Abstract
Background
Preeclampsia is the second leading cause of maternal death in Uganda. WHO recommends using magnesium sulphate (MgSO4) to prevent and treat preeclampsia with severe features (PEC) and eclampsia. MgSO4 is used to prevent eclampsia and treat women who experience an eclamptic convulsion to avoid severe maternal/infant illnesses and death. We set out to assess MgSO4 administration patterns in women with PEC or eclampsia and the immediate newborn outcomes of neonates.
Methods
This was an analytical observational cohort study at Kawempe National Referral Hospital in Uganda. Two hundred ten pregnant mothers with PEC or eclampsia were recruited in the study after receiving the loading dose of MgSO4 and then followed through labour and delivery to observe MgSO4 administration patterns and immediate newborn outcomes using Apgar and Thompson scores. SPSS version 23 was used to analyse data, and both bivariate and multivariate logistic regressions were used to determine factors associated with the low Apgar score at five minutes.
Results
Overall, majority of the patients received more than one dose with 33.3% received a sixth dose of MgSO4. The majority, 84.8%, of the mothers delivered live babies, 31.0% babies had complications, and were admitted to the neonatal intensive care unit (NICU). NICU admissions were mostly due to respiratory distress21.4%, preterm delivery21.0%, and 5.5% died within seven days. Majority 93.3% of the newborns had an Apgar score of seven and above at five minutes, of the newborns who were Thompson scored, 70.4% scored between 1 to 10 which is mild HIE. Initiation of MgSO4 treatment within one hour from prescription (AOR = 0.49, CI: 0.01–1.94), 4-hourly timing of the first maintenance dose (AOR = 0.22, CI: 0.06–0.79) and having complete doses of MgSO4 treatment (AOR = 0.89, CI: 0.03–3.05) decreased the likelihood of having low Apgar scores at five minutes.
Conclusions
Timely administration of the first maintenance dose of MgSO4 decreases the likelihood of low Apgar score at 5 min and NICU admission in newborns, and most NICU admissions were due to respiratory distress and preterm delivery.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06915-z.
Keywords: Preeclampsia, Intrapartum, Magnesium sulphate, Newborn outcomes, Apgar score, Uganda
Background
Pre- eclampsia (PE) is a life-threatening hypertensive disorder affecting women after 20 weeks of pregnancy with or without proteinuria [1]. It is a leading cause of maternal/foetal morbidity and mortality globally [2, 3]. Estimates of 2019 indicated that there were 18.08 million incidences of pregnancy-related hypertensive disorders with over 30.05% deaths by 2020 globally [4]. Moreover, 3 to 5% of all pregnancies are complicated by the condition [5], causing preterm labour, convulsions, seizures and undesirable immediate newborn outcomes such as low Apgar scores [6]. Moreover, the incidence of hypertensive disorder is estimated to be seven times higher in developing countries compared to developed countries [3] and in Uganda, preeclampsia contributes to 25% of maternal deaths and is a key determinant of fetal growth and survival with an overall maternal mortality rate of 189/100,000 live birth in 2022.
Magnesium sulphate (MgSO4) administration has proven effective in the prevention of morbidity and mortality from hypertensive disorders [7, 8]. Therefore, the World Health Organisation (WHO) recommends the use of Magnesium sulphate (MgSO4) for the prevention of seizures in pre-eclamptic women or prevention of recurrence in eclamptic women [9]. As such, MgSO4 is administered as a 14 g loading dose, followed by 5 g every four hours for 24 h after the mother has given birth or until the last convulsion in eclampsia, whatever of the two comes last.
Like many other countries, Uganda has adopted use of MgSO4 in managing preeclampsia in the same [10]. In Uganda this is done using the Pritchard Regimen that is, loading bolus dose of 4 g of MgSO4 is given slowly intravenously over 5–10 min and this is followed by 10 g given intramuscularly (5 g in each buttock). Then a maintenance dose of 5 g is given intramuscularly into alternate buttocks every 4 h [10]. However, the administration of MgSO4 seems to be a challenge given the congestion common in public facilities. Therefore, recipients of the treatment are likely to face dose non-completion, overdosing and toxicities [11–13]. Moreover, the administration of MgSO4 has also been associated with undesirable side effects in mothers; such as patellar reflex loss, oliguria, and respiratory depression [14]. On the other hand, given that the drug is capable of crossing the placenta, previous studies have associated adverse birth outcomes such as low calcium, higher risk of neonatal death, seizures, neonatal encephalopathy, and increased likelihood of neonatal intensive care admissions [15, 16].
There seems to be a dearth of studies to assess the administration patterns of MgSO4 among pregnant women in Uganda. Moreover, the immediate newborn outcomes such as Apgar scores in babies whose mothers received MgSO4 during pregnancy have not been well documented despite evidence suggesting the potential of MgSO4 to cross the placenta and affect the foetus [17]. Therefore, the current study aimed to assess the administration patterns of MgSO4 among pregnant mothers with preeclampsia in Kawempe National Referral Hospital as well as their immediate newborn outcomes. The study site was chosen because it is a national referral hospital where almost all mothers with severe obstetric conditions are referred hence easy to obtain the study participants.
Methods
Study area and design
The study used an analytical observational cohort design to assess pregnant women in Kawempe National Referral Hospital, Uganda. The hospital is a government national referral facility with a 200-bed capacity that serves as a clinical, training, and research facility. The study was conducted in the labour ward, severe PET ward, obstetric theatre, HDU, ICU, postnatal ward, and neonatal intensive care unit.
Study participants
The primary respondents were pregnant women diagnosed with PEC or eclampsia and had received magnesium sulphate during labour. Newborn babies to these mothers were the secondary targets.
Inclusion and exclusion criteria
Mothers at 28 weeks of gestation diagnosed with PEC or eclampsia during triaging and being treated with MgSO4 intrapartum or prelabour were recruited in the study and their newborn babies. All mothers fitting the inclusion criteria but for various reasons missed receiving MgSO4 meaning that they were never initiated on MgSO4 so did not receive even a loading dose were excluded from the study and the unconscious mothers who could not consent or answer any questions asked by the researcher were also excluded from the study.
Sample size calculation
The sample size was determined by the Kish Leslie formula [18] for survey studies at a 16.0% prevalence of eclampsia documented by Milln and Nakimuli from a study that quantified the burden of complex medical conditions on the obstetric high-dependency unit at Mulago National referral Hospital in Uganda [19] for a 95% confidence interval and a precision of 0.05 thus yielding a total targeted sample of 210 mothers with preeclampsia.
Sampling procedure
Given that the labour ward at the hospital is the admission point for all mothers in labour irrespective of the underlying condition, it was desirable to use a consecutive sampling technique as it allows every participant who meets the inclusion criteria to be selected [20] until the requires sample size is achieved.
Data collection methods and tools
Structured questionnaires with mainly close-ended questions, socio-demographic, obstetric characteristics, and delivery characteristics were obtained from the mothers through interviews that lasted for 30 to 45 min. Prior to the actual assessment, research assistants were trained and deployed with the drafted tools between 22nd and 28th August 2022. Actual data collection commenced on 29 August 2022 and ended on 16 October 2022. The total follow-up time from the time of recruitment of the mother to the final data collection from the newborn took seven days. The Principal Investigator was part of the entire process of data collection including the actual observation of MgSO4 administration patterns and Apgar, as well as Thompson scoring.
A checklist was used for MgSO4 drug administration patterns as stipulated in the Uganda Clinical Guidelines (UCG) and EMNCG [7, 21] to assess patterns and methods of drug patterns that are in line with the WHO treatment guidelines for preeclampsia with severe features and eclampsia [6]. When actual examination was not possible, reviews from patient files and displayed treatment charts were done and if not documented, the mother was asked.
Apgar scoring: the scoring was done by the midwife who delivered the mother and the research assistant only copied the score that the midwife gave, therefore, newborn parameters such as the heart rate, muscle tone and other signs were captured and recorded in the patient files to ascertain the need for extra medical care or emergencies. This was done twice; at 1 min after birth, and repeated 5 min after birth.
Thompson scoring: Data on administration patterns of magnesium sulphate such as the dose administered and time given were obtained through direct observations from medicine administration and treatment sheets. Actual examination of the newborns after delivery was done by the principal investigator and midwives on the ward for Apgar and Thompson scores.
Data analysis
Analysis of MgSO4 administration patterns; upon collection of the data, categories were created including the timing of when the loading dose was administered from prescription time, the timing of maintenance doses interval, the total number of administered doses, and dose completion.
Apgar scores were categorised as low for scores less than seven and normal for scores between 7–10. On the other hand, Thompson's scores were categorized as 0 for normal, between 1 and 10 for mild neonatal encephalopathy, between 11 and 14 for moderate neonatal encephalopathy, and 15 and 22 for severe neonatal encephalopathy.
We included all variables; descriptive analysis was done and reported in the form of means and standard deviations. We also did a bivariate analysis and got unadjusted p-values for every variable collected. Afterwards, we had all variables in a logistic regression model and removed the non-statistically significant predictors step-wise to give odds ratios and confidence intervals. Finally, we retained the independent risk factors for low Apgar scores and significant Thompson scores at five minutes. The significance level was set at 5% (p < 0.05) at a 95% confidence interval.
Data availability declaration
Both the acquired and analyzed data are not publicly available because of the lack of authorization from the agreement made with the Research Ethics Committee that the database would remain with the corresponding author only. However, all data can be made available by the corresponding author upon reasonable request and authorization from the Research Ethics Committee.
Study questionnaire
The questionnaire which was used in this study was specifically developed for the study although it incorporated time tested tools of newborn scores such as Apgar score which was developed by Virginia Apgar (1962), Thompson score by (Thompson et, al, 1997) which were used to assess the immediate newborn outcomes in this study. The tool which were used to asses MgSO4 administration patterns were developed based on the guidelines and protocols of preeclampsia management in the Uganda clinical guidelines (2016) and The Essential maternal and newborn care guidelines of Uganda (2022) that were both adopted from WHO (2016) guidelines.
Results
Characteristics of study participants
As shown in Table 1 below, 210 mothers were assessed. The mean (SD) age of the mothers was 26.9 (± 5.8) years, with most of the mothers 125/210 (59.5%) being aged at least 25 years and married 162/210 (77.1%). The mean (SD) gestational age was 35.7 (± 4.1) weeks, with 181/210 (86.2%) of the mothers having 34 weeks of gestation and below, while 29/210 (13.8%) were 34 weeks of gestation and above. The mean (SD) BP was 168.7/00.0 (22.7/00.) mmHg with 184/210 (87.6%) of the mothers having PEC whereas 26/210 (12.4%) had eclampsia.
Table 1.
Characteristics of study participants
| Characteristic | Frequency | Percentage (%) | Mean (SD.) |
|---|---|---|---|
| Age (years) | |||
| ≤ 24 | 85 | 40.5 | |
| 25 to 35 | 103 | 49.0 | 26.9 (5.8) |
| > 35 | 22 | 10.5 | |
| Marital status | |||
| Single | 48 | 22.9 | |
| Married | 162 | 77.1 | |
| Gestational age | |||
| 33 weeks & below | 47 | 22.4 | |
| 34 to 36 weeks | 41 | 19.5 | 35.7 (4.1) |
| 37 weeks and above | 122 | 58.1 | |
| Systolic B.P | |||
| < 149 | 23 | 11.0 | |
| 150 to 159 | 47 | 22.4 | 168.7 (22.7) |
| > 160 | 140 | 66.7 | |
| Diastolic B.P | |||
| < 89 | 15 | 7.1 | |
| 90 to 100 | 55 | 26.2 | 107.0 (15.1) |
| > 110 | 140 | 66.7 | |
| Diagnosis | |||
| Pre-eclampsia with severe features | 184 | 87.6 | |
| Eclampsia | 26 | 12.4 | |
Drug administration patterns
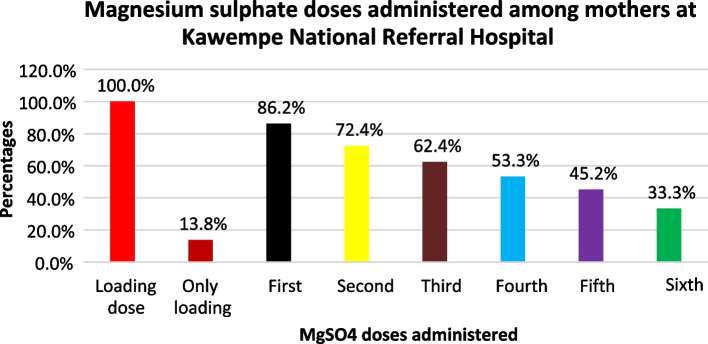
As would be expected, all the respondents received the loading dose. Of the maintenance doses, 181/210 (86.2%), 152/210 (72.4%), 131/210 (62.4%), 112/210 (53.3%), and 95/210 (45.2%), second, third, fourth, and fifth doses respectively. Only 70/210 (33.3%) mothers completed the six maintenance doses. The proportion of mothers receiving the Magnesium sulphate doses reduced with an increase in the number of maintenance doses which showed poor adherence to MgSO4 drug administration protocol (Fig. 1).
Fig. 1.
Magnesium sulphate doses administered to mothers
As shown in Table 2, the time of treatment initiation from prescription among respondents was as follows; within the first 30 min 49/210 (23.3%), after 30 min to 1 h 33/210 (15.7%), and long after 1 h 128/210 (61.0%) among the mothers. On the other hand, the four-hourly administration of the subsequent maintenance doses was inappropriate among all respondents. The first maintenance dose was administered within four hours among only 42/210 (20.0%) of the mothers. The rest of the mothers received the first dose either earlier than 4 h 12/210 (5.7%) or after 4 h 127/210 (60.5%).
Table 2.
Magnesium sulphate administration patterns among mothers in KNRH
| Characteristic | Frequency (N = 210) | Percentage (%) |
|---|---|---|
| Time of treatment initiation from prescription Within the first 30 min | 49 | 23.3 |
| After 30 min to one hour | 33 | 15.7 |
| After one hour and beyond | 128 | 61.0 |
| Timing of first maintenance dose | ||
| 4-hourly | 42 | 20.0 |
| < 4 h | 12 | 5.7 |
| > 4 h | 127 | 60.5 |
| Only loading | 29 | 13.8 |
| Administration status | ||
| Full dose | 70 | 33.3 |
| Partial dose | 140 | 66.7 |
Labour, delivery characteristics and newborn outcomes among women in KNRH
The majority 87/210 (41%) of the mothers had artificial membrane rapture while 66/210 (31.4%) of the mothers were in the second stage of labour when their membranes raptured whereas 20/210 (9.5%) and 37/210 (17.6%) were in the latent and first stages of labour respectively. Of these, (70.5%) of the mothers were not induced while 29.5% were induced. Deliveries were mainly by c-section 127/210 (60.5%) and SVD 81/210 (38.6%) whereas only two mothers were produced by other methods. In addition, the liquor state was clear, meconium stained, and blood stained among 153/210 (72.9%), 45/210 (21.4%) and 12/210 (5.7%) of the mothers, resptively (Table 3).
Table 3.
Labor and delivery characteristics among eclamptic and pre-eclamptic women in KNRH
| Characteristic | Frequency | Percentage (%) | Mean (SD.) |
|---|---|---|---|
| Birth mode | |||
| SVD | 81 | 38.6 | |
| Emergency C-section | 127 | 60.5 | |
| Others | 2 | 1.0 | |
| Labour inducing | |||
| Yes | 62 | 29.5 | |
| No | 148 | 70.5 | |
| Stage of membrane rapture | |||
| Latent | 20 | 9.5 | |
| Active | 66 | 31.4 | |
| Second | 37 | 17.6 | |
| Liquor state | |||
| Clear | 66 | 72.9 | |
| Meconium stained (I, II, III) | 45 | 21.4 (8.1, 3.8, 9.5) | |
| Bloodstained | 12 | 5.7 | |
Newborn outcomes among eclamptic and pre-eclamptic women in KNRH
The majority, 178/210 (84.8%), of the mothers delivered live babies, while 32/210 (15.2%) had stillbirths, of which 6.2% were FSBs while 9.1% were MSBs. There were more males, 108/210 (51.4%) than females, 102/210 (48.6%) weighing >2.5 kgs; 120/210 (57.1%), 1.5 to 2.5 kgs; 32/210 (27.6%), and <1.5kg 32/210 (15.2%) who were admitted in the postnatal ward 113/210 (53.8%) and the NICU 65/210 (31.0%). The mean (SD) birth weight was 2.5 (0.8). Diagnoses for the neonates in the NICU included respiratory distress (21.4%), preterm (21.0%), low birth weight (7.6%), and others (6.2%) (Table 4).
Table 4.
Newborn outcomes among eclamptic and pre-eclamptic women in KNRH
| Characteristic | Frequency | Percentage (%) | Mean (SD.) |
|---|---|---|---|
| Birth outcome | |||
| Alive | 178 | 84.8 | |
| Stillbirth | 32 | 15.2 | |
| Sex | |||
| Male | 108 | 51.4 | |
| Female | 102 | 48.6 | |
| Weight (kgs) | |||
| < 1.5 | 32 | 15.2 | |
| 1.5 to 2.5 | 58 | 27.6 | 2.5 (0.8) |
| > 2.5 | 120 | 57.1 | |
| Admission to NICU | |||
| Yes | 65 | 31.0 | |
| No | 145 | 69.0 | |
| Apgar at five minutes (N = 178) | |||
| ≤ 6 | 12 | 6.7 | 7.7 (3.6) |
| 7 to 10 | 166 | 93.3 | |
| Reasons for NICU admission | |||
| Preterm | 44 | 21.0 | |
| Respiratory distress | 45 | 21.4 | |
| Low birth weight | 16 | 7.6 | |
| Others | 13 | 6.2 | |
| Treatment administered | |||
| Respiratory support | 58 | 27.6 | |
| Antibiotics | 65 | 31.0 | |
| Fluid/nutrition support | 62 | 29.5 | |
| Others | 12 | 5.7 | |
Thompson score of newborns admitted to NICU
Of the 65/210 (31.0%) newborns that were admitted in NICU, 55/65 (84.6%) were Thompson scored on the fifth day following admission, 21/55 (38.2%), while others were scored on the day of discharge 35/55 (63.6%). On the other hand, ten early neonatal deaths occurred and were not scored. Still, 38/54 (70.4%) of the Thompson-scored neonates had a score of 1 to 10 (mild HIE), and 16/54 (29.6%) had a normal score. Characteristics that showed deviations from the normal were posture (17/55 fisting or cycling and 4/55 strong distal flexion), moro (partial 21/55 and 1/55 absent), grasping (poor 25/55 and absent 1/55), suckling (poor 32/55 and absent ± bite 2/55), and respiration (hyperventilation 4/55 and brief apnoea 2/55) (Table 5).
Table 5.
Thompson score of newborns admitted to NICU
| Day of scoring | Frequency | Percentage (%) | Mean (SD) |
|---|---|---|---|
| O day | 1 | 1.8 | |
| Day one | 4 | 7.4 | |
| Day two | 7 | 12.7 | |
| Day three | 9 | 16.3 | 3.3 (4.6) |
| Day four | 13 | 23.6 | |
| Day five | 21 | 38.2 | |
| Total | 55 | 100.0 | |
|
Characteristics that deviated from normal Posture Fisting/cycling |
17 | 30.9 | |
| Strong distal flexion | 4 | 7.3 | |
| Moro | |||
| Partial | 21 | 38.2 | |
| Absent | 1 | 1.8 | |
| Grasping | |||
| Poor | 25 | 45.5 | |
| Absent | 1 | 1.8 | |
| Suckling | |||
| Poor | 32 | 58.2 | |
| Absent ± bite | 2 | 3.6 | |
| Respiration | |||
| Hyperventilation | 4 | 7.3 | |
| Brief apnoea | 2 | 3.6 | |
Magnesium sulphate administration patterns and newborn outcomes
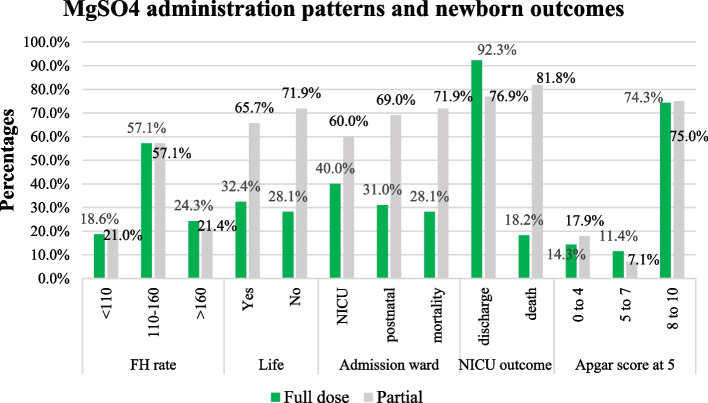
As shown in Fig. 2 below, there were more stillbirths 23/32 (71.9%) among mothers who had partial MgSO4 doses compared to the mothers who completed their MgSO4 doses 9/32 (28.1%). In addition, the proportion of early neonatal deaths among neonates admitted to the NICU was higher among mothers who had incomplete MgSO4 doses 9/11 (81.8%) compared to mothers who had complete doses 2/11 (18.2%).
Fig. 2.
Variations in newborn outcomes by MgSO4 administration patterns
Factors associated with Apgar score at five minutes among newborns
Having SVD deliveries (COR=0.61, CI: 0.160-2.361), PEC diagnosis (COR=0.16, CI: 0.46-0.559) and membrane rapture during active labour (COR=0.73, CI: 0.062-8.765) were protective against low Apgar scores at five minutes. Adjusted analysis highlighted an increased likelihood of low Apgar scores at five minutes among mothers aged 25 or below (AOR=3.23, CI: 0.296-35.449) compared to older mothers. Inducing mothers for labour also increased the likelihood of producing a baby with a low fifth-minute Apgar score (AOR=2.08, CI: 0.180-22.889).
MgSO4 administration patterns associated with low Apgar scores at five minutes
In adjusted analysis, three MgSO4 treatment initiation within the first hour from the prescription (AOR=0.49, CI: 0.124-1.944), proper timing of the four-hourly administration of the first maintenance dose (AOR=0.22, CI: 0.063-0.794) and completing of the MgSO4 doses (AOR=0.89, CI:0.259-3.055) were protective against low Apgar scores (Table 6).
Table 6.
MgSo4 administration patterns associated with low apgar score at 5 minutes
| Characteristic | Low > 6 |
High < 7 |
COR (CI) | AOR (CI) |
|---|---|---|---|---|
| Treatment initiation from prescription | ||||
| Within 1 h | 3 | 67 | 0.49 (0.129–1.887) | 0.49 (0.124–1.944) |
| Beyond 1 h | 9 | 99 | 1.00 | |
| Timing of first maintenance dose | ||||
| 4-hourly | 6 | 28 | 0.22 (0.063–0.774) | 0.22 (0.063–0.794) |
| < 4 h | 1 | 9 | 0.42 (0.045–4.037) | 0.39 (0.040–3. 784) |
| > 4 h | 5 | 106 | 1.00 | |
| Administration status | ||||
| Full dose | 5 | 56 | 0.71 (0.216–2.347) | 0.89 (0.259–3.055) |
| Partial dose | 7 | 110 | 1.00 | |
Discussion
In this study, we assessed MgSO4 administration patterns in women with PEC or eclampsia and the immediate newborn outcomes of their neonates. It was found that women with preeclampsia prescribed MgSO4 were more likely to receive incomplete doses of MgSO4 with inappropriate frequency either earlier or later than the four hourly recommendation, and were also more likely to have a caesarian section delivery. Neonates born to these women were more likely to be born with low birth weight, premature, with respiratory distress, and these were the most common reasons why these newborns were admitted to the neonatal intensive care unit. Maternal age below twenty-five years was an important predictor of low newborn Apgar scores at five minutes, while labour induction increased the likelihood of producing a baby with a low Apgar score by two times at five minutes.
Drug administration patterns
Proper drug administration reduces illness and death rates to provide safer and more reliable healthcare services [22]. Effective drug administration patterns can ensure magnesium sulfate's safe and optimal use in managing preeclampsia and eclampsia, thereby improving maternal and fetal outcomes. In this study, it was found that the practice in the hospital was to administer MgSO4 loading dose and six maintenance doses irrespective of when the mother gave birth or last had a seizure. This was contrary to the WHO recommendation and UCG guidelines which says that the mother should receive a loading dose of MgSO4 followed by six maintenance doses every four hours for 24 h after the mother has given birth or until the last convulsion in eclampsia, whatever of the two comes last [9, 10]. This deviation from WHO recommendation could be associated with poor interpretation of the guidelines hence the need to re orient both midwives and prescribing doctors of WHO guidelines and UCG guidelines on treatment of preeclampsia and eclampsia.
Only one-third of the mothers completed the six maintenance doses as per the hospital practice, and even these were not administered as per the recommended after every four hours [10, 23]. Furthermore, the timing of the first maintenance dose was inappropriate among two-thirds of the mothers which is 66.2%. this puts the mother at risk of both maternal and fetal complications such as eclampsia for the mother, intra uterine fetal death and birth asphyxia for the newborn. It is recommended that drug administration of MgSO4 should be initiated as soon as the diagnosis of PEC or eclampsia is made and maintenance doses given timely at a four hourly interval [13]. This is smiler to what was reported about clinical use of MgSO4 for eclampsia prevention and treatment in LMIC that varied widely, and was largely inconsistent with current international recommendations [24]. This untimely drug administration could be associated with the health worker's familiarity with clinical procedures and a belief that higher dosage may result in greater clinical efficacy, as suggested in some studies.
Moreover, the untimely administration of the MgSO4 to the mothers with very close intervals of less than the recommended four hours could result in drug overload(Arumugam, 2021) characterised by hypotension, cardiac arrest [21] and respiratory failure [25].
Inappropriate administration of drugs can cause unpleasant or dangerous side effects, render the medication ineffective, or even worse result in the death of patients [22]. Administration of incomplete doses of MgSO4 leads to undesirable birth outcomes on the newborn heart rate and reduced foetal oxygen [26]. As was found in the current study, undesirable MgSO4 administration patterns seem to be a common challenge. A study by Long and colleagues revealed that only the loading dose and the loading plus a maximum of four maintenance doses (6.1% and 21.7%, respectively) were administered among women in Latin, America and Asia [11].This is also validated by another study in Malawi which showed poor timing and incomplete administration of MgSO4 [13].
The incomplete administration of MgSO4 in the studies could be attributed to factors such as insufficient human resources and supplies as well as high costs of equipment to monitor patients [24, 27, 28] especially in developing countries respectively. A multi- country survey that was done in LMIC to assess clinical practice patterns on the use of MgSO4 for treatment of PE and eclampsia revealed that in spite of the availability of a clinical protocol for treatment of PE and eclampsia in most facilities, the MgSO4 dosing regimens in use varied widely and were largely inconsistent with current international recommendations with overall, around one-fifth of the surveyed health facilities in the African region, one-third of those in the Latin American region and half of those in the Asian region used MgSO4 regimens for treatment of PE in keeping with current recommendations [24]. These findings are consistent with the findings in our study.
A study that was done on Adherence to established clinical guidelines and protocols is essential to ensure the safe and optimal use of MgSO4 in the management of preeclampsia and eclampsia, leading to improved maternal and fetal outcomes. There is need healthcare professionals must adhere to established protocol and guidelines to safely and effectively treat preeclampsia and eclampsia.
Immediate newborn outcomes
Neonates born to women with preeclampsia were more likely to be low birth weight, small for gestational age or premature, have a lower mean 5 min Apgar score and be admitted to the neonatal intensive care unit compared to neonates of normotensive women [29]. Smillary a study done in Mulago hospital Uganda to assess adverse neonatal outcomes in women with PE adverse neonatal outcome included; delivery of a stillborn baby, an early neonatal death, a need to admit the baby to the NICU, a baby who needed oxygen resuscitation and, a baby weighing less than 2500gm [30]. Findings in this study showed that almost all the newborns had Apgar scores at five minutes of seven and above. This contrasts with the above findings and findings from a study by Sherwine and colleagues, in which the majority (82.7%) of the newborns to mothers admitted to a facility in the United States had Apgar scores of above seven at five minutes [30].
The high Apgar scores in the study could be linked to the high proportion of women with non-complicated PEC and caesarean deliveries. Sirenden et al. found that women with PEC with no complications who delivered by caesarean section had newborns with high Apgar scores at five minutes [31]. On the other hand, our findings revealed that only 6.7% of the newborns had low Apgar scores at five minutes. This was higher than findings documented in previous studies conducted in West and sub-Saharan Africa (3.9%), North Africa (1.8%), and resource constrained settings (2.3%) [32–34].
Contrary to our finding, results of a study conducted in North-west Ethiopia revealed a 13.8% proportion of Apgar above seven at five minutes among newborns which is low compared to our findings [31].The contradiction could be associated with the fact that our study was conducted in a national referral hospital where mothers previously managed from lower facilities before referral causing a delay in receiving appropriate care which negatively affects the newborns’ Apgar scores as also seen in a study done in Tanzania east Africa [35]. 5.5% on babies that were admitted in NICU died within seven days of hospital stay which is consistent with other studies done in LMIC which state that PE is more prevalent in resource-limited settings and contributes significantly to not only maternal but also fetal and neonatal morbidity and mortality, causing a high rate of early neonatal deaths [35]. Hence our recommendation that pregnant women diagnosed with pre-eclampsia during antenatal period should immediately be referred to appropriate hospitals who can manage their condition to continue antenatal care in those facilities as it reduces the delay to access care thus improving birth outcomes for the mother baby pair.
Factors associated with Apgar score at five minutes among newborns
Maternal age below twenty-five years was an important predictor of low newborn Apgar scores at five minutes. A previous study found that maternal age below twenty years predicts low Apgar score in newborns [35].Similarly, studies in hospitals in Ethiopia and in the Netherlands found a positive association between maternal age and Apgar scores [36, 37]. However, in obstetrics low maternal age is below twenty and high maternal age is above thirty-five which leaves room for further research to find out what could be the associated factors for those above twenty.
Contrary to our findings, some studies done in developed countries have documented a higher risk of low Apgar scores among women of advanced age in [38] This discordance could be attributed to the heterogeneity of study populations, differences in the definition of pregnancy outcomes, and confounder effects during data analysis.
Inducing mothers for labour increased by two times the likelihood of producing a baby with a low Apgar score at five minutes. Similarly, Mersha and colleagues found that induction of mothers for labour increased the likelihood of a baby having a low Apgar score at five minutes in facilities in Arba Minch town, Southern Ethiopia [39]. This could be associated to the many failed induction rate that resulted into prolonged labor, fetal distress and cesarean section delivery.
MgSO4 treatment initiation within the first one hour from prescription, proper timing of the four-hourly administration of the first maintenance dose and the completion of the MgSO4 doses among the mothers lowered the likelihood of having low Apgar scores at five minutes among newborns. Previous studies have highlighted a relationship between MgSO4 treatment with newborn Apgar scores. Nadim et al. reported that Magnesium sulphate treatment in mothers was important in reducing the risk of low Apgar scores at five minutes [40]. This could be attributed to the efficacy of MgSO4 which leads to lowered blood pressure and prevention of seizures hence reduced adverse effects to the fetus and newborn.
Contrary to our findings, Abbassi-Ghanavati and colleagues found that babies born to mothers exposed to MgSO4 were more likely to have low Apgar scores at birth [41].This discrepancy could be associated with the failure to control for potential confounders like gestational age, length of labour and mode of delivery which have been reported to affect Apgar scores.
Another study on single postpartum women in Egypt found that MgSO4 was ineffective in reducing the risk of an Apgar score of less than seven at 5 min [42]. Still, other studies which revealed that MgSO4-exposed newborns had a higher risk of low Apgar scores [43, 44]. This could be attributed to the MgSO4 property of muscle relaxant and since MgSO4 crosses the placenta burrier, this also extends to the fetus hence affecting its blood pressure and respiration thus causing low Apgar score.
Study strength and limitations
The study site hospital had a good stock of MgSO4 and was readily available for use which made it easy to rule out drug availability as reason for untimely drug administration, we were able to follow up and observe mothers twenty-four hours hence making it easy to observe MgSO4 administration.
We were not in a position to take cord blood to measure neonatal serum MgSO4 concentrations, hence we did not analyze it to determine concentrations of MgSO4 in the newborn. Furthermore, given that most mothers received incomplete doses of MgSO4, it was difficult to measure the outcomes which might have affected our results. Since the unit does not monitor the intrapartum mothers on labour progress charts like patographs, it was difficult to ascertain if the low apgar score in induced mothers were due to other factors such as prolonged labour. In addition, we had limited time and resources hence could not take on a bigger sample size or follow up newborns that were not admitted in NICU since they got discharged to go home with twenty-four hours of being born.
Conclusions
With respect to the treatment of preeclampsia with severe features and eclampsia, a wide variation in the administration patterns of MgSO4 was observed in terms of treatment initiation from time of prescription, timely administration and dosage completion, most of which were not congruent with the established evidence-based international recommendations and local guidelines. This highlights an inappropriate adoption of current recommendations into local protocols; this can be attributed to the perception of clinical midwives towards administration of MgSO4 or drug in general, familiarity of health workers with their tasks and may be partly linked to their attitude towards service delivery.
On the other hand, the study found that preterm delivery along with respiratory distresses syndrome were common among newborns which were also the main reported reasons for admission into the neonatal intensive care unit. The proportion of women completing the doses for MgSO4 was higher for mothers of the newborns that were admitted to the NICU than those that were in good condition to go with their mothers to the postnatal ward. Most of the mothers who were admitted to the HDU in the postnatal period where treatment was prioritized received full dose of MgSO4 unlike those sent to postnatal ward, where there are overwhelming numbers of mothers with few staff.
Recommendations
In order to improve MgSO4 use in the management of pre-eclampsia and eclampsia, it requires understanding the perceptions towards MgSO4 use among clinical midwives yet such information is scanty in Uganda hence a need to carry out a study to that effect.
MgSO4 administration is a demanding activity due to inadequate staffing and training. While periodic in-service training needs to be intensified to improve MgSO4 use, necessary resources should be provided especially adequate human resource and protocols.
Use of the midwifery model that allows the midwife to manage all mothers with no complications, monitoring mothers on labor progress tools such as partograph, which will guide labor management and alert the midwife when complications arise that may need doctors’ involvement in management. This will reduce the proportion of low risk mothers managed in tertiary facilities like our study site hence reducing on the workload for midwives in that facility therefore giving them ample time to monitor and administer drugs such as MgSO4 to the few mothers effectively. It will also reduce caesarean sections done in the facility for mothers suffering from pre-eclampsia with no complications hence reduce on the time of hospital stay of mothers and also reduce staff burnout due to poor midwife- patient ratio hence improving the quality of care offered to these mothers.
To bridge the identified recommendation-to-practice gap, future studies should focus on understanding midwives’ practices and perceptions towards MgSO4 and drug administration in general which will reveal the reasons for not adhering to drug administration recommendations as highlighted in the current study.
Supplementary Information
Acknowledgements
A special thanks goes to Case Western University Stroke Project for the research lessons and mentorship offered to the primary investigator during the writing of this paper, and to Shapiro Norman for all the data analysis lessons he offered to the principal investigator of this paper.
Data collection tool used and data set
The questionnaire that was used to collect data for this study and a data set have been provided as Appendix 1 and 2 respectively in the supplementary information section of this file.
Abbreviations
- AOR
Adjusted Odds Ratio
- EMNCGU
Essential Maternal and Newborn Clinical Care Guidelines for Uganda
- FSB
Fresh Still Birth
- LBW
Low Birth Weight
- LMIC
Low to Middle Income Countries
- MgSO4
Magnesium Sulphate
- MSB
Macerated Still-Birth
- NICU
Neonatal Intensive Care Unit
- UCG
Uganda Clinical Guidelines
- WHO
World Health Organization
- PEC
Pre-eclampsia with severe feature
- PE
Pre-eclampsia
Authors’ contributions
M.B is the primary author, she carried out the study right from proposal writing to data collection to dissertation and manuscript writing, she also identified the journal for publication. S.N.M is the primary supervisor and mentor of the lead author, contributed in conceptualizing the topic of research and was the primary reviewer of the whole research project writing. J.N, M. k, J.N, C.B, S.M, C.B, E.T.K, and M.S were all supervisors and advisers from proposal writing and through out the dissertation writing process. All authors approved the final draft of the paper.
Funding
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS118544.
Data availability
The acquired and/or analyzed data are not publicly available because of the lack of authorization from the agreement with the Research Ethics Committee that the database would remain with the corresponding author only. However, all data can be made available by the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethical review and approval were obtained from the Research Ethics Committee (REC) of the School of Health Sciences, College of Health Sciences at Makerere University under REC number MAKSHSREC-2022–330. Administrative clearance and permission to collect data were obtained from the management of Kawempe National Referral hospital. All participants gave written informed consent to participate voluntarily in the study prior to data collection and were informed that there would be no consequences if they withdraw from the study at any given point of the study if they wished not to continue. Participants who could not read or write were asked to provide informed verbal consent after the script was read to them by the research assistant in order to assist in the comprehension of the research and informed verbal consent process, and these participants signed the written consent form by putting a thumb print. Information collected from the respondents was held with confidentiality through the use of anonymous questionnaires which were filed and kept under lock and key throughout the whole data collection and analysis process.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tochio A, Obata Y, Saigusa R, Shindo E, Aoki S. "Does pre‐eclampsia without proteinuria lead to different pregnancy outcomes than pre‐eclampsia with proteinuria? J Obstetr Gynaecol Res. 2019;45(8):1576–15. [DOI] [PubMed] [Google Scholar]
- 2.Nakimuli A, Chazara O, Byamugisha J, Elliott A. M., Kaleebu P, Mirembe F, Moffet A. “Pregnancy, parturition and preeclampsia in women of African ancestry,.” Am J Obstetr Gynecol. 2014;210(6):510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vata P. K., Chauhan N. M., Nallathambi A, Hussein F. “Assessment of prevalence of preeclampsia from Dilla region of Ethiopia,.” BMC res notes. 2015;8(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, Zhang H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC pregnancy childbirth. 2021;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartal MF, Sibai BM. Eclampsia in the 21st century. Am J Obstet Gynecol. 2022;226:S1237–53. [DOI] [PubMed] [Google Scholar]
- 6.Laskowska M. Eclampsia: A critical pregnancy complication demanding enhanced maternal care: A review. Medical Science Monitor. Int Med J Experim Clin Res. 2023;29:e939919–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal S, Gupta R, Pandey K, Gupta N, Lal P, Verma M. Is low dose magnesium sulfate regimen a better option for treatment of hypertensive disorders of pregnancy: Our experience at tertiary care centre. Int J Clin Obstetr Gynaecol. 2020;4(1):213–7. [Google Scholar]
- 8.J. Padda, K. Khalid, L. B. Colaco, S. Padda, N. L. Boddeti, A. S. Khan and G. Jean-Charles, "Efficacy of magnesium sulfate on maternal mortality in eclampsia.," Cureus, 13(8), p. 13(8), 2021. [DOI] [PMC free article] [PubMed]
- 9.WHO, "WHO Recommendations for Prevention and Treatment of Pre-eclampsia and Eclampsia.," World Health Organization, Geneva, Switzerland, 2011. http://whqlibdoc.who.int/hq/2011/WHO_RHR_11.25_eng.pdf. [PubMed]
- 10.MOH, "Ministry_of_Health Uganda. Uganda Clinical guidelines. In: December 2016 edn," Ministry of Health, Kampala, 2016.
- 11.Long Q, Oladapo OT, Leathersich S, Vogel JP, Carroli G, Lumbiganon P, Mugerwa K. Clinical practice patterns on the use of magnesium sulphate for treatment of pre-eclampsia and eclampsia: a multi-country survey. An Int J Obstetr Gynaecol. 2017;124(12):1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Y. B. A. M. A. Alsiddig, A. W. Salah and B. R. Rifaat, "Assessment of magnesium sulphate usage in pre-eclamptic and eclamptic women in Omdurman Maternity Hospital, 2017: A cross-sectional study," F1000Research, pp. 8, 447, 2019.
- 13.Chikalipo MC, Phiri LK, Mndolo N, Mbiza CR, Khisi P, Golombe E, Maluwa A. Perception of midwives towards magnesium sulfate use at Chatinkha Maternity Wing in Blantyre, Malawi: a qualitative study. Int J Women's Health. 2020;12:187–96. 10.2147/IJWH.S223029. [DOI] [PMC free article] [PubMed]
- 14.Esfaye AG, Tefera BL, Sena BK. Pregnancy Induced Hypertension and Associated Factors among Pregnant Women Receiving Antenatal Care Service at Jimma Town Public Health Facilities, South West Ethiopia. J Gynecol Women’s Health. 2018:10(3):555792. 10.19080/JGWH.2018.10.555792.
- 15.Lingam I, Robertson NJ. Magnesium as a neuroprotective agent: a review of its use in the fetus, term infant with neonatal encephalopathy, and the adult stroke patient. Develop neurosci. 2018;40(1):1–12. [DOI] [PubMed] [Google Scholar]
- 16.Montori MGMAÁ, Álvarez CL, Cuchí NA, Alcalá PM, Ruiz-Martínez S. Advanced maternal age and adverse pregnancy outcomes: A cohort study. Taiwanese J Obstetr Gynecol. 2021;60(1):119–24. [DOI] [PubMed] [Google Scholar]
- 17.Marín R, Abad C, Rojas D, Chiarello DI, Rangel H, Teppa-Garrán A, Ruette F. Magnesium salts in Pregnancy. J Trace Elem Miner. 2023;4:100071.
- 18.Kish L. Sampling organizations and groups of unequal sizes. Am Sociol Rev. 1965;30(4):564–72. [PubMed]
- 19.Milln JM, Nakimuli A. Medical complications in pregnancy at Mulago Hospital, Uganda’s national referral hospital. Obstetr Med. 2019;12(4):168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omair A. Sample size estimation and sampling techniques for selecting a representative sample. J Health Special. 2014;2(4):142. [Google Scholar]
- 21.Wex F, Luze R, Franke J, Ramoni A, Gizewski E, Lucovnik M, Mörtl M. Magnesium sulfate overdose resulting in maternal cardiac arrest: a case report. Clin Obstet Gynecol Reprod Med. 2020;6:1–4. [Google Scholar]
- 22.M. J. F. S. F. K. S. A. S. S. M. A. &. P. A. S. Tarrahi, "Medication safety climate from the perspectives of healthcare providers: A cross-sectional study," journal of education and health promotion, vol. 12, no. 22, p. 195, 2023. [DOI] [PMC free article] [PubMed]
- 23.Ministry of Health. Essential Maternal and Newborn Clinical Care Guidelines for Uganda. Kampala: Ministry of health; 2022.
- 24.Firoz T, Sanghvi H, Merialdi M, von Dadelszen P. Pre-eclampsia in low and middle income countries. Best Pract ResClin Obstet Gynaecol. 2011;25:537–48. [DOI] [PubMed] [Google Scholar]
- 25.Elkady GA, GabAllah RR, Mansour AZ. Magnesium in intensive care unit: a review. The Egypt J Hospital Med. 2017;68(3):1497–504. [Google Scholar]
- 26.Richter AE, Scherjon SA, Dikkers R, Bos AF, Kooi EM. Antenatal magnesium sulfate and preeclampsia differentially affect neonatal cerebral oxygenation. Neonatol. 2020;117(3):331–40. [DOI] [PubMed] [Google Scholar]
- 27.Barua A, Mundle S, Bracken H, Easterling T, Winikoff B. Facility and personnel factors influencing magnesium sulfate use for eclampsia and pre-eclampsia in 3 Indian hospitals. Int J Gynecol Obstetr. 2011;115(3):231–4. [DOI] [PubMed] [Google Scholar]
- 28.Oguntunde O, Charyeva Z, Cannon M, Sambisa W, Orobaton N, Kabo IA. Factors influencing the use of magnesium sulphate in pre-eclampsia/eclampsia management in health facilities in North Nigeria: a mixed methods study. BMC pregnancy childbirth. 2015;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerri-Ann McKenzie H. T. “A Retrospective Study of Neonatal Outcome in Preeclampsia at the University Hospital of the West Indies: A Resource-limited Setting,,.” J Trop Pediatr. 2019;65(1):78–83. [DOI] [PubMed] [Google Scholar]
- 30.Sherwin CM, Balch A, Campbell SC, Fredrickson J, Clark EA, Varner M, Spigarelli MG. Maternal magnesium sulphate exposure predicts neonatal magnesium blood concentrations. Basic clin pharmacol toxicol. 2014;114(4):318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirenden H, Sunarno I, Arsyad MA, Idris I. Birth weight, Apgar score, and fetal complications in mothers with severe preeclampsia. Enfermeria clinica. 2020;30:533–6. [Google Scholar]
- 32.Olusanya BO, Solanke OA. Correlates of birth asphyxia using two Apgar score classification methods. Nig Q J Hosp Med. 2010;20(4):153–61. [PubMed] [Google Scholar]
- 33.Cacciani L, Asole S, Polo A, Franco F, Lucchini R, De Curtis M, Guasticchi G. Perinatal outcomes among immigrant mothers over two periods in a region of central Italy. BMC public health. 2011;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudayu TW. Proportion and factors associated with low fifth minute Apgar score among singleton newborn babies in Gondar University referral hospital; North West Ethiopia. African health sci. 2017;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarimo CS, Bhuyan SS, Zhao Y, Ren W, Mohammed A, Li Q, Wu J. Prediction of low Apgar score at five minutes following labor induction intervention in vaginal deliveries: machine learning approach for imbalanced data at a tertiary hospital in North Tanzania. BMC Pregnancy Childbirth. 2022;22(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehari MA, Maeruf H, Robles CC, Woldemariam S, Adhena T, Mulugeta M, Kumsa H. Advanced maternal age pregnancy and its adverse obstetrical and perinatal outcomes in Ayder comprehensive specialized hospital, Northern Ethiopia, 2017: a comparative cross-sectional study. BMC pregnancy childbirth. 2020;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rademaker D, Hukkelhoven CW, van Pampus MG. Adverse maternal and perinatal pregnancy outcomes related to very advanced maternal age in primigravida and multigravida in The Netherlands: a population-based cohort. Acta obstetricia et gynecologica Scandinavica. 2021;100(5):941–8. [DOI] [PubMed] [Google Scholar]
- 38.Leader J, Bajwa A, Lanes A, Hua X, White RR, Rybak N, Walker M. The effect of very advanced maternal age on maternal and neonatal outcomes: a systematic review. J obstetr gynaecol Canada. 2018;40(9):1208–18. [DOI] [PubMed] [Google Scholar]
- 39.Mersha A, Shibiru S, Bante A. Meconium-stained liquor and low birth weight increases the odds of low fifth-minute Apgar scores in public health facilities of Arba Minch town, southern Ethiopia: a cross-sectional study. J Pediatr Neonatal Care. 2020;10(3):86–90. [Google Scholar]
- 40.Nadim AA, ElBohoty AE, Mahmoud FM. Using of Magnesium Sulphate for Fetal Neuroprotection in Patients Presenting by Intrapartum Fetal Distress at Term: A Randomized Controlled Trial. Egypt J Hosp Med. 2017;69(2):1794-802.
- 41.Abbassi-Ghanavati M, Alexander JM, McIntire DD, Savani RC, Leveno KJ. Neonatal effects of magnesium sulfate given to the mother. Am J Perinatol. 2012;29(10):795–800. [DOI] [PubMed] [Google Scholar]
- 42.Aboshama RA, Ezzat L. Using of Mg Sulphate for Fetal Neuroprotection in women Presenting by Fetal Distress during labour at Term: A Randomized Controlled Trial. Multidiscip Academic J Publ. 2020;10(4).
- 43.Girsen A. I., Greenberg M. B., El-Sayed Y. Y., Lee H, Carvalho B, Lyell D. J. “Magnesium sulfate exposure and neonatal intensive care unit admission at term,.” J Perinatol. 2015;35(3):181–5. [DOI] [PubMed] [Google Scholar]
- 44.M. I. Akbar, D. A. Yoseph, M. A. Bachnas, E. G. Dachlan, G. A. Dekker and Ernawati, "Magnesium intoxication in women with preeclampsia with severe features treated with magnesium sulfate," Hyperten Pregnancy. 39(3), 221–227, 2020. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Both the acquired and analyzed data are not publicly available because of the lack of authorization from the agreement made with the Research Ethics Committee that the database would remain with the corresponding author only. However, all data can be made available by the corresponding author upon reasonable request and authorization from the Research Ethics Committee.
The acquired and/or analyzed data are not publicly available because of the lack of authorization from the agreement with the Research Ethics Committee that the database would remain with the corresponding author only. However, all data can be made available by the corresponding author upon reasonable request.