Abstract
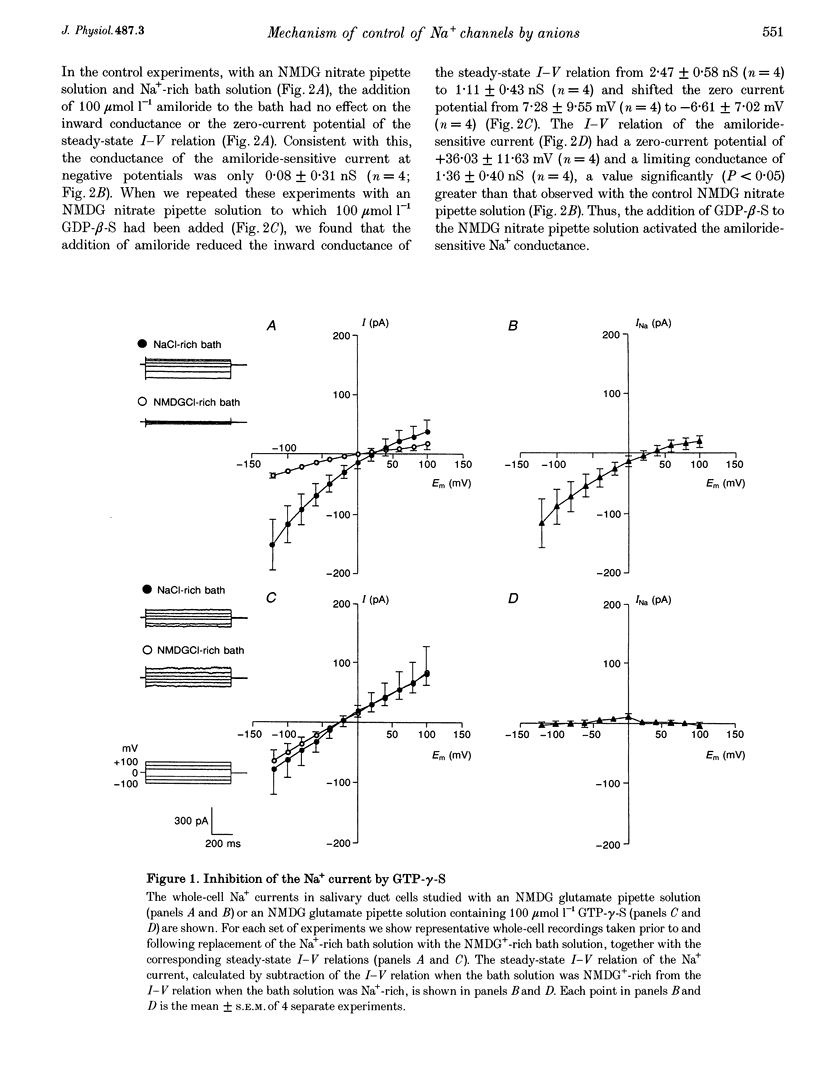
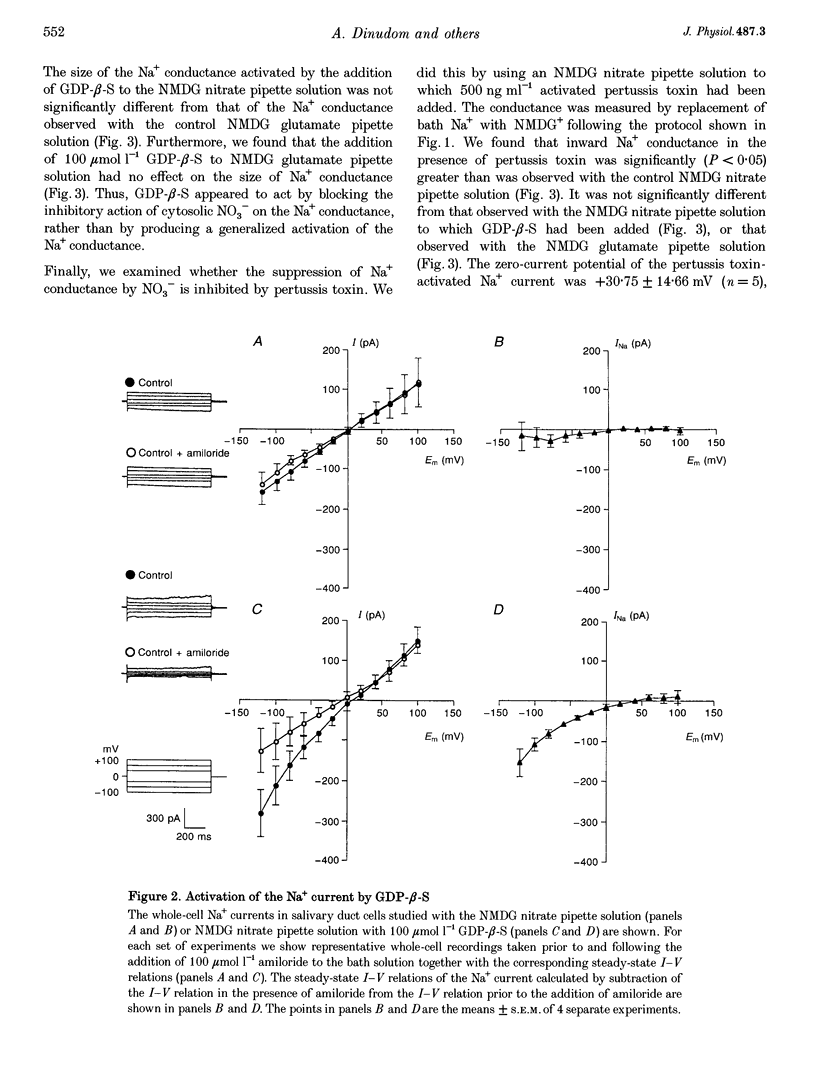
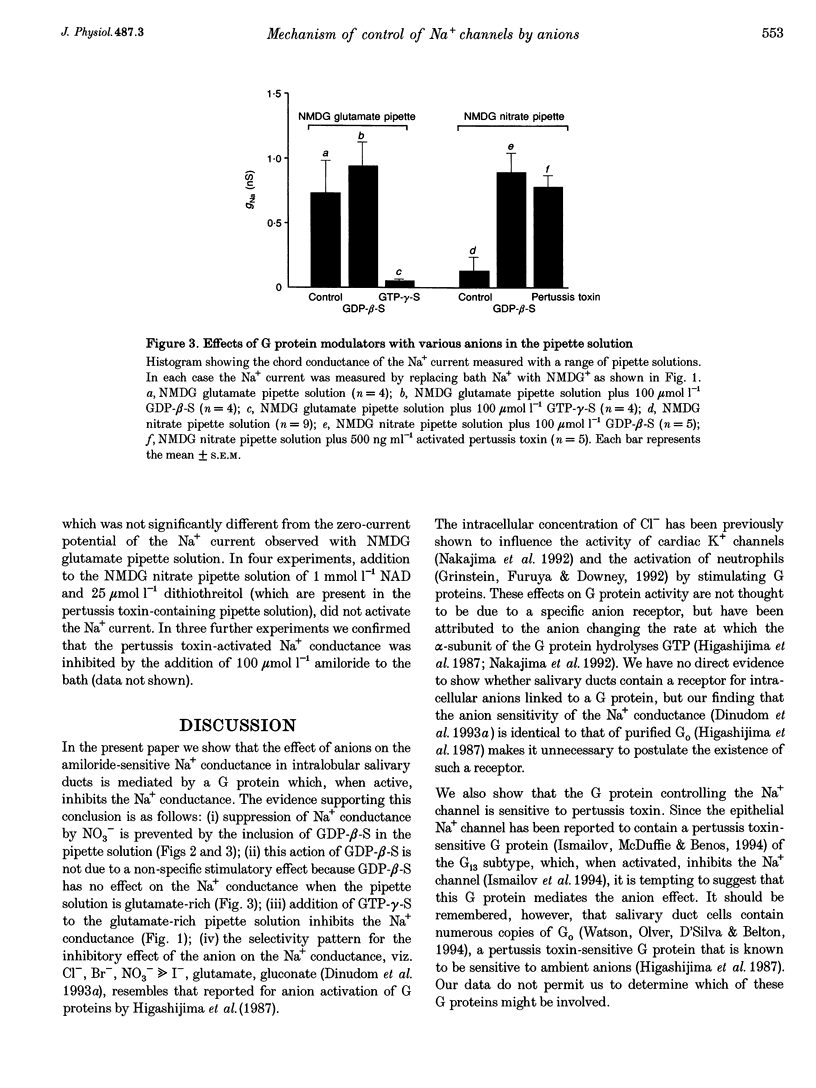
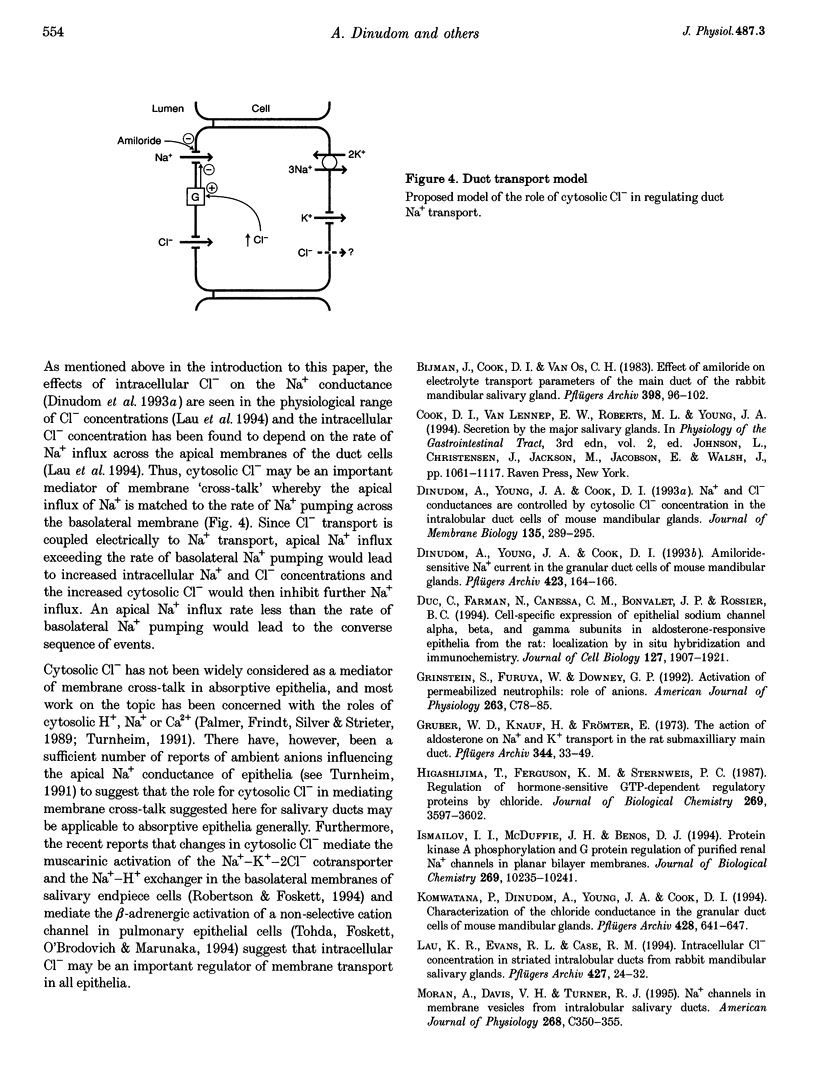
1. We have previously reported that the Na+ conductance in mouse intralobular salivary duct cells is controlled by cytosolic anions, being inhibited by high cytosolic concentrations of Cl- and NO3- but not of glutamate. In the present paper, we use whole-cell patch-clamp methods to investigate whether this anion effect is mediated by a G protein. 2. Inclusion of 100 mumol l-1 GTP-gamma-S, a non-hydrolysable GTP analogue, in the glutamate-containing pipette solution, i.e. when the Na+ conductance is active, reduced the size of the Na+ conductance whereas inclusion of 100 mumol l-1 GDP-beta-S, a non-hydrolysable GDP analogue, had no effect. 3. Inclusion of 100 mumol l-1 GDP-beta-S in the NO3(-)-containing pipette solution, i.e. when the Na+ conductance is inhibited, reactivated the conductance. Inclusion of 500 ng ml-1 activated pertussis toxin in the NO3(-)-containing pipette solution had a similar effect on the Na+ conductance. 4. We conclude that the inhibitory effect of intracellular anions such as NO3- and Cl- on the amiloride-sensitive Na+ conductance in mouse mandibular intralobular duct cells is mediated by a G protein sensitive to pertussis toxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijman J., Cook D. I., van Os C. H. Effect of amiloride on electrolyte transport parameters of the main duct of the rabbit mandibular salivary gland. Pflugers Arch. 1983 Jul;398(2):96–102. doi: 10.1007/BF00581055. [DOI] [PubMed] [Google Scholar]
- Dinudom A., Young J. A., Cook D. I. Amiloride-sensitive Na+ current in the granular duct cells of mouse mandibular glands. Pflugers Arch. 1993 Apr;423(1-2):164–166. doi: 10.1007/BF00374977. [DOI] [PubMed] [Google Scholar]
- Dinudom A., Young J. A., Cook D. I. Na+ and Cl- conductances are controlled by cytosolic Cl- concentration in the intralobular duct cells of mouse mandibular glands. J Membr Biol. 1993 Sep;135(3):289–295. doi: 10.1007/BF00211100. [DOI] [PubMed] [Google Scholar]
- Duc C., Farman N., Canessa C. M., Bonvalet J. P., Rossier B. C. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994 Dec;127(6 Pt 2):1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Downey G. P. Activation of permeabilized neutrophils: role of anions. Am J Physiol. 1992 Jul;263(1 Pt 1):C78–C85. doi: 10.1152/ajpcell.1992.263.1.C78. [DOI] [PubMed] [Google Scholar]
- Gruber W. D., Knauf H., Frömter E. The action of aldosterone on Na+ and K+ transport in the rat submaxillary main duct. Pflugers Arch. 1973 Nov 15;344(1):33–49. doi: 10.1007/BF00587440. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Sternweis P. C. Regulation of hormone-sensitive GTP-dependent regulatory proteins by chloride. J Biol Chem. 1987 Mar 15;262(8):3597–3602. [PubMed] [Google Scholar]
- Ismailov I. I., McDuffie J. H., Benos D. J. Protein kinase A phosphorylation and G protein regulation of purified renal Na+ channels in planar bilayer membranes. J Biol Chem. 1994 Apr 8;269(14):10235–10241. [PubMed] [Google Scholar]
- Lau K. R., Evans R. L., Case R. M. Intracellular Cl- concentration in striated intralobular ducts from rabbit mandibular salivary glands. Pflugers Arch. 1994 May;427(1-2):24–32. doi: 10.1007/BF00585938. [DOI] [PubMed] [Google Scholar]
- Moran A., Davis V. H., Turner R. J. Na+ channels in membrane vesicles from intralobular salivary ducts. Am J Physiol. 1995 Feb;268(2 Pt 1):C350–C355. doi: 10.1152/ajpcell.1995.268.2.C350. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Sugimoto T., Kurachi Y. Effects of anions on the G protein-mediated activation of the muscarinic K+ channel in the cardiac atrial cell membrane. Intracellular chloride inhibition of the GTPase activity of GK. J Gen Physiol. 1992 May;99(5):665–682. doi: 10.1085/jgp.99.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara A., Matsunaga H., Eaton D. C. G protein activation inhibits amiloride-blockable highly selective sodium channels in A6 cells. Am J Physiol. 1993 Feb;264(2 Pt 1):C352–C360. doi: 10.1152/ajpcell.1993.264.2.C352. [DOI] [PubMed] [Google Scholar]
- Robertson M. A., Foskett J. K. Na+ transport pathways in secretory acinar cells: membrane cross talk mediated by [Cl-]i. Am J Physiol. 1994 Jul;267(1 Pt 1):C146–C156. doi: 10.1152/ajpcell.1994.267.1.C146. [DOI] [PubMed] [Google Scholar]
- Tohda H., Foskett J. K., O'Brodovich H., Marunaka Y. Cl- regulation of a Ca(2+)-activated nonselective cation channel in beta-agonist-treated fetal distal lung epithelium. Am J Physiol. 1994 Jan;266(1 Pt 1):C104–C109. doi: 10.1152/ajpcell.1994.266.1.C104. [DOI] [PubMed] [Google Scholar]
- Turnheim K. Intrinsic regulation of apical sodium entry in epithelia. Physiol Rev. 1991 Apr;71(2):429–445. doi: 10.1152/physrev.1991.71.2.429. [DOI] [PubMed] [Google Scholar]
- Watson E. L., Oliver C., D'Silva N., Belton C. M. Localization of the G-protein G(o) in exocrine glands. J Histochem Cytochem. 1994 Jan;42(1):41–47. doi: 10.1177/42.1.7505300. [DOI] [PubMed] [Google Scholar]
- Zong X., Lux H. D. Augmentation of calcium channel currents in response to G protein activation by GTP gamma S in chick sensory neurons. J Neurosci. 1994 Aug;14(8):4847–4853. doi: 10.1523/JNEUROSCI.14-08-04847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


