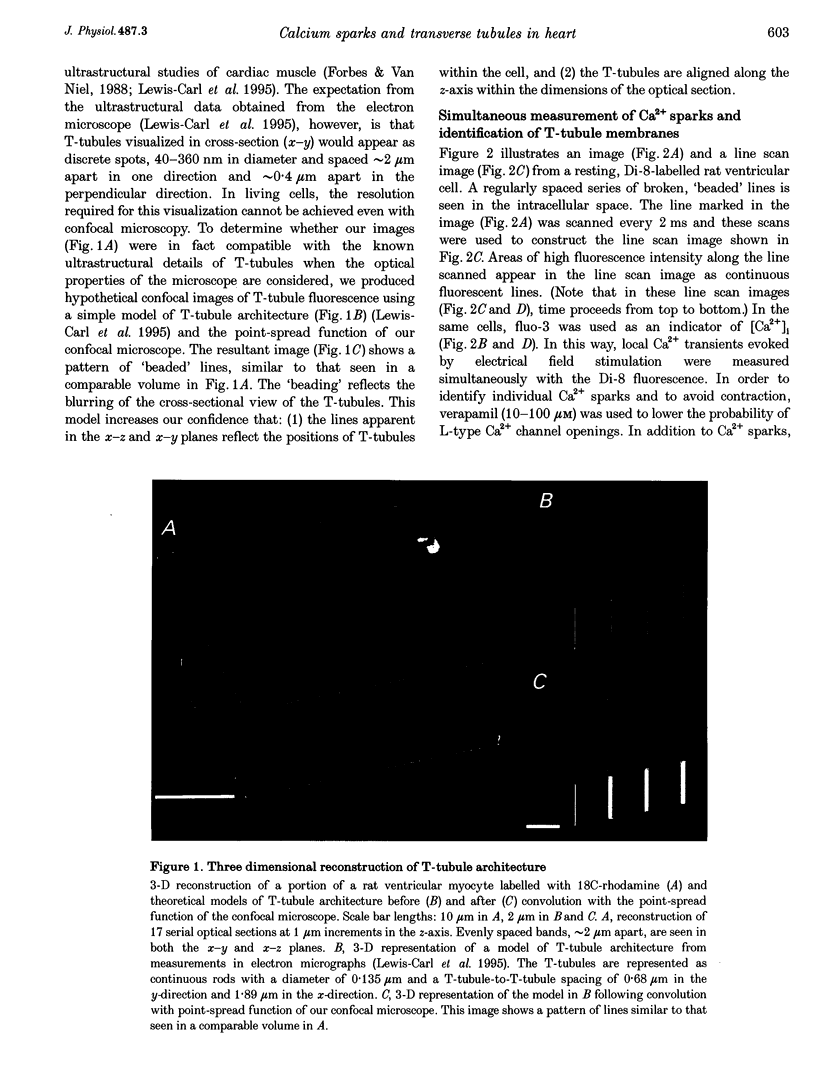
Abstract
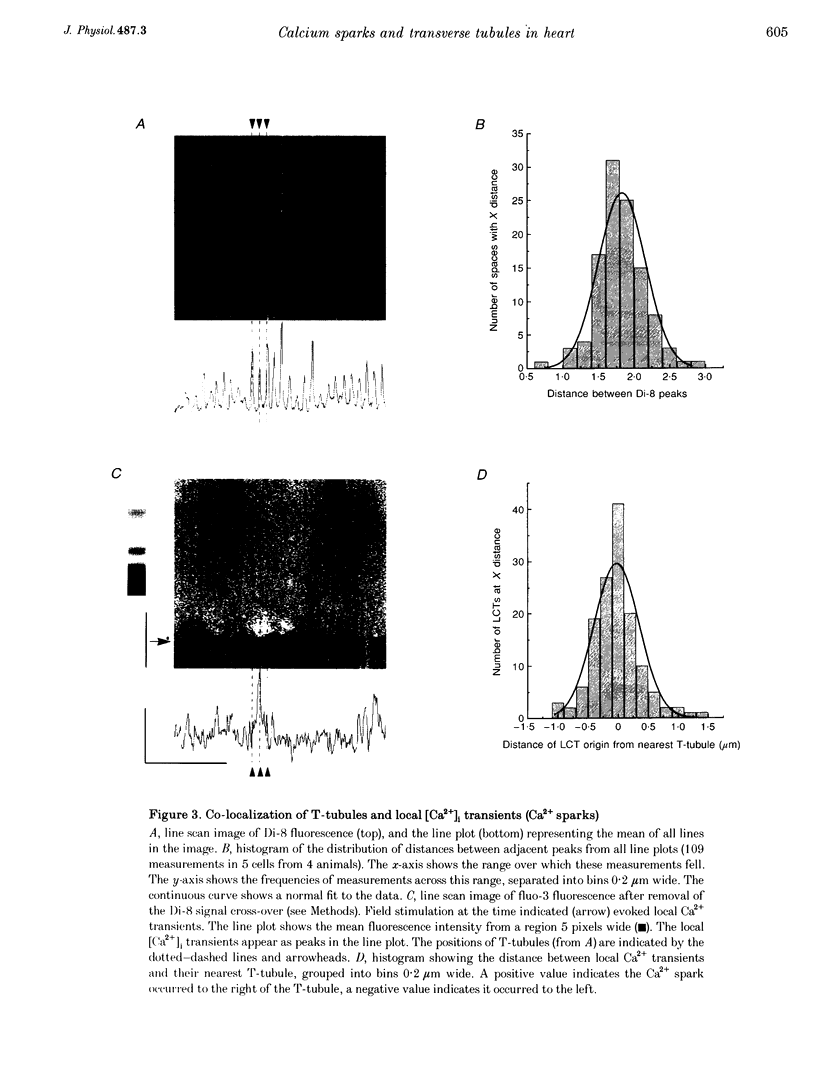
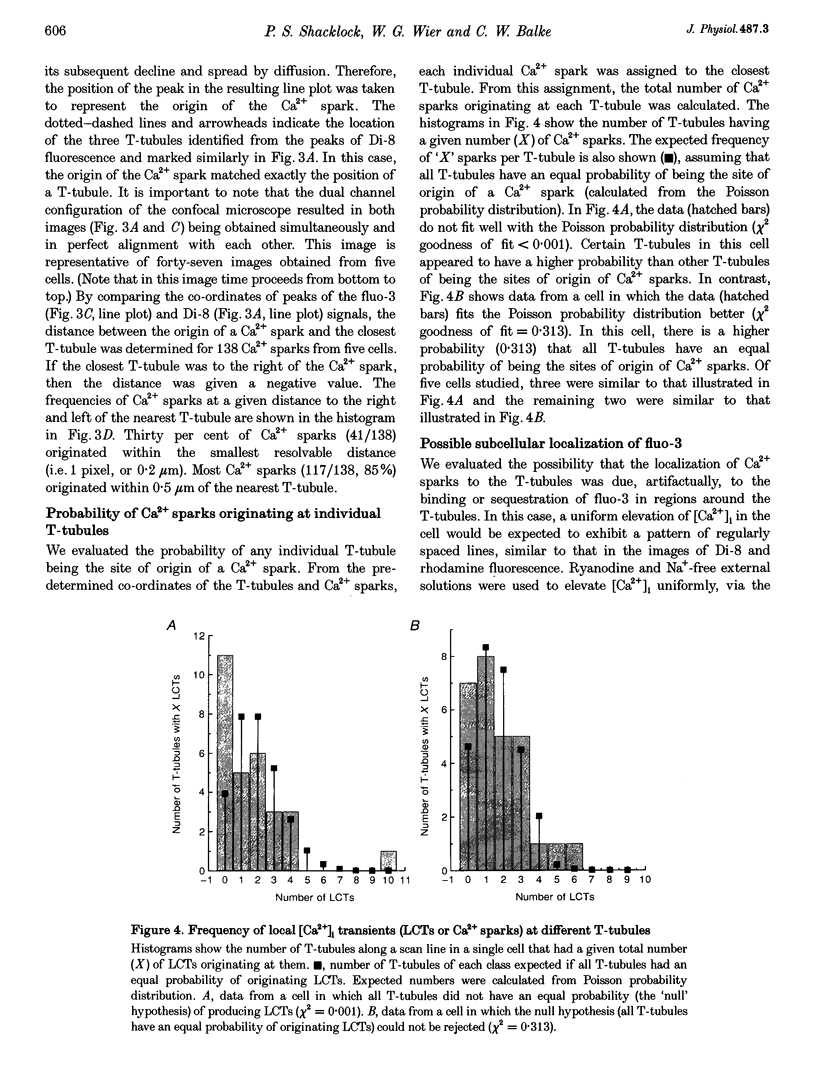
1. The origins of local [Ca2+]i transients (Ca2+ sparks) were studied using dual-channel confocal laser scanning microscopy. Line scan images showing [Ca2+]i (as fluo-3 fluorescence) and the transverse tubule membranes (as Di-8 fluorescence) were obtained simultaneously in single rat cardiac ventricular cells. 2. Line scan images of Di-8 fluorescence showed peaks regularly spaced at intervals of 1.83 +/- 0.30 microns (mean +/- S.D.). These peaks corresponded to the transverse tubules (T-tubules) in cross-section. 3. Line scan images of fluo-3 fluorescence showed local [Ca2+]i transients (LCTs or Ca2+ sparks) evoked by electrical stimulation. 4. Eighty-five per cent (85%) of all Ca2+ sparks evoked by electrical stimulation (n = 138, in 5 cells) occurred within 0.5 micron of a T-tubule. Thirty per cent (30%) occurred within 1 pixel (0.20 micron) of a T-tubule. 5. In some cells studied (3 out of 5), certain T-tubules had a higher probability of being sites of origin of Ca2+ sparks than others. 6. These results support local control theories of excitation-contraction coupling in which Ca2+ release from the sarcoplasmic reticulum (SR) is triggered by a high local [Ca2+]i established between the L-type Ca2+ channels in the T-tubules and associated ryanodine receptor(s) in the junctional SR.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr, Vaghy P. L., Meissner G., Ferguson D. G. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995 May;129(3):673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Escobar A. L., Monck J. R., Fernandez J. M., Vergara J. L. Localization of the site of Ca2+ release at the level of a single sarcomere in skeletal muscle fibres. Nature. 1994 Feb 24;367(6465):739–741. doi: 10.1038/367739a0. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Bredt D. S., Cameron A. M., Snyder S. H. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes M. S., Hawkey L. A., Sperelakis N. The transverse-axial tubular system (TATS) of mouse myocardium: its morphology in the developing and adult animal. Am J Anat. 1984 Jun;170(2):143–162. doi: 10.1002/aja.1001700203. [DOI] [PubMed] [Google Scholar]
- Forbes M. S., van Neil E. E. Membrane systems of guinea pig myocardium: ultrastructure and morphometric studies. Anat Rec. 1988 Dec;222(4):362–379. doi: 10.1002/ar.1092220409. [DOI] [PubMed] [Google Scholar]
- Jorgensen A. O., Shen A. C., Arnold W., McPherson P. S., Campbell K. P. The Ca2+-release channel/ryanodine receptor is localized in junctional and corbular sarcoplasmic reticulum in cardiac muscle. J Cell Biol. 1993 Feb;120(4):969–980. doi: 10.1083/jcb.120.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieval R. S., Bloch R. J., Lindenmayer G. E., Ambesi A., Lederer W. J. Immunofluorescence localization of the Na-Ca exchanger in heart cells. Am J Physiol. 1992 Aug;263(2 Pt 1):C545–C550. doi: 10.1152/ajpcell.1992.263.2.C545. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Modulation of Ca2+ release in cultured neonatal rat cardiac myocytes. Insight from subcellular release patterns revealed by confocal microscopy. Circ Res. 1994 May;74(5):979–990. doi: 10.1161/01.res.74.5.979. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994 Oct 1;480(Pt 1):21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Asai J., Hama K. The transverse tubular system of rat myocardium: its morphology and morphometry in the developing and adult animal. Anat Embryol (Berl) 1986;173(3):307–315. doi: 10.1007/BF00318914. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lipp P. Subcellular features of calcium signalling in heart muscle: what do we learn? Cardiovasc Res. 1995 Apr;29(4):441–448. [PubMed] [Google Scholar]
- Severs N. J., Slade A. M., Powell T., Twist V. W., Jones G. E. Morphometric analysis of the isolated calcium-tolerant cardiac myocyte. Organelle volumes, sarcomere length, plasma membrane surface folds, and intramembrane particle density and distribution. Cell Tissue Res. 1985;240(1):159–168. doi: 10.1007/BF00217570. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]