and PCR molecular testing for Mtb. The final diagnosis was Warthin’s tumor of the parotid gland with concomitant tuberculosis. One month after removal of the parotid mass, the patient’s chest CT showed cavitary tuberculosis. Subsequently, the patient received anti-tuberculosis treatment; however, due to severe gastrointestinal adverse effects, the patient stopped the medication in less than a month and did not receive regular treatment. Four months after stopping the medication, the patient’s pulmonary tuberculosis progressed and worsened.
Conclusion
Combined tuberculosis in Warthin’s tumor is extremely rare, with only 14 cases reported to date. However, the specific pathogenesis of this condition is not yet fully understood, and the preliminary treatment and prognosis have not been conclusively determined. Early diagnosis of tuberculosis, standardized and effective use of anti-tuberculosis drugs, and personalized treatment are crucial in the management of tuberculosis. We have reviewed the treatment progress of this rare disease and analyzed the potential pathogenesis of the condition. Furthermore, we have summarized the current understanding of the pathogenesis of tuberculosis, drug resistance mechanisms, and the latest treatment advances. These studies have important clinical implications for better understanding and treating extrapulmonary tuberculosis and tuberculosis within Warthin’s tumor of the parotid gland. This comprehensive analysis sheds light on the complexities of tuberculosis and provides valuable insights for improved management and care of affected individuals.
Keywords: Mycobacterium tuberculosis, Parotid gland, Warthin’s tumor, Anti-tuberculosis treatment, Cavitary pulmonary tuberculosis, Case report
Background
Warthin’s tumor (WT) is the second most common benign tumor of the parotid gland [1], after pleomorphic adenoma, accounting for approximately 14–30% of all parotid tumors [2]. It typically presents as a slow-growing, painless, nodular mass in the lower pole of the parotid gland, and is more common in middle-aged or elderly males, especially smokers (> 50 years old) [3]. WT is an encapsulated tumor composed of double-layered oncocytic epithelial cells and lymphoid stroma, with both cystic and solid areas. TB is a leading cause of global infectious disease mortality, with over 10 million new cases reported annually [4]. Parotid tuberculosis is a rare disease, even in countries with a high prevalence of tuberculosis [5]. The coexistence of tuberculosis within a Warthin tumor of the parotid gland is extremely rare, with only 15 cases reported in English literature, including the cases discussed in this article [2, 6–14].
Here, we report a rare case of combination of tuberculosis within a Warthin’s tumor in the parotid gland. Six months after surgery, the patient did not receive regular treatment, which led to further progression and deterioration of cavitary pulmonary tuberculosis.
Case presentation
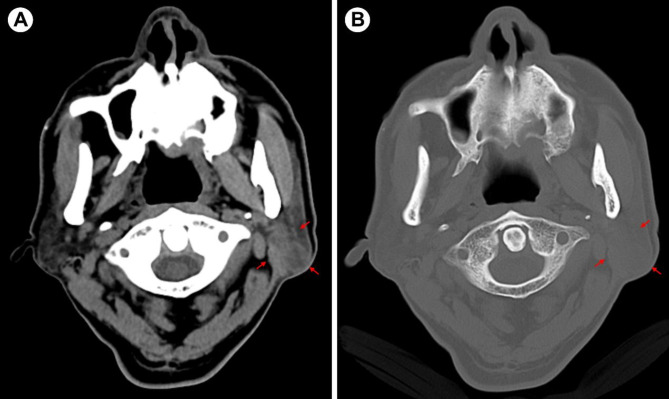
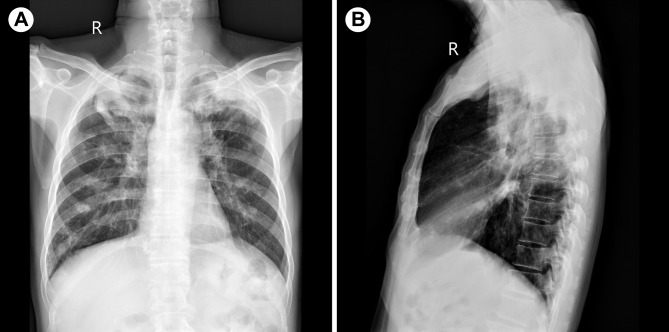
A 65-year-old elderly male presented with a painless mass in the left parotid region for over a month. The patient had a history of diabetes and long-term smoking, with no family history of tuberculosis. Additionally, during the more than ten years of having diabetes, the patient has been regularly taking metformin, and their blood sugar levels have remained within a controllable range. On specialized examination, a mass measuring approximately 30 mm × 25 mm was observed in the lower posterior part of the left parotid region. The overlying skin appeared normal without any signs of redness or fever. The mass was firm, mobile, and had clear borders, with no fluctuation or tenderness. Oral hygiene was average. Computed tomography (CT) examination revealed a circular soft tissue density lesion measuring approximately 16 mm × 15 mm in the superficial lobe of the left parotid gland (Fig. 1A, B), suggestive of a cystic mixed tumor. Chest X-ray showed secondary fibrotic tuberculosis in both lungs (Fig. 2A, B), while sputum smears were culture-negative for Mtb. Laboratory testing showed a white blood cell count within the normal range, with a slight increase in neutrophils, lymphocytes, and monocytes.
Fig. 1.
(A, B) CT shows a class of rounded soft tissue density shadows in the superficial lobe of the left parotid gland (shown by red arrows), with no destruction of the adjacent bone
Fig. 2.
(A, B) Chest X-ray shows thickening of the texture of both lungs, with patchy, mottled cords and nodular-like hyperdense shadows; the upper lung fields are prominent
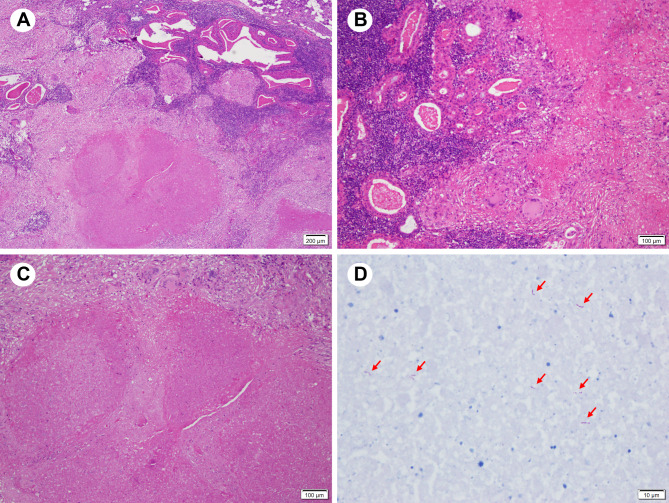
Under general anesthesia, the patient underwent excision of the left parotid region mass. Gross examination of the specimen revealed a tumor measuring approximately 20 mm × 16 mm × 15 mm, with intact capsule and multiple nodules on the cut surface, some of which showed necrosis. Histopathological examination with hematoxylin and eosin (HE) staining showed tumor tissue composed of bilayered oncocytic epithelium and lymphoid stroma, surrounded by normal salivary gland tissue (Fig. 3A). The epithelial component formed glandular or cystic structures with secretions in the lumens (Fig. 3B). Numerous granulomatous nodules consisting of caseous necrosis, epithelioid cells, and Langhan’s giant cells were observed in between (Fig. 3B, C). Acid-fast staining (Fig. 3D) and polymerase chain reaction (PCR) testing confirmed the presence of Mtb. The final pathological diagnosis was combination of tuberculosis within a Warthin’s tumor.
Fig. 3.
Hematoxylin and eosin stain (HE) staining and acid-fast staining show characteristic parotid gland lesions. (A) HE staining shows that the tumor tissue is composed of epithelial cells and lymphoid stroma, with mixed granulomatous lesions, surrounded by normal salivary gland tissue. (B) The bilayered eosinophilic epithelial component forms glandular or cystic structures, with secretions within the lumina. (C) Granulomas consist of large areas of caseous necrosis and Langhans giant cells. (D) Acid-fast staining shows a positive result (shown by red arrows)
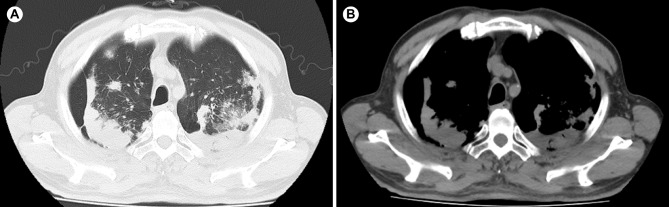
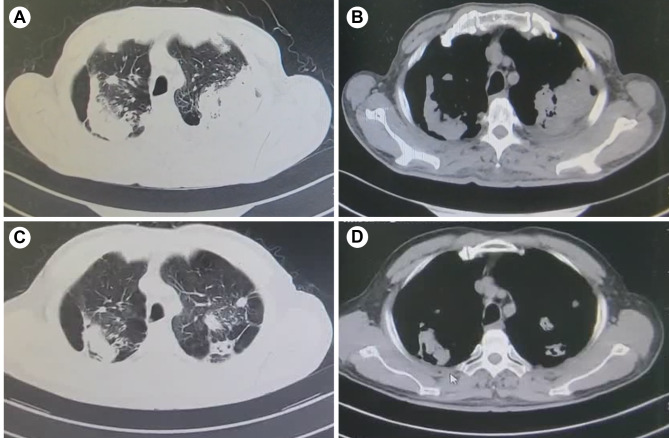
The patient’s wound healed well, but experienced facial nerve paralysis. One month after removal of the parotid mass, the patient’s chest CT showed cavitary pulmonary tuberculosis (Fig. 4A, B). Subsequently, underwent an Xpert MTB/RIF test, which showed RIF sensitivity. Therefore, the patient received a combination of isoniazid (INH), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA), which are anti-tuberculosis drugs for the treatment of tuberculosis, while the patient stopped the medication in less than a month due to severe gastrointestinal adverse effects. Four months after stopping the medication, the patient’s pulmonary tuberculosis progressed and worsened (Fig. 5A, B). Subsequently, the patient voluntarily sought treatment at a specialized infectious disease hospital in another province. Currently, the patient has undergone 10 months of anti-tuberculosis treatment and and the pulmonary tuberculosis cavity has significantly resolved with well-controlled tuberculosis symptoms (Fig. 5C, D). The patient is now in good health and will continue to receive 2 more months of anti-tuberculosis treatment.
Fig. 4.
(A, B) CT scan reveals pulmonary lesions one month after parotid gland surgery
Fig. 5.
CT scan reveals pulmonary lesions. (A, B) CT scan shows bilateral cavitary pulmonary tuberculosis in the patient. (C, D) CT scan demonstrates significant reduction in bilateral cavitary lesions after 10 months of standard anti-tuberculosis treatment
Discussion
WT is a well-known benign tumor. Although WT typically occurs in the parotid gland, approximately 8% of these tumors may originate from sites other than the parotid gland, with the most commonly accepted site being cervical lymph nodes [15]. Its aetiopathogenesis continues to be an unresolved and controversial question. Initially, Thompson and Bryant propose that tumor due to the neoplastic proliferation of parotid ductal epithelium and the concomitant accumulation of lymphoid tissue [16]. Subsequently, this theory was supported by immunohistochemistry (IHC) finding that luminal and basal epithelial cells of WT posse characteristics similar to those of the striated duct cells and basal cells of the excretory duct of salivary gland [17]. A retrospective study on salivary gland tumors originating from heterotopic salivary inclusions revealed that among 24 patients, 15 developed tumor lesions. The benign tumors were predominantly Warthin’s tumor (8) and pleomorphic adenoma (1), suggesting a significant correlation between heterotopic salivary glands in periparotid and cervical lymph nodes and the occurrence of Warthin’s tumor [18]. Orabona et al. proposed that that hypersensitive/allergic reaction may play a crucial role in the epithelial proliferation during the formation of WT and potentially stimulate reactive proliferation in the germinal centers of lymphoid stroma [19]. The metaplastic theory has been associated with the impact of long-term cigarette smoking, which has also been suggested as a potential explanation for the historical male predominance and higher incidence of head and neck squamous cell carcinoma in patients with WTs [20, 21]. While the exact cause is still uncertain, it is believed that damage to mitochondrial DNA, possibly resulting from chronic nicotine abuse, and abnormalities in mitochondrial enzymes contribute to the development of WTs [22, 23]. Studies have shown that the lymphoid stromal components of WTs, including B cells and T cells, are polyclonal, suggesting that the lymphoid component may represent a hypersensitive reaction to the proliferating ducts and their secretions, which manifests itself in a manner similar to that of regional lymph nodes [14, 23].
As a common infectious disease, TB typically manifests with pulmonary infection. Extrapulmonary tuberculosis represents approximately 15% of all TB infections, lymphatic TB represents approximately 15% of extrapulmonary TB cases [24]. It affects the cervical lymph nodes most frequently. Besides cervical lymph nodes, tuberculous involvement of head and neck is extremely uncommon. In a study by Prasad et al., out of 165 patients of head and neck tuberculosis, only 3 patients were found to have parotid tuberculosis [25]. WT with coexistent TB is extremely rare.
When a person inhales aerosol droplets containing Mtb, infection begins. After entering the lungs, Mtb is engulfed by phagocytic cells and then transported to the lymph nodes on the same side of the body. Subsequently, phagocytic cells containing Mtb present antigens to naïve lymphocytes, triggering an immune response. Activated lymphocytes return to the lungs and suppress tuberculosis infection. Live Mtb either gets transported by phagocytic cells to the lymph nodes or carried by lymphatic fluid, where it starts to replicate and form granulomas [26]. Lymph nodes serve as sites for the persistence, dissemination, and reactivation of Mtb, and the reactivation of latent pulmonary tuberculosis can also originate from lymph nodes [27, 28]. Although lymph nodes generate immune responses against Mtb infection, studies have shown that they are poor killers of the bacteria and often fail to eliminate the infection [27]. Granuloma is a typical characteristic of TB, primarily composed of central caseous necrosis surrounded by macrophages and other immune cells. This lesion not only prevents the spread of Mtb but also acts as a reservoir, encapsulating and sheltering the bacteria to maintain its dormant state [29]. However, it is worth noting that one of the challenges in current tuberculosis treatment is targeting this resilient pathogen within the granuloma.
Apart from cervical lymph nodes, tuberculosis lesions in the head and neck region are extremely rare. Involvement of the salivary glands is even more uncommon, as the continuous flow of saliva prevents the accumulation of tubercle bacilli in this area. Additionally, saliva has antimicrobial activity, making it more resistant to infection [30]. The salivary glands and peripheral lymph nodes may be affected in two ways. Firstly, primary involvement is likely to occur through the oral route, with dental trauma or infection being possible triggering factors [30]. The slow flow of saliva in the salivary glands allows Mtb to ascend through its ducts into the salivary gland or enter the associated lymph nodes through lymphatic drainage [30, 31]. Secondly, it can occur through distant hematogenous or lymphatic spread from a primary pulmonary source [32]. Salivary gland tuberculosis involvement is more likely to be a result of systemic dissemination of pulmonary tuberculosis compared to primary extrapulmonary tuberculosis [31].
In the past 20 years, only 9 documented case reports of WT with TB have been reported in the English literature (Table 1). These cases predominantly occurred in men over 40 years old. Among these cases, two patients had a history of smoking, while information regarding smoking history was not available for the remaining patients. Diagnosis of TB in these patients posed a challenge prior to surgery. Most of them had no previous history of pulmonary tuberculosis, and did not exhibit typical symptoms of TB such as fever, chills, night sweats, and weight loss. Since 2011, the TB diagnostic landscape has been revolutionized by the introduction of rapid molecular tests that are highly specific and sensitive. This has replaced the reliance on more traditional microscopy and culture methods. Only three of these cases, including ours, utilized PCR methods to confirm the presence of Mtb.
Table 1.
Reported cases of Warthin’s tumor of the parotid gland concomitant with TB infection in the past 20 years
| Authors (year) | Sex | Age | Smoking | Other focus of TB | Complications | TB culture | PCR |
|---|---|---|---|---|---|---|---|
| Ozcan et al. [6] | Male | 53 | NA | NA | Nil | NA | Positive |
| Wen et al. [7] | Male | 81 | NA | Bilateral lung nodules | Nil | Positive | NA |
| Cobb et al. [7] | Male | 44 | Yes | NA | Facial nerve paralysis | Negative | NA |
| Lee et al. [7] | Female | 51 | NA | Bilateral lung linear nodular opacities | Nil | Negative | NA |
| Maheshwari et al. [8] | Male | 81 | NA | Lung calcified hilar lymph nodes | Nil | Positive | Positive |
| Wu et al. [9] | Male | 92 | Yes | Miliary pulmonary TB | Nil | Positive | Negative |
| Ulusan et al. [2] | Male | 46 | NA | NA | Nil | NA | NA |
| Aaronson et al. [10] | Male | 79 | NA | Neck level II adenopathy | Nil | Positive | NA |
| Chen et al. [11] | Female | 82 | NA | Bilateral lung consolidation | Facial nerve paralysis | Negative | NA |
| Present case, 2023 | Male | 65 | Yes | Secondary pulmonary tuberculosis | Cavitary tuberculosis, facial nerve paralysis | NA | Positive |
NA = data not available, PCR = polymerase chain reaction, TB = tuberculosis, WT = Warthin’s tumor
The patient is a 65-year-old male with a history of diabetes mellitus (DM). DM significantly increases the risk of developing severe TB forms. Individuals with DM are about three times more likely to develop active TB [33, 34] and have approximately double the risk of latent TB infection (LTBI) [35]. Additionally, DM contributes to a higher likelihood of TB recurrence following preventive treatment [33] and leads to poorer outcomes after therapeutic interventions [33, 35]. This includes elevated rates of treatment failure, relapse, recurrence of infection, and increased mortality [34, 36, 37]. The patient underwent an Xpert MTB/RIF test, which showed RIF sensitivity. Therefore, the patient was treated with first-line anti-tuberculosis drugs. For elderly patients with rifampicin-sensitive pulmonary tuberculosis, the preferred chemotherapy regimen is 2 months of INH, RIF, PZA, and EMB, followed by 4 months of INH and RIF (2HRZE/4HR), with an alternative option of 9 months of INH, RIF, and EMB (9HRE). For patients with hematogenous disseminated pulmonary TB, intrathoracic lymph node TB, tuberculous pleurisy, tracheobronchial TB, DM, silicosis, and severe pulmonary TB, a treatment regimen of 2HRZE/10HRE is recommended [38]. Diabetes and the status of glycemic control can influence the progression of TB. Maintaining good glycemic control is critical for patients with both TB and DM, as it helps reduce the occurrence of cavities, supports sputum conversion to a negative state, and aids in the absorption of lesions [39].
Standard treatment for drug-sensitive tuberculosis (DS-TB) includes four drugs: INH, RIF, EMB, and PZA. These four drugs are used in combination for two months, followed by continued use of INH and RIF for four months [40]. Despite the effectiveness of these first-line anti-tuberculosis drugs against DS-TB, patients may experience corresponding adverse reactions, including liver dysfunction, peripheral neuropathy, erythema nodosum, ocular toxicity, central nervous system toxicity, gastrointestinal intolerance, and rash [41–43]. Due to the long duration of anti-tuberculosis treatment, intolerable adverse reactions, and factors such as excessive or misuse of antibiotics, patients have poor compliance, resulting in poor treatment outcomes and even drug resistance [29]. The reported case patient in our study discontinued treatment due to intolerable gastrointestinal adverse reactions, which may also be one of the reasons for the rapid progression of pulmonary tuberculosis.
Mtb is a bacterium with a rich history, and it has developed resistance to all known drugs that target it, including those that have been newly introduced into clinical practice. Its genome is well-preserved compared to other bacteria, as it is an obligate human pathogen that has adapted to the human environment over a period of five to ten thousand years [44]. The main mechanisms that confer a drug-tolerant phenotype in Mtb include [45]: (i) Metabolic slowdown, achieved by reducing the metabolism and growth rate. This strategy is successful because antibiotics generally target active cellular processes. (ii) Metabolic shifting, which involves rerouting metabolite fluxes to maintain homeostasis when specific pathways are targeted by antibiotic drugs. (iii) Cell wall thickening, which reduces the concentration of drugs in mycobacterial cells. (iv) Upregulation of efflux pumps, which increases the clearance of antibiotics from the mycobacterial cell.
Treatment for individuals diagnosed with rifampicin-resistant TB (RR-TB), isoniazid-resistant TB and multidrug-resistant TB (MDR-TB, defined as resistance to INH and RIF) requires regimens that include more expensive second-line drugs like bedaquiline (BDQ) and fluoroquinolones (FQ). These regimens have a higher cost per person (≥ US$ 1000) and are associated with more side effects compared to the first-line treatments used for DS-TB [46]. Pre-extensively drug-resistant TB (pre-XDR-TB, defined as TB that is resistant to RIF and any FQ) and XDR-TB (resistance to RIF, any FQ and at least one of BDQ or linezolid (LZD)) are even harder to treat [46]. Although TB is curable, the treatment success rates in 2021 were only 88% for DS-TB and 63% for MDR/ RR-TB [46]. In the Global tuberculosis report 2023, WHO recommendations emphasize a new all-oral 6-month regimen as the key change. This regimen, known as BPaLM for those with MDR/RR-TB and BPaL for those with pre-XDR-TB, includes bedaquiline (B), pretomanid (Pa), linezolid (L), and moxifloxacin (M) [46]. The shorter duration, lower cost, reduced pill burden, and high efficacy of this novel regimen are expected to significantly improve treatment outcomes for individuals with MDR/RR-TB or pre-XDR-TB. With the in-depth research on Mtb, an increasing number of countries are turning to targeted sequencing and whole-genome sequencing (WGS) to diagnose Mtb infection and identify drug resistance. It is important to be aware of the impact of drug resistance evolution.
Conclusions
The coexistence of TB within WT in the parotid gland is extremely rare, and there are few reports on this condition. Based on the pathogenesis of WT, it may be considered as a special form of tuberculosis involving the lymph nodes around the parotid gland. Lymph node tuberculosis is the most common extrapulmonary manifestation of tuberculosis and is also the source of reactivation of latent pulmonary tuberculosis. However, it is often overlooked in the course of tuberculosis treatment. Studies have shown that the current anti-tuberculosis treatment regimen has significantly reduced effectiveness against Mtb within lymph nodes compared to granulomatous lesions in pulmonary tuberculosis [27, 47]. This may be due to poor penetration of drugs into the affected lymph nodes [48, 49]. Early diagnosis of tuberculosis, standardized and effective use of anti-tuberculosis drugs, and personalized treatment are crucial in the management of tuberculosis. Furthermore, improving and enhancing the delivery efficiency of vaccines or anti-tuberculosis drugs to target the lymph nodes is also necessary to benefit individual patients and ultimately achieve the goal of ending tuberculosis.
Acknowledgements
Not applicable.
Abbreviations
- TB
Tuberculosis
- WT
Warthin’s tumor
- Mtb
Mycobacterium tuberculosis
- DS-TB
Drug-sensitive tuberculosis
- MDR-TB
Multidrug-resistant tuberculosis
- pre-XDR-TB
Pre-extensively drug-resistant tuberculosis
- INH
Isoniazid
- RIF
Rifampin
- EMB
Ethambutol
- PZA
Pyrazinamide
- PCR
Polymerase chain reaction
- WGS
Whole-genome sequencing
Author contributions
SH and QW wrote the main manuscript text. YW and XL prepared Figs. 1, 2 and 3. FL and BW put great effort and made many constructive comments during the revision and writing of this article. SH, YW and BW acquired funding, provided resources and supervised all the work. All authors contributed to the review and editing process. All authors approved the final work.
Funding
This work was supported by Major Science and Technology Program of Hainan Province (ZDKJ2021040), Hainan Provincial Natural Science Foundation of China (820QN387, 820QN389), National Natural Science Fund Cultivating 530 Project of Hainan General Hospital (2021MSXM13).
Data availability
Data relating to this study are contained and presented in this document. Other materials are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written and informed consent to publish was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sha-Sha Hu, Qing-Chen Wei and Yu Wu contributed equally to this work and are co-first authors.
Contributor Information
Fu-Jin Liu, Email: 1955093270@qq.com.
Bo Wang, Email: wangqugans@163.com.
References
- 1.Tunç O, et al. Change in Warthin’s tumor incidence: a 20-year joinpoint trend analysis. Eur Arch Otorhinolaryngol. 2020;277(12):3431–4. [DOI] [PubMed] [Google Scholar]
- 2.Ulusan M, Abul Y, Bakır S. Mycobacterium Tuberculosis infection within a Warthin Tumor: a Case Report and Literature Review. N Am J Med Sci. 2013;5(10):617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quer M et al. Current trends and controversies in the management of Warthin Tumor of the parotid gland. Diagnostics (Basel), 2021. 11(8). [DOI] [PMC free article] [PubMed]
- 4.Furin J, Cox H, Pai M. Tuberculosis Lancet. 2019;393(10181):1642–56. [DOI] [PubMed] [Google Scholar]
- 5.Cataño JC, Robledo J. Tuberculous lymphadenitis and Parotitis. Microbiol Spectr, 2016. 4(6). [DOI] [PubMed]
- 6.Ozcan C, et al. Mycobacterium tuberculosis infection within parotid gland Warthin tumor. J Craniofac Surg. 2008;19(6):1561–5. [DOI] [PubMed] [Google Scholar]
- 7.Cobb CJ, Greaves TS, Raza AS. Fine needle aspiration cytology and diagnostic pitfalls in Warthin’s tumor with necrotizing granulomatous inflammation and facial nerve paralysis: a case report. Acta Cytol. 2009;53(4):431–4. [DOI] [PubMed] [Google Scholar]
- 8.Maheshwari V et al. Warthin’s tumour with coexistent tuberculosis. BMJ Case Rep, 2011. 2011. [DOI] [PMC free article] [PubMed]
- 9.Wu KC, Chen BN. Mycobacterial tuberculosis superimposed on a Warthin tumor. Ear Nose Throat J. 2012;91(5):E4–6. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson NL, Adam SI, Boey HP. Warthin’s tumor with superimposed mycobacterium tuberculosis infection. Conn Med. 2014;78(2):85–9. [PubMed] [Google Scholar]
- 11.Chen SL, et al. Warthin’s tumor with necrotizing tuberculous granulomatous inflammation causing severe facial nerve adhesion in parotid gland: a case report and literature review. Med (Baltim). 2020;99(7):e18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Suh S. Tuberculosis infection within a Warthin’s Tumor of the parotid gland: a Case Report. J Korean Soc Radiol. 2011;65:213. [Google Scholar]
- 13.Wen Y-H, Chen P-R, Wu H-P. Tuberculosis infection within a Warthin’s Tumor of the parotid gland. Tzu Chi Med J. 2008;20(4):332–4. [Google Scholar]
- 14.Watanabe M, et al. Mycobacterium tuberculosis infection within Warthin’s tumor: report of two cases. Pathol Int. 2001;51(10):797–801. [DOI] [PubMed] [Google Scholar]
- 15.Patterson JW, Wright ED, Camden S. Extraparotid Warthin’s tumor. J Am Acad Dermatol. 1999;40(3):468–70. [DOI] [PubMed] [Google Scholar]
- 16.Thompson AS, Bryant HC Jr. Histogenesis of papillary cystadenoma lymphomatosum (Warthin’s tumor) of the parotid salivary gland. Am J Pathol. 1950;26(5):807–49. [PMC free article] [PubMed]
- 17.Segami N, Fukuda M, Manabe T. Immunohistological study of the epithelial components of Warthin’s tumor. Int J Oral Maxillofac Surg. 1989;18(3):133–7. [DOI] [PubMed] [Google Scholar]
- 18.Daniel E, Sr. McGuirt WF. Neck masses secondary to heterotopic salivary gland tissue: a 25-year experience. Am J Otolaryngol. 2005;26(2):96–100. [DOI] [PubMed] [Google Scholar]
- 19.Orabona GD, et al. Warthin’s tumour: aetiopathogenesis dilemma, ten years of our experience. J Craniomaxillofac Surg. 2015;43(4):427–31. [DOI] [PubMed] [Google Scholar]
- 20.Freedman LS, Oberman B, Sadetzki S. Using time-dependent covariate analysis to elucidate the relation of smoking history to Warthin’s tumor risk. Am J Epidemiol. 2009;170(9):1178–85. [DOI] [PubMed] [Google Scholar]
- 21.Zaccarini DJ, Khurana KK. Incidence of Non-salivary Gland neoplasms in patients with Warthin Tumor: a study of 73 cases. Head Neck Pathol. 2020;14(2):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis PD, et al. Detection of damage to the mitochondrial genome in the oncocytic cells of Warthin’s tumour. J Pathol. 2000;191(3):274–81. [DOI] [PubMed] [Google Scholar]
- 23.Mandic R et al. Aberrant expression of glyceraldehyde-3-Phosphate dehydrogenase (GAPDH) in Warthin Tumors. Cancers (Basel), 2020. 12(5). [DOI] [PMC free article] [PubMed]
- 24.Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary Tuberculosis: pathophysiology and imaging findings. Radiographics. 2019;39(7):2023–37. [DOI] [PubMed] [Google Scholar]
- 25.Prasad KC, et al. Tuberculosis in the head and neck: experience in India. J Laryngol Otol. 2007;121(10):979–85. [DOI] [PubMed] [Google Scholar]
- 26.Ganchua SKC, et al. Lymph Nodes-the neglected battlefield in tuberculosis. PLoS Pathog. 2020;16(8):e1008632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganchua SKC, et al. Lymph nodes are sites of prolonged bacterial persistence during Mycobacterium tuberculosis infection in macaques. PLoS Pathog. 2018;14(11):e1007337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esmail H, et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[(18)F]fluoro-D-glucose positron emission and computed tomography. Nat Med. 2016;22(10):1090–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alsayed SSR, Gunosewoyo H. Tuberculosis: Pathogenesis, current treatment regimens and new drug targets. Int J Mol Sci, 2023. 24(6). [DOI] [PMC free article] [PubMed]
- 30.Gupta N, et al. Parotid gland tuberculosis. QJM. 2020;113(7):500–1. [DOI] [PubMed] [Google Scholar]
- 31.Bakir M, et al. Parotid gland tuberculosis: a Case Report and Literature Review. Cureus. 2022;14(8):e27590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myer C, Cotton RT. Salivary gland disease in children: a review. Part 1: acquired non-neoplastic disease. Clin Pediatr (Phila). 1986;25(6):314–22. [DOI] [PubMed] [Google Scholar]
- 33.Oglesby W, et al. Metformin in Tuberculosis: beyond control of hyperglycemia. Infection. 2019;47(5):697–702. [DOI] [PubMed] [Google Scholar]
- 34.Esmail A, et al. Management of drug-resistant tuberculosis in special sub-populations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J Thorac Dis. 2018;10(5):3102–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonio-Arques V, Franch-Nadal J, Caylà JA. Diabetes and tuberculosis: a syndemic complicated by COVID-19. Med Clin (Engl Ed). 2021;157(6):288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Crevel R, Critchley JA. The Interaction of Diabetes and Tuberculosis: translating Research to Policy and Practice. Trop Med Infect Dis, 2021. 6(1). [DOI] [PMC free article] [PubMed]
- 37.Cáceres G, Calderon R, Ugarte-Gil C. Tuberculosis and comorbidities: treatment challenges in patients with comorbid diabetes mellitus and depression. Ther Adv Infect Dis. 2022;9:20499361221095831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.[Expert consensus on diagnosis and treatment of elderly pulmonary tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi, 2023. 46(11): pp. 1068–1084. [DOI] [PubMed]
- 39.Meng F, et al. Impact of diabetes itself and glycemic control status on tuberculosis. Front Endocrinol (Lausanne). 2023;14:1250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grace AG et al. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst Rev, 2019. 12(12): p. Cd012918. [DOI] [PMC free article] [PubMed]
- 41.Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology. 2010;15(3):413–32. [DOI] [PubMed] [Google Scholar]
- 42.Sotgiu G, et al. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med. 2015;5(5):a017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yee D, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–7. [DOI] [PubMed] [Google Scholar]
- 44.Nimmo C, et al. Evolution of Mycobacterium tuberculosis drug resistance in the genomic era. Front Cell Infect Microbiol. 2022;12:954074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goossens SN, Sampson SL, Van Rie A. Mechanisms of Drug-Induced Tolerance in Mycobacterium tuberculosis. Clin Microbiol Rev, 2020. 34(1). [DOI] [PMC free article] [PubMed]
- 46.WHO. 2023. Global tuberculosis report 2023. WHO, Geneva, Switzerland. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
- 47.Lin PL, et al. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proc Natl Acad Sci U S A. 2012;109(35):14188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kislitsyna NA. [Comparative evaluation of rifampicin and isoniazid penetration into the pathological foci of the lungs in tuberculosis patients]. Probl Tuberk, 1985(4): pp. 55–7. [PubMed]
- 49.Dartois V, Barry CE. Clinical pharmacology and lesion penetrating properties of second- and third-line antituberculous agents used in the management of multidrug-resistant (MDR) and extensively-drug resistant (XDR) tuberculosis. Curr Clin Pharmacol. 2010;5(2):96–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data relating to this study are contained and presented in this document. Other materials are available from the corresponding authors on reasonable request.