Abstract
Background
To investigate whether an additional gastrojejunostomy reduces the incidence of delayed gastric emptying (DGE) following a distal segmental duodenectomy for duodenal and proximal jejunal gastrointestinal stromal tumors (GIST).
Materials and methods
This retrospective review of the GIST database at Peking University Cancer Hospital included 50 patients who underwent distal segmental duodenectomies for primary GIST in the duodenum or proximal jejunum within 20 cm of Treitz’s ligament between January 2008 and December 2023. The patients were divided into two groups: non-bypass (without gastrojejunostomy) and bypass (with gastrojejunostomy and Braun’s jejunojejunostomy). Perioperative characteristics and postoperative complications were analyzed.
Results
Among the 50 patients, 27 underwent duodenojejunostomies without gastrojejunostomies and 23 with gastrojejunostomies and Braun’s jejunojejunostomies. The incidence of grade B-C DGE was significantly lower in the bypass group (43.5% vs. 74.1%, p = 0.028). In addition, non-bypass surgery was an independent risk factor for increased grade B-C DGE (OR 3.67, 95% CI 1.07–12.64, p = 0.039). The bypass group showed a trend towards a shorter postoperative hospital stay (median: 14 days, range: 10–56) compared to the non-bypass group (median: 28 days, range: 6–75), but this difference did not reach statistical significance (p = 0.070). Operative time (min) was significantly longer in the multi-visceral resection group (381.0 ± 108.8 vs. 227.3 ± 87.6, p < 0.001), for tumors ≥ 6.3 cm compared to < 6.3 cm (337.0 ± 116.4 vs. 228.3 ± 99.8, p = 0.002), and in patients with positive preoperative symptoms versus asymptomatic patients (319.9 ± 118.0 vs. 210.2 ± 90.3, p = 0.031).
Conclusion
The addition of gastrojejunostomy and Braun’s jejunojejunostomy in distal segmental duodenectomy can reduce the incidence of grade B-C DGE, potentially facilitating timely adjuvant imatinib therapy. Future multicenter studies are needed to confirm these findings.
Keywords: Gastrointestinal stromal tumor, Delayed gastric emptying, Surgery, Duodenum, Proximal jejunum
Introduction
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors of the gastrointestinal tract. Although GISTs can develop anywhere in the digestive tract, only 3–5% of GISTs occur in the duodenum [1]. Currently, surgery is the cornerstone of therapy for these localized or potentially resectable GISTs [2]. Local resection (LR) for duodenal GISTs should be performed when feasible, as it does not result in inferior survival outcomes [3]. However, the complex anatomy of the pancreaticoduodenal region and varied clinical presentations make diagnosing and resecting duodenal GISTs challenging [4]. Surgical resection methods for duodenal GISTs thus vary significantly depending on their location and possible invasion of the Vater’s ampulla or pancreas [5].
Distal segmental duodenectomy is often necessary for tumors located in the D3/D4 duodenum and proximal jejunum, which can impair gastrointestinal motility and lead to delayed gastric emptying (DGE) in patients [6–8]. The duodenum, which stores motilin, plays an important role in initiating gastrointestinal motility in humans. Duodenectomies not only decrease motilin secretion, impacting the contraction of the gastric antrum, but also influences the neuromodulation of the gastrointestinal tract, thereby affecting gastrointestinal motility [9, 10]. Postoperative ischemia or edema of the duodenal stump may represent another potential cause of DGE following distal segmental duodenectomy. Extensive mesopancreas dissection is often necessary, which involves severing the duodenal branches of the superior mesenteric vessels. This disruption may increase the risk of ischemia or edema, potentially contributing to a higher incidence of DGE [11]. The literature reports that the incidence of DGE following distal segmental duodenectomy varies between 23-67% [5, 6, 11, 12].
DGE can prolong hospital stays and delay necessary postoperative treatments [13]. Delayed postoperative imatinib may lead to worse recurrence-free survival in patients with a high risk of recurrence [14]. Although the impact of DGE following segmental duodenectomy on adjuvant imatinib therapy remains unclear due to the lack of available data, in other settings, patients with DGE after pancreaticoduodenectomy for pancreatic cancer were reported to be less likely to have received postoperative chemotherapy or radiation therapy compared to those without DGE [15].
Therefore, we designed a study investigating the impact of an additional gastrojejunostomy in reducing the incidence of DGE following a distal segmental duodenectomy for duodenal and proximal jejunal GISTs.
Materials and methods
Study population
A retrospective review of the institutional GIST database was conducted. The data of the patients with primary GIST located in the duodenum and proximal jejunum within 20 cm of Treitz’s ligament who underwent surgical resection between January 2008 and December 2023 at Peking University Cancer Hospital (PKUCH) were selected.
The inclusion criteria for patient selection were as follows: (1) between 18 and 80 years of age; (2) distal segmental duodenectomy; and (3) pathologically confirmed primary GIST located in the D3/D4 duodenum or jejunum within 20 cm of the Treitz’s ligament. The exclusion criteria were: (1) operations conducted in an emergency setting; (2) locally recurrent or metastatic GIST involving duodenum or proximal jejunum; (3) other duodenal resection methods beyond distal segmental duodenectomy, including distal gastrectomy with proximal duodenectomy, pancreaticoduodenectomy, wedge duodenal wall resection or endoscopic duodenal resection; (4) merely jejunal resection without duodenal resection for proximal jejunal GIST; and (5) history or presence of any severe, unstable, systemic disease.
Until the end of 2017, our standard practice for cases of GISTs involving the proximal jejunum or the D3/D4 duodenum that required distal segmental duodenectomies was to perform only an end-to-side duodenojejunostomy without a gastrojejunostomy. However, we frequently encountered DGE, leading to many patients having to receive long-term gastrointestinal decompression therapy and being unable to consume food, potentially delaying subsequent imatinib therapy. In early 2018, we began exploring a modified approach. This new strategy included the addition of a gastrojejunostomy and a Braun’s jejunojejunostomy. For this study, patients were divided into two groups: the non-bypass group (patients who had undergone surgery by the end of 2017 without a gastrojejunostomy and Braun’s jejunojejunostomy) and the bypass group (those who were treated thereafter with a gastrojejunostomy and a Braun’s jejunojejunostomy).
Surgical approach
The same surgical team carried out the following surgical procedure with the same main steps:
After a thorough exploration of the abdominal cavity, the Cattell–Braasch maneuver was employed to mobilize the entire small intestine and mesocolon to facilitate exposure of the distal duodenum.
The distal duodenum and proximal jejunum were mobilized and sectioned distal to the tumor with the mesentery. The tumor, along with the duodenum and jejunum, was then dissected and repositioned posterior to the superior mesenteric vessels on the right side.
In instances where pancreas involvement was detected, the decision would be made to proceed with pancreaticoduodenectomy, partial pancreatic resection, or distal pancreatectomy based on the extent of the involvement. For large tumors near the junction of D2 and D3, catheterization through the cystic duct after a cholecystectomy was used to identify and protect the Vater’s ampulla and to delineate a safe resection margin.
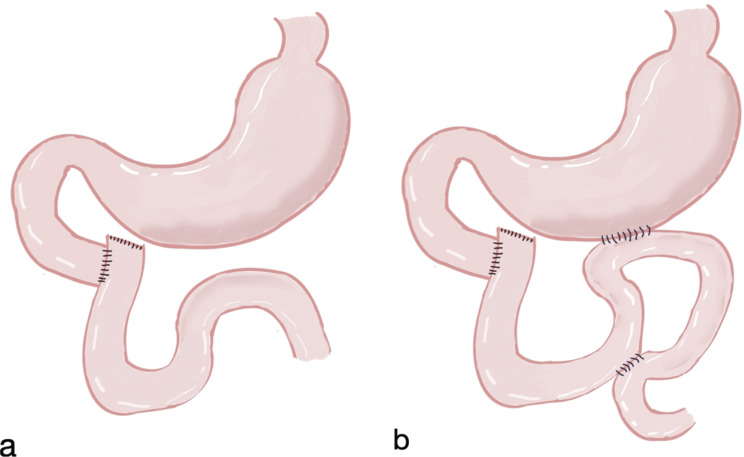
The duodenum was sectioned proximal to the tumor and distal to the Vater’s ampulla. Jejunal ascension was performed retrocolically, followed by an end-to-side anastomosis with the duodenal stump (Fig. 1a). For patients with the gastrojejunostomy procedure, an antecolic side-to-side gastrojejunostomy was stapled 40 cm distal to the duodenojejunal anastomosis along the greater curvature. A Braun’s antimesenteric jejunojejunostomy was then performed 15 cm proximal to the gastrojejunostomy for the afferent limb and 40 cm distal for the efferent limb (Fig. 1b).
Fig. 1.
(a). Patients in the non-bypass group underwent end-to-side duodenojejunostomy. (b). Patients in the bypass group also underwent an antecolic side-to-side gastrojejunostomy and a Braun antimesenteric jejunojejunostomy
Postoperative care
The patients had routine nasogastric tubes (NGT) inserted during the operation. According to the institutional protocol, it is standard practice for patients to receive prophylactic nasogastric decompression after segmental duodenectomy and undergo routine upper gastrointestinal series (UGIS) between postoperative days (POD) 5 and 7 for the early detection of anastomotic leakage. Once the UGIS did not reveal any anastomotic fistula and the contrast agent transmitted rapidly into the distal jejunum, the NGT was removed immediately, and the patient was advised to resume eating.
Complication definition
Postoperative clinical data concerning any complications and deviation from the normal postoperative course was also collected. DGE and other incidents such as postoperative pancreatic fistula (POPF) were graded following the standards published by the International Study Group of Pancreatic Surgery (ISGPS) [16, 17].
It should be noted that the true incidence of grade A DGE could not be determined in the study as all the patients were allowed to remove the nasogastric tube after the UGIS was done 5–7 days postoperatively due to our institutional protocol. Considering that grade A DGE generally only causes minor disturbances during the resumption of solid food intake, it typically has minimal clinical impact and may cause only a slight deviation from the clinical pathway [16, 18]. We thus mainly compared the incidence grades B-C (moderate and severe) DGE between the non-bypass group and the bypass group. The other postoperative complications were graded by the Clavien–Dindo classification and considered “major” if they were Grade III or higher [19].
Data handling and statistical analysis
We collected and analyzed demographic information for variables including age, gender, and body mass index (BMI). Clinicopathologic variables such as symptoms, diabetes, smoking, preoperative hemoglobin (HGB), tumor size, tumor location, liver metastases, and American Society of Anesthesiologists (ASA) classifications were collected as well, and treatment variables such as neoadjuvant imatinib therapy, operation time, estimated blood loss, multi-visceral resection (MVR), intensive care unit (ICU) stays, NGT removal interval, NGT reinsertion, highest body temperature, and postoperative stays count were also incorporated into our study. It is important to note that according to the criteria of the Chinese Working Group on Obesity and thus our study, BMI is classified into the following three groups: low and normal weight (18.5–24), overweight (24 to < 28), and obesity [20].
Statistical analysis was performed with SPSS Statistics (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.). Standard descriptive statistics were calculated for categoric data (i.e., frequency and percentage) and continuous data (i.e., median and range), as listed in Table 1. Independent sample t-tests, Chi-square tests, and nonparametric Mann-Whitney U tests were used to compare variables between the non-bypass group and the bypass group for significant differences.
Table 1.
Clinicopathologic characteristics of patients
| Characteristics | Without bypass (n = 27) | With bypass (n = 23) | p-value |
|---|---|---|---|
| Age, years | 53 (27–76) | 51 (28–76) | 0.498 |
| Gender | 0.665 | ||
| Male | 16 (59.3) | 15 (65.2) | |
| Female | 11 (40.7) | 8 (34.8) | |
| BMI | 0.859 | ||
| Normal | 12 (44.4) | 10 (43.5) | |
| Overweight | 12 (44.4) | 9 (39.1) | |
| Obesity | 3 (11.1) | 4 (17.4) | |
| Symptoms | 0.005 | ||
| Bleeding | 10 (37.0) | 3 (13.0) | |
| Abdominal discomfort | 5 (18.5) | 12 (52.2) | |
| Palpable mass | 0 | 3 (13.0) | |
| Asymptomatic | 12 (44.4) | 5 (21.7) | |
| Diabetes | 0.460 | ||
| Yes | 0 | 1 (4.3) | |
| No | 27 (100) | 22 (95.7) | |
| Smoking | 0.670 | ||
| Yes | 9 (33.3) | 9 (39.1) | |
| No | 18 (66.7) | 14 (60.9) | |
| Hemoglobin, g/L | 118 (81–166) | 118 (69–157) | 0.943 |
| Tumor size, cm | 6 (2–22) | 9 (3–28) | 0.005 |
| Risk classification | 0.104 | ||
| Very low | 1 (3.7) | 0 | |
| Low | 9 (33.3) | 3 (13.0) | |
| Intermediate | 0 | 0 | |
| High | 17 (63.0) | 20 (87.0) | |
| Location | 0.449 | ||
| Duodenum | 23 (85.2) | 22 (95.7) | |
| Proximal jejunum | 4 (14.8) | 1 (4.3) | |
| Liver metastases | 0.094 | ||
| No | 18 (66.7) | 20 (87.0) | |
| Yes | 9 (33.3) | 3 (13.0) | |
| Neoadjuvant therapy | 0.449 | ||
| No | 16 (59.3) | 16 (69.6) | |
| Yes | 11 (40.7) | 7 (30.4) | |
| ASA | 0.225 | ||
| I | 1 (3.7) | 4 (17.4) | |
| II | 24 (88.9) | 16 (69.6) | |
| III | 2 (7.4) | 3 (13.0) | |
| Operative time, min | 230 (100–490) | 311 (142–557) | 0.017 |
| Intraoperative bleeding, ml | 100 (20-2000) | 200 (20-3000) | 0.084 |
| Multi-visceral resection | 0.869 | ||
| No | 17 (63.0) | 15 (65.2) | |
| Yes | 10 (37.0) | 8 (34.8) | |
| ICU admission | 0.159 | ||
| No | 25 (92.6) | 17 (73.9) | |
| Yes | 2 (7.4) | 6 (26.1) | |
| Complication | 1.000 | ||
| < 3 | 24 (88.9) | 21 (91.3) | |
| ≥ 3 | 3 (11.1) | 2 (8.7) | |
| Highest body temperature, ℃ | 37.8 (36.9–39.1) | 37.7 (36.8–39.0) | 0.453 |
| Postoperative stays, days | 28 (6–75) | 14 (10–56) | 0.070 |
| DGE | 0.028 | ||
| 0-A | 7 (25.9) | 13 (56.5) | |
| B-C | 20 (74.1) | 10 (43.5) | |
| NGT removal, days | 8 (5–70) | 7 (3–39) | 0.090 |
| NGT reinsertion | 0.435 | ||
| No | 20 (74.1) | 20 (87.0) | |
| Yes | 7 (25.9) | 3 (13.0) |
Each clinicopathological variable associated with DGE was analyzed using binary logistic regression models, with results presented as odds ratios (ORs) and 95% confidence intervals (95% CIs). Initially, each covariate was evaluated in a univariate model and retained if the p-value was less than 0.1. Subsequently, a stepwise selection method was employed to identify significant covariates. For our usage, a p-value of less than 0.05 was considered statistically significant for all analyses.
Continuous variables (age, hemoglobin level, tumor size, operation time, estimated blood loss, highest body temperature) and categorical variables (gender, symptoms, BMI, smoking status, tumor location, liver metastases, ASA classification, neoadjuvant imatinib therapy, multi-visceral resection, ICU admission, GIST risk classification, complication grade) were analyzed for associations with grade B-C delayed gastric emptying (DGE) using univariate logistic regression. Results are reported as ORs with 95% CIs. Variables with a p < 0.1 in univariate analysis were included in a multivariable logistic regression model to identify independent predictors of grade B-C DGE.
Independent sample t-tests assessed correlations between operative time and various factors, including age, gender, symptoms, BMI, smoking status, HGB levels, tumor size, risk classification, location, liver metastases, neoadjuvant imatinib therapy, ASA classification, MVR, and bypass. Variables with a p < 0.05 were included in multiple linear regression analysis. Tumor size and hemoglobin levels were categorized by mean values.
Results
Patient characteristics
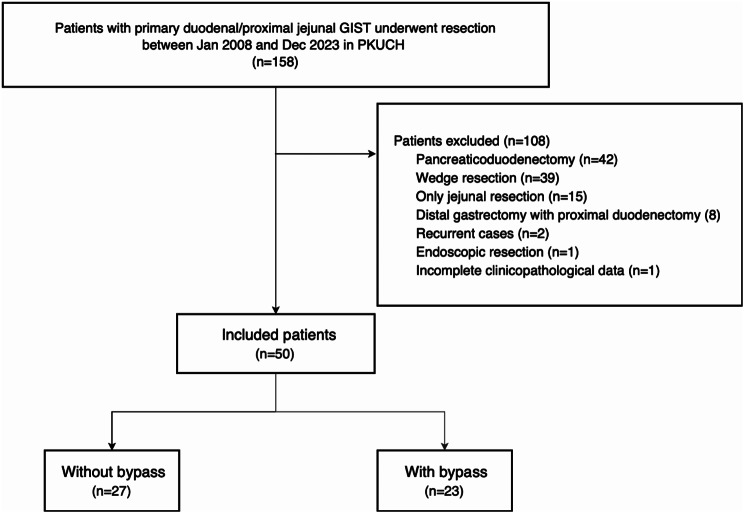
During the study period, 158 primary GISTs located in the duodenum or jejunum within 20 cm of Treitz’s ligament underwent surgical treatment at our institute. One hundred eight of them were excluded from the study for the following reasons: 42 patients who underwent pancreaticoduodenectomy, 39 patients with wedge duodenal wall resection, eight patients with distal gastrectomy with proximal duodenectomy for tumors located at the duodenal bulb, 15 patients with segmental jejunal resection without duodenectomy, two patients with recurrent GISTs involving the duodenum, one patient with endoscopic resection, and one patient with incomplete clinicopathological data (Fig. 2).
Fig. 2.
Flow chart of patient selection in the study
According to the abovementioned criteria, 50 patients who underwent distal segmental duodenectomy for duodenal or proximal jejunal GIST at our institution were finally enrolled in the study, including 31 males and 19 females. The median age was 52.5 years (range: 27–76). Two patients presented with histories of concurrent cancers. Specifically, one patient had a previous diagnosis of breast cancer, while the other had been diagnosed with endometrial cancer. Notably, both patients had undergone successful treatment without any evidence of tumor relapse.
The most common symptoms reported were abdominal discomfort including pain, bloating, and indigestion(affecting 17 patients (34%)). Thirteen patients (26%) presented with symptoms related to gastrointestinal bleeding such as anemia and melena. Three patients (6%) were diagnosed following medical consultations for self-detected palpable masses. Meanwhile, 17 asymptomatic patients (34%) were incidentally diagnosed with GISTs.
The median tumor size was 6.3 cm (range: 2.0–28.0 cm). The tumors were found both located in the duodenum (n = 45) and proximal jejunum (n = 5). Twelve patients presented with concurrent liver metastases, and 18 patients received neoadjuvant therapy. According to the modified National Institutes of Health Consensus criteria [21], the patients with very low, low, and high-risk GIST were 1 (2%), 12 (24%), and 37 (74%), respectively.
Operative characteristics and complications
Among the 50 patients who underwent segmental duodenectomy, 27 underwent duodenojejunostomy without gastrojejunostomy, and 23 with an antecolic gastrojejunostomy plus a Braun’s jejunojejunostomy. Regarding multi-visceral resection, a total of 30 organs other than duodenum and gallbladder in 18 patients were resected, including 11 pancreatic resections (7 partial resections of head/uncinate process, and four distal pancreatectomies), seven liver resections, ten colectomies, and two nephrectomies.
The median operative time in all cases was 272 min (range: 100–557 min), and the median estimated blood loss was 200 ml (range: 20-3000 ml). Eight patients were transferred to the ICU postoperatively, with stays ranging only from one to two days. No patients required reoperation, and there was no death within 90 days post-operation. The median highest body temperature after surgery was 37.8 °C (range 36.8–39.1 °C), and the median postoperative hospital stay was 21 days (6–75 days). Among thirteen patients whose postoperative hospital stay exceeded 30 days, two patients did not experience grade B or C DGE. The primary reasons for their prolonged hospital stay were postoperative pneumonia caused by a COVID-19 infection and a grade B postoperative pancreatic fistula (POPF) leading to an intra-abdominal abscess, respectively. The remaining 11 patients experienced grade B or C DGE. Among them, the extended hospital stay of eight patients was primarily due to DGE, while two patients had a combination of grade B POPF and grade B or C DGE, and one patient had a prolonged hospital stay primarily due to an anastomotic leakage of the duodenojejunostomy.
The patients’ NGT was removed on a median POD length of 7 days (range: 3–70), and ten patients (20%) required reinsertion after removal. Thirty patients (60%) experienced grade B-C DGE. The incidence of moderate-severe DGE was significantly lower in the bypass group compared to the non-bypass group (43.5% vs. 74.1%, p = 0.028). The patients in the bypass group had a higher incidence of positive clinical symptoms (p = 0.005), larger tumor size (p = 0.005), and longer operation times (p = 0.017) (Table 1). Among the patients with B-C grade DGE, six patients underwent gastroscopy during postoperative hospitalization. Five demonstrated patent anastomotic openings without edema on the gastroscopies, while one exhibited mucosal edema at the anastomotic site of duodenojejunostomy. In addition, six patients exhibited radiological evidence of intestinal wall thickening and edema at the anastomosis on postoperative CT scans.
Besides DGE, other major postoperative complications occurred in five patients (10%) who had all undergone multi-visceral resection. The incidence of grade B POPF was 27.3% (3/11) in the patients with pancreatic resection. Two of them developed intra-abdominal abscesses that were successfully treated with abdominal paracentesis drainage and none progressed to grade C. One patient experienced an anastomotic leakage of the duodenojejunostomy detected by UGIS on POD 7. Subsequent CT scans of this patient revealed the presence of an abdominal abscess, which was successfully treated with abdominal paracentesis drainage, as well as the anastomotic leakage. One patient underwent thoracic paracentesis drainage because of postoperative atelectasis. All patients eventually recovered during their hospital stays.
Predictors of incidence of grade B-C DGE and operating time
Univariate and multivariate logistic regression analyses revealed that non-bypass surgery was an independent risk factor for an increased incidence of grade B-C DGE (OR 3.67, 95% CI 1.07–12.64, p = 0.039). No correlation was found between the other clinicopathological characteristics and the occurrence of severe DGE, except for bypass operations (Table 2).
Table 2.
Univariate and multivariate logistic regression analysis of the correlation between clinicopathological characteristics and incidence of grade B-C DGE
| Characteristics | Mean (range)/N (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | ||
| Age, years | 52.5 (27–76) | 1.01 (0.97–1.06) | 0.609 | ||
| Gender | 0.812 | ||||
| Female | 19 (38) | Reference | |||
| Male | 31 (62) | 1.15 (0.36–3.68) | |||
| Symptoms | 0.466 | ||||
| No | 17 (34) | Reference | |||
| Yes | 33 (66) | 1.56 (0.47–5.10) | |||
| BMI | 0.642 | ||||
| Overweight or obesity | 28 (56) | Reference | |||
| Normal weight | 22 (44) | 1.31 (0.42–4.13) | |||
| Smoking | 0.904 | ||||
| Yes | 18 (36) | Reference | |||
| No | 32 (64) | 0.93 (0.29–3.03) | |||
| Hemoglobin, g/L | 118 (69–166) | 1.00 (0.97–1.02) | 0.776 | ||
| Tumor size, cm | 6.3 (2.0–28.0) | 0.94 (0.85–1.04) | 0.248 | ||
| Risk classification | 0.895 | ||||
| High | 37 (74) | Reference | |||
| Very low or low | 13 (26) | 1.10 (0.30–3.99) | |||
| Location | 0.348 | ||||
| Duodenum | 45 (90) | Reference | |||
| Proximal jejunum | 5 (10) | 0.41 (0.06–2.67) | |||
| Liver metastases | 0.892 | ||||
| Yes | 12 (24) | Reference | |||
| No | 38 (76) | 1.10 (0.29–4.10) | |||
| Neoadjuvant therapy | 0.904 | ||||
| Yes | 18 (36) | Reference | |||
| No | 32 (64) | 0.93 (0.29–3.03) | |||
| ASA | 0.088 | 0.104 | |||
| III | 5 (10) | Reference | Reference | ||
| I or II | 45 (90) | 7.25 (0.75–70.51) | 7.09 (0.67–75.14) | ||
| Operative time, min | 272 (100–557) | 1.00 (1.00-1.01) | 0.219 | ||
| Intraoperative bleeding, ml | 200 (20-3000) | 1.00 (0.99-1.00) | 0.555 | ||
| ICU admission | 0.169 | ||||
| Yes | 8 (16) | Reference | |||
| No | 42 (84) | 3.00 (0.63–14.34) | |||
| Bypass | 0.031 | 0.039 | |||
| With | 23 (46) | Reference | Reference | ||
| Without | 27 (54) | 3.71 (1.13–12.2) | 3.67 (1.07–12.64) | ||
| Multi-visceral resection | 0.904 | ||||
| Yes | 18 (36) | Reference | |||
| No | 32 (64) | 0.93 (0.29–3.03) | |||
| Complication | 1.000 | ||||
| ≥ 3 | 5 (10) | Reference | |||
| <3 | 45 (90) | 1.00 (0.15–6.59) | |||
| Highest body temperature, ℃ | 37.8 (36.8–39.1) | 1.88 (0.77–4.63) | 0.167 | ||
Univariate analysis showed significant differences in operative time based on clinical symptoms (p = 0.002), smoking status (p = 0.011), tumor size (p = 0.001), risk classification (p = 0.001), bypass (p = 0.033), and multi-visceral resection (p < 0.001) (Table 3). Subsequent multiple linear regression analysis indicated that multi-visceral resection (β = 124.626, t = 4.885, p < 0.001), tumor size (β = 77.127, t = 3.201, p = 0.002), and clinical symptoms (β = -57.864, t = -2.219, p = 0.031) significantly affected the operative time (Table 4). Patients undergoing multi-visceral resection, with tumor size ≥ 6.3 cm, or presenting with positive clinical symptoms also had longer operative times.
Table 3.
Correlation between clinicopathological characteristics and operative time
| Characteristics | N (%) | Operative time | p-value |
|---|---|---|---|
| Age, years | 0.955 | ||
| <65 | 12 (24) | 280.9 ± 109.8 | |
| ≥65 | 38 (76) | 283.2 ± 125.0 | |
| Gender | 0.301 | ||
| Female | 19 (38) | 259.9 ± 117.3 | |
| Male | 31 (62) | 296.6 ± 122.2 | |
| Symptoms | 0.002 | ||
| No | 17 (34) | 210.2 ± 90.3 | |
| Yes | 33 (66) | 319.9 ± 118.0 | |
| BMI | 0.059 | ||
| Overweight or obesity | 28 (56) | 311.1 ± 121.8 | |
| Normal weight | 22 (44) | 246.4 ± 111.1 | |
| Smoking | 0.011 | ||
| Yes | 18 (36) | 339.6 ± 125.8 | |
| No | 32 (64) | 250.6 ± 106.4 | |
| Hemoglobin, g/L | 0.119 | ||
| <118 | 24 (48) | 310.8 ± 138.6 | |
| ≥118 | 26 (52) | 256.6 ± 96.5 | |
| Tumor size, cm | 0.001 | ||
| <6.3 | 25 (50) | 228.3 ± 99.8 | |
| ≥6.3 | 25 (50) | 337.0 ± 116.4 | |
| Risk classification | 0.001 | ||
| High | 37 (74) | 314.1 ± 116.1 | |
| Very low or low | 13 (26) | 193.0 ± 84.2 | |
| Location | 0.709 | ||
| Duodenum | 45 (90) | 280.5 ± 117.8 | |
| Proximal jejunum | 5 (10) | 302.0 ± 156.7 | |
| Liver metastases | 0.224 | ||
| Yes | 12 (24) | 327.7 ± 149.8 | |
| No | 38 (76) | 268.4 ± 108.1 | |
| Neoadjuvant therapy | 0.297 | ||
| Yes | 18 (36) | 306.6 ± 122.4 | |
| No | 32 (64) | 269.2 ± 119.2 | |
| ASA | 0.758 | ||
| III | 5 (10) | 298.6 ± 159.4 | |
| I or II | 45 (90) | 280.9 ± 117.6 | |
| Bypass | 0.033 | ||
| With | 23 (46) | 321.7 ± 107.1 | |
| Without | 27 (54) | 249.3 ± 123.1 | |
| Multi-visceral resection | < 0.001 | ||
| Yes | 18 (36) | 381.0 ± 108.8 | |
| No | 32 (64) | 227.3 ± 87.6 |
Table 4.
Multivariate linear regression analysis of the operative time
| Characteristics | B | SE | β | t | p-value |
|---|---|---|---|---|---|
| Multi-visceral resection | 124.626 | 25.514 | 0.502 | 4.885 | < 0.001 |
| Tumor size | 77.127 | 24.096 | 0.323 | 3.201 | 0.002 |
| Symptoms | -57.864 | 26.079 | -0.230 | -2.219 | 0.031 |
Discussion
In this study, we investigated the significance of adding a gastrojejunostomy to distal segmental duodenectomy on the effects of DGE and other complications. Firstly, by adding a gastrojejunostomy, we found significant evidence signaling it can lower the incidence of DGE. This can potentially be explained by the fact that the gastrojejunostomy creates a direct passage between the stomach and the jejunum, facilitating more efficient gastric emptying by reducing the functional load on the pylorus and the duodenum, which are often the sites of postoperative motility issues. In addition, the gastrojejunostomy may mitigate the effects of postoperative inflammation and edema around the pyloric and duodenal regions, which are common contributors to DGE. By providing an alternative route for gastric contents, this procedure may help maintain gastrointestinal continuity and function, thereby reducing the risk of delayed gastric emptying. This improvement in gastric emptying may lead to earlier resumption of oral intake, improved nutritional status, and more timely initiation of adjuvant therapies such as imatinib. As delayed initiation of adjuvant therapy has been associated with poorer outcomes, the use of gastrojejunostomy could potentially improve overall treatment efficacy and patient prognosis.
Previous studies demonstrate that DGE has a high incidence in distal segmental duodenectomy procedures. However, there is currently no established method for the prevention of DGE. Kato et al. reported an incidence rate of as high as 42% incidence of grade C DGE in a cohort of 24 patients with distal duodenal malignancies who underwent distal segmental duodenectomy. Furthermore, compared to side-to-side duodenojejunostomy, other reconstruction techniques, such as end-to-end or end-to-side anastomosis, were also associated with a higher incidence of grade C DGE [11]. However, Liu et al. demonstrated the inverse relationship between the so-called E-style duodenojejunostomy (end-to-end or end-to-side) and the incidence of DGE, with a significantly lower rate observed in the E-style group (7.7% vs. 52.4%) compared to the side-to-side group [6]. Our previous standard protocol for distal segmental duodenectomy involved performing an end-to-side duodenojejunostomy without a gastrojejunostomy, which was associated with a high incidence of DGE. Evidence from prophylactic gastrojejunostomy in patients with unresectable periampullary carcinoma has demonstrated a reduction in long-term gastric outlet obstruction [22], prompting us to consider whether adding a prophylactic gastrojejunostomy to distal duodenectomy could reduce the high postoperative incidence of DGE. As a result, in this study, patients who underwent gastrojejunostomy experienced a lower incidence of grade B-C DGE, which may have contributed to a trend toward shorter postoperative hospital stays. Regarding the operative feature results, patients who underwent an additional gastrojejunostomy had a longer median operation time than those who did not. However, statistical analysis indicated that the bypass itself was not an independent risk factor for prolonged operative time. In fact, the most significant risk factors for increased operative time were tumor size, multi-visceral resection, and positive symptoms. In addition, other surgical parameters, such as intraoperative bleeding and postoperative major complication rates, were not notably increased by the addition of a gastrojejunostomy and a Braun’s jejunojejunostomy.
There might be a concern that this study didn’t accurately assess the incidence of DGE grade A and the proportion of patients without DGE. As an institutional protocol, all patients received postoperative nasogastric decompression and underwent a UGIS until POD 5–7, and only after that would the decision to remove or retain the NGT be made. Notably, as it is a rare entity, segmental duodenectomy for duodenal or proximal jejunal GIST is not well studied, especially for patients with multi-visceral resections. In our study, the overall rate of multi-visceral resection was as high as 30%, with 22% of the patients combined with pancreatic resection and 14% with liver resection. Hence, implementing a cautious and relatively conservative postoperative treatment strategy is rational since patient safety is our top priority.
Recently, some reports showed routine UGIS may be of limited value for the detection of anastomotic leakage after gastric bypass or sleeve gastrectomy as bariatric procedures [23, 24]. Given that duodenal resection is more complicated and has higher risks compared to bariatric surgery, as well as the potentially catastrophic occurrence of an anastomotic fistula of duodenojejunostomy, it is inappropriate to directly apply clinical experience from bariatric surgery to postoperative care for segmental duodenectomy. In future clinical practice, attempts could be made to advance the timing of UGIS to POD 4 or earlier to facilitate early removal of NGT in those patients who do not experience any grade of DGE; it may be helpful to clarify the true incidence of grade A DGE, reduce unnecessary gastrointestinal decompression, and improve recovery strategies, shorten hospital stays, and reduce medical costs.
This study has several limitations. First, it is a retrospective analysis conducted by a single surgical team. The practices and outcomes observed may reflect only our specific practice and expertise, limiting the generalizability of the study’s conclusions to other settings. However, given the rarity of duodenal GISTs within the already rare category of GISTs, it is particularly challenging for surgeons to conduct prospective randomized trials, meaning starting points must be initialized anyhow to improve general clinical understanding of the treatment of this disease. Second, the extended study period presents an additional challenge, as surgical methods and quality may not remain consistent throughout. We must consider the potential impact of the team’s learning curve; the surgical skills, and decision-making abilities of team members. These are likely to improve over the study period, and consequently, operation times and complication rates may gradually decrease, introducing variability that can affect the consistency and reliability of the study results. Third, the long-term outcomes, complications, and potential disadvantages of adding a gastrojejunostomy in patients undergoing distal segmental duodenectomy remains currently unclear due to low numbers of previous attempts. However, insights from bariatric surgery suggest that bypass procedures may lead to postoperative complications such as malnutrition, gallstones, gastric ulcers, and dumping syndrome [25]. Future research should focus on implementing multi-center collaboration and involving a larger number of patients with extended follow-up periods to achieve more reliable and generalizable conclusions, with a particular focus on long-term gastrointestinal function and nutritional outcomes.
Conclusions
Adding a gastrojejunostomy and Braun’s jejunojejunostomy after distal segmental duodenectomy for duodenal and proximal jejunal GIST significantly reduces the incidence of grade B-C DGE. This modification may help to facilitate the timely initiation of adjuvant imatinib therapy without increasing intraoperative bleeding or major postoperative complication rates. Future studies should investigate these findings in a prospective, multicenter setting to confirm the effectiveness and safety of this procedure on patient survivability and recovery.
Acknowledgements
The authors would like express their gratitude to Mr. Tony Leihui Tong from Boston University for his invaluable assistance in proofreading and correcting the English in this paper.
Abbreviations
- ASA
American Society of Anesthesiologists
- BMI
Body mass index
- CI
Confidence intervals
- DGE
Delayed gastric emptying
- GIST
Gastrointestinal stromal tumors
- HGB
Hemoglobin
- ICU
Intensive care unit
- ISGPS
International Study Group of Pancreatic Surgery
- LR
Local resection
- MVR
Multi-visceral resection
- NGT
Routine nasogastric tubes
- OR
Odds ratio
- POD
Postoperative days
- POPF
Postoperative pancreatic fistula
- UGIS
Upper gastrointestinal series
Author contributions
Study conception and design: WWJ, JHW and CPL; Acquisition of data: WWJ, DNL, XPW and RZS; Analysis and interpretation of data: WWJ and CY; Drafting of the manuscript: WWJ, JHW, and CPL; Critical revision of the manuscript: CYH and CPL.
Funding
This study was funded by the Science Foundation of Peking University Cancer Hospital (Grant No.XKFZ2421 and JC202407), National Natural Science Funding (Grant No. 91959120, Capital’s Funds for Health Improvement and Research (Grant No. 2024-4-2159), and Beijing Xisike Clinical Oncology Research Foundation (Grant No. Y-Young2020-0539, 2021-0111, and 2022-0117).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study received approval from the institutional ethics committee and adhered to the principles outlined in the 1964 Helsinki Declaration, along with its subsequent amendments or comparable ethical standards. Written informed consent was obtained from each participating patient, ensuring compliance with ethical guidelines. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We have obtained consent from all authors and they have agreed to publish the results of this study.
Consent for publication
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei-Wei Jia and Jian-Hui Wu contributed equally to this work.
Contributor Information
Cheng-Peng Li, Email: chengpengli@bjmu.edu.cn.
Chun-Yi Hao, Email: haochunyi@bjmu.edu.cn.
References
- 1.Marano L, Boccardi V, Marrelli D, Roviello F. Duodenal gastrointestinal stromal tumor: from clinicopathological features to surgical outcomes. Eur J Surg Oncol. 2015;41:814–22. [DOI] [PubMed] [Google Scholar]
- 2.Swallow CJ. The enduring decision-making role of the surgeon in the multidisciplinary management of GIST. Ann Oncol. 2022;33:17–9. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Chen G, Lin L, Jin X, Kang M, Zhang Y, et al. Resection of GIST in the duodenum and proximal jejunum: a retrospective analysis of outcomes. Eur J Surg Oncol. 2019;45:1950–6. [DOI] [PubMed] [Google Scholar]
- 4.Uppal A, Wang M, Fischer T, Goldfarb M. Duodenal GI stromal tumors: is radical resection necessary? J Surg Oncol. 2019;120:940–5. [DOI] [PubMed] [Google Scholar]
- 5.Fu X, Wang X, Xiong J, Yao Y, Tan C, Liu X. Surgical strategies for duodenal gastrointestinal stromal tumors. Langenbecks Arch Surg. 2022;407:835–44. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Wang J, Ma L, Zhuang A, Xu J, He J, et al. Which style of duodenojejunostomy is better after resection of distal duodenum. BMC Surg. 2022;22:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv A, Qian H, Qiu H, Wu J, Li Y, Li Z, et al. Organ-preserving surgery for locally advanced duodenal gastrointestinal stromal tumor after neoadjuvant treatment. Biosci Trends. 2017;11:483–9. [DOI] [PubMed] [Google Scholar]
- 8.Fujii T, Kanda M, Kodera Y, Nagai S, Sahin TT, Kanzaki A, et al. Comparison of pancreatic Head Resection with Segmental Duodenectomy and Pylorus-preserving pancreatoduodenectomy for Benign and Low-Grade Malignant neoplasms of the pancreatic head. Pancreas. 2011;40:1258–63. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T. Interdigestive migrating motor complex -its mechanism and clinical importance. J Smooth Muscle Res. 2013;49:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood-Van Meerveld B, Johnson AC, Grundy D. Gastrointestinal Physiology and Function. In: Greenwood-Van Meerveld B, editor. Gastrointest Pharmacol [Internet]. Cham: Springer International Publishing; 2017 [cited 2024 Feb 25]. pp. 1–16. http://link.springer.com/10.1007/164_2016_118 [DOI] [PubMed]
- 11.Kato T, Ono Y, Oba A, Sato T, Ito H, Inoue Y, et al. Treatment strategy of pancreas-sparing distal duodenectomy for distal duodenal malignancies with adjustable dissection levels according to Disease Progression (with video). World J Surg. 2023;47:1752–61. [DOI] [PubMed] [Google Scholar]
- 12.Yang F, Jin C, Du Z, Subedi S, Jiang Y, Li J, et al. Duodenal gastrointestinal stromal tumor: clinicopathological characteristics, surgical outcomes, long term survival and predictors for adverse outcomes. Am J Surg. 2013;206:360–7. [DOI] [PubMed] [Google Scholar]
- 13.Duan P, Sun L, Kou K, Li X-R, Zhang P. Surgical techniques to prevent delayed gastric emptying after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int. 2023;S1499387223002047. [DOI] [PubMed]
- 14.Qianyi W, Mei X, Rui Z, Yong W, Yutao W, Xiaoding S, et al. Delayed adjuvant imatinib in patients with high risk of recurrence of gastrointestinal stromal tumor after radical surgery: a retrospective cohort study. J Cancer Res Clin Oncol. 2022;148:1493–500. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez OH, Grigorian A, Wolf RF, Imagawa DK, Nahmias JT, Jutric Z. Delayed gastric emptying is associated with increased risk of mortality in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma. Updat Surg. 2023;75:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of pancreatic surgery (ISGPS). Surgery. 2007;142:761–8. [DOI] [PubMed] [Google Scholar]
- 17.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91. [DOI] [PubMed] [Google Scholar]
- 18.Tan WJ, Kow AWC, Liau KH. Moving towards the New International Study Group for pancreatic surgery (ISGPS) definitions in pancreaticoduodenectomy: a comparison between the old and new. HPB. 2011;13:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien P-A. Classification of Surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Wang Y, Zhang X, Zhang Z, Zhao N, Ou W, et al. Obesity and Cardiac Conduction Block Disease in China. JAMA Netw Open. 2023;6:e2342831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9. [DOI] [PubMed] [Google Scholar]
- 22.Gurusamy KS, Kumar S, Davidson BR. Prophylactic gastrojejunostomy for unresectable periampullary carcinoma. Cochrane Upper GI and Pancreatic Diseases Group, editor. Cochrane Database Syst Rev [Internet]. 2013 [cited 2024 Oct 6]; https://doi.wiley.com/10.1002/14651858.CD008533.pub3 [DOI] [PMC free article] [PubMed]
- 23.Musella M, Cantoni V, Green R, Acampa W, Velotti N, Maietta P, et al. Efficacy of postoperative Upper Gastrointestinal Series (UGI) and computed tomography (CT) scan in bariatric surgery: a Meta-analysis on 7516 patients. Obes Surg. 2018;28:2396–405. [DOI] [PubMed] [Google Scholar]
- 24.Dayma K, David A, Omer A, Abdel-Dayam H, Tawil A, Socci N, et al. Routine Upper Gastrointestinal Series post-bariatric surgery: predictors, usage, and Utility. Obes Surg. 2024;34:1552–60. [DOI] [PubMed] [Google Scholar]
- 25.Kassir R, Debs T, Blanc P, Gugenheim J, Ben Amor I, Boutet C, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg. 2016;27:77–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.