Abstract
Background
No specific triglyceride-lowering therapy is recommended in patients with hypertriglyceridemia-associated acute pancreatitis (HTG-AP), primarily because of the lack of quality evidence. This study aimed to describe practice variations in triglyceride-lowering therapies for early HTG-AP patients and assess whether more rapid triglyceride decline is associated with improving organ failure.
Methods
This is a multicentre, prospective cohort study recruiting HTG-AP patients with elevated plasma triglyceride (> 11.3 mmol/L) admitted within 72 h from the onset of symptoms. Patients were dichotomised on study day 3 into either target reaching (plasma triglyceride ≤ 5.65 mmol/L) or not. The primary outcome was organ failure-free days (OFFD) to 14 days of enrolment. The association between target-reaching and OFFD was modelled. Additionally, the slope in plasma triglyceride over the first three days in response to treatment was calculated, and its association with OFFD was assessed as a sensitivity analysis.
Results
Among the 300 enrolled patients, 211 underwent exclusive medical treatment, and 89 underwent various blood purification therapies. Triglyceride levels were available in 230 patients on study day 3, among whom 122 (53.0%) had triglyceride levels of ≤ 5.65 mmol/l. The OFFD was not different between these patients and those in whom plasma triglyceride remained > 5.65 mmol/L [median (IQR): 13 (10–14) vs. 14 (10–14), p = 0.46], even after adjustment for potential confounders. For the decline slopes, there was no significant change in OFFD with a steeper decline slope [risk difference, − 0.088, 95% CI, − 0.334 to 0.158, p = 0.48].
Conclusions
Triglyceride-lowering therapies vary greatly across centres. More rapid triglyceride decline was not associated with improving incidence and duration of organ failure.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03755-8.
Keywords: Acute pancreatitis, Hypertriglyceridemia, Triglyceride, Organ failure, Blood purification
Background
Acute pancreatitis (AP) is an inflammatory process of the pancreas caused by various aetiologies, among which gallstones and alcohol abuse are the leading causes globally [1]. Hypertriglyceridemia was reported as another common cause, accounting for 5.9% of cases in North America, according to a recent international cohort study [2]. Of note, hypertriglyceridemia is often under-recognised as an aetiology in patients with acute pancreatitis when there are other etiological risk factors [3]. In China, recent studies showed that hypertriglyceridemia-associated acute pancreatitis (HTG-AP) accounted for 14.3% to 23.9% of AP cases [4, 5]. Rapidly changing lifestyles and genetic variants might explain this phenomenon [6, 7].
Previous studies showed that compared with other causes, patients with HTG-AP are more likely to have severe disease courses and to have an incidence of organ failure as high as more than 40% [8, 9]. However, in a recent study using data from an international registry, the results showed that HTG-AP patients had similar outcomes compared to other aetiologies, with 42% of the HTG-AP patients having moderately severe/severe AP [10]. The severity of organ failure seems to depend partly on the intensity of the inflammatory response and partly on the injury caused by toxic fatty acids [11, 12], a lipase-mediated decomposition product of triglycerides. In this regard, efforts have been made to reduce the triglyceride level to prevent or improve organ failure [13].
The optimal therapeutic target for plasma triglyceride level is unclear, although previous studies have suggested lowering plasma triglyceride < 5.65 mmol/L to improve disease prognosis [14–17]. Currently, international guidelines do not recommend specific triglyceride-lowering therapy or a specific triglyceride target due to the lack of high-quality evidence. Therefore, we conducted this multicentre, prospective cohort study to describe the current practice variations in triglyceride-lowering therapy for HTG-AP patients and to assess whether more rapid triglyceride decline was associated with improving incidence and duration of organ failure.
Methods
Study design and oversight
This is a multicentre, prospective cohort study (the PERFORM study) conducted by the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG). The study was approved by the local ethics committees of the participating hospitals and registered with the Chinese Clinical Trial Registry (ChiCTR2000039541) before enrolment commenced, and the study protocol was published in 2021 [18]. This study was funded by the Key Research and Development Programme Foundation of Jiangsu Province of China (No. BE 2016749) and the National Science Foundation of China (No. 81900592). The funders were not involved in the study’s design, data collection, interpretation, manuscript preparation, and choice of submission.
Study population
Patients diagnosed with AP aged 18–70 years admitted to the participating sites within 72 h of the onset of abdominal pain were screened. The diagnosis of AP was based on the Revised Atlanta Classification criteria [19]. The inclusion criteria were as follows: (1) plasma triglyceride > 11.3 mmol/L at admission and (2) the presence of any one or more of the following clinical worrisome features described by Gelrud et al. on the UpToDate [20]: (1) signs of hypocalcaemia, (2) lactic acidosis, (3) presence of systemic inflammatory response syndrome (SIRS), and (4) signs of worsening organ dysfunction or multiorgan failure. Patients were excluded if they were pregnant or were expected to die within 48 h after enrolment, which was defined as patients with norepinephrine usage at a dose of 25 mg/min or more despite sufficient fluid resuscitation, with a systolic blood pressure < 90 mmHg and serum pH values < 7.0. The full inclusion and exclusion criteria are provided in the Supplementary Protocol. At each hospital, written informed consent was obtained from the patients or their next of kin before enrolment. The study patients were enrolled from November 7, 2020, to January 30, 2022.
Patient management and triglyceride-lowering therapies
All patients received standard treatment for AP, which included intravenous fluid hydration, pain control, nutrition therapy and mechanical ventilation, and continuous renal replacement therapy if required. Intervention for pancreatic/peripancreatic collections was indicated when infection was suspected or confirmed, preferably after 4 weeks from the onset of the symptoms, as recommended in international guidelines [19, 21].
In addition, triglyceride-lowering therapy was administered at the discretion of the treating physicians, including blood purification treatment (e.g. plasma exchange, haemoperfusion, and haemofiltration) and medical treatment (e.g. insulin and heparin).
Clinical outcomes
The primary outcome was organ failure-free days (OFFD) to study day 14. The diagnosis of organ failure was made when an individual sequential organ failure assessment (SOFA) score [22] of two or more for the respiration, cardiovascular, or renal system. In patients with transient organ failure resolution, only the final periods of OFFD were counted. Patients who had organ failure on day 14 or died before day 14 were assigned zero OFFD.
Secondary outcomes included a composite endpoint of death from any cause by day 28 and the presence of at least one organ failure at day 7, SOFArank, ΔSOFAmax, and ΔSOFA14 to day 14, ICU-free days to day 14, hospital-free days to day 14, day 28, and day 60, new-onset organ failure (defined as organ failure that is no present at any time in the first 24 h of enrolment) to day 14, requirement of blood purification and ICU admission during the index admission, and mortality and incidence of infected pancreatic necrosis (IPN) by day 60. SOFArank was calculated as a sum of the daily delta SOFA score (defined as the daily total SOFA score minus the baseline SOFA score) over the first 14 days of enrolment [23]. Discharge was counted (from the day of discharge forward) as a score of 0 and death as 24. ΔSOFAmax was defined as the maximum SOFA score within 14 days minus the baseline, and ΔSOFA14 as the SOFA score at day 14 minus the baseline [24].
Data collection
A web-based electronic database (access through https://capctg.medbit.cn/) was used for data collection and storage. Before the first enrolment at each site, a start-up meeting was organised for data entry training. The coordinating centre of the CAPCTG was responsible for overall data management, monitoring, and communication among the study sites.
Data collected in the PERFORM study included demographic characteristics, baseline characteristics at enrolment, daily laboratory tests, technical aspects of daily triglyceride-lowering treatment like mode, dose, and frequency, daily SOFA score, and follow-up measures. The detailed data collected were listed in the Supplementary Protocol.
Statistical analysis
Based on the patient volume of the participating sites, a sample size of 300 patients was expected for 2 years. Considering an estimated 20% rate of incomplete data or losses of follow-up, our expected sample size (240 patients with complete data) would provide 87% power to detect a 2-day (SD [standard deviation]: 5) or 82.5% for 1.5-day (SD: 4) improvement of OFFDs between patients achieving target TG and those not if equally distributed [18].
Continuous data were reported as means and SDs or medians and interquartile ranges (IQR) as appropriate, depending on their normality. The normality of data was determined by Shapiro–Wilk tests. Categorical data were summarised by counts and percentages. The intergroup differences were compared by Student’s t-test for normally distributed data or Wilcoxon rank-sum test for skewed data. Fisher’s exact test was used for categorical data.
Two statistical approaches were applied to assess the association between triglyceride-lowering effects (triglyceride decline) and clinical outcomes. First, all the study individuals with triglyceride levels on study day 3 were dichotomised depending on whether the triglyceride level was ≤ 5.65 (target-reaching). Comparisons were made between those who reached the target and those who did not. For the primary outcome comparison, Wilcoxon rank-sum test was employed, and the median difference (95% CI [confidence intervals]) was calculated by the Hodges–Lehmann estimation. Additionally, the adjusted generalised linear model (GLM) was performed to account for potential confounders. We followed three rules to choose the confounding variables: (1) potential baseline differences with a p-value less than 0.1; (2) potentially relevant variables based on previous studies and clinical considerations; (3) missing data no more than 10%. Collinearity was additionally tested to ensure the independence of each variable. As a result, age [25, 26], sex [17, 26], body mass index (BMI) [26], baseline triglyceride level [26], and baseline Acute Physiology and Chronic Health Evaluation II (APACHE II) score [27] were involved. Furthermore, the Fine and Gray competing risk model was additionally adopted to account for dead cases.
A second statistical approach was performed as a sensitivity analysis. Briefly, the decline rate of triglyceride for the first three days of enrolment was obtained from the linear regression, and the regression coefficient was the slope of the triglyceride decline. The adjusted GLMs were used to assess the association between decline slope and OFFD, and confounders were the same as the first approach. We tested the proportional hazard assumption using Schoenfeld residuals, suggesting proportional hazards for the primary outcome based on the direct effect model.
For the comparison of secondary outcomes, the adjusted GLM was performed to identify its association with target-reaching. The variables included in the model were the same as the adjusted analysis for the primary outcome. The association between blood purification and clinical outcomes was additionally assessed. Propensity score matching (PSM) analysis was used to control potential confounders. Patients who received blood purification were matched 1:1 with patients who received exclusive medical treatment based on the same confounding variables for the adjusted GLM. Genetic matching with a calliper width of 0.2 was used in the PSM. Comparisons of differences between groups in the PSM cohort were performed using Wilcoxon signed-rank test and McNemar’s test for matched data. To evaluate the robustness of our findings, we also performed the inverse probability of treatment weighting (IPTW) analysis [28]. Comparisons of differences between groups in the IPTW cohort were performed using Wilcoxon rank-sum test and Fisher’s exact test.
We performed data conversions (including log, reciprocal, and square root transformations) for the continuous outcomes with skewed distribution in the GLM models. Statistical tests were two-sided, and p values < 0.05 were deemed significant unless otherwise stated. All statistical analyses were done in the SPSS 26.0 software and the R 4.2.1 software.
Results
Patient recruitment and variations in triglyceride-lowering therapy
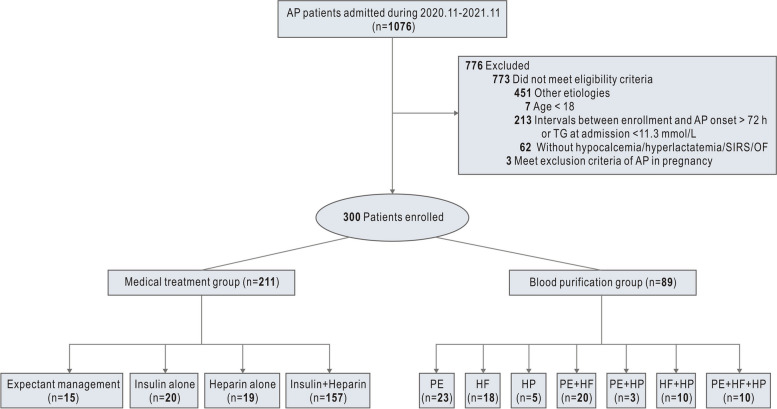
The PERFORM study was commenced on November 7, 2020, and it reached the phase I goal with the 300th patient being recruited on November 30, 2021 (end of 60-day follow-up, January 30, 2022). During the study period, 1076 consecutive AP patients from 28 sites across China were assessed for inclusion, of whom 300 were formally enrolled (Fig. 1). The numbers of cases from each site are shown in Supplemental Table S1. The diagnoses of AP were confirmed by CT scan among all the enrolled patients. The timing of CT scan and the diagnostic procedure were based on the local practice. The demographics and baseline characteristics of the study patients were summarised in Table 1. The study population was mostly male (69.7%), with a mean age of 38.1 (SD 8.9). The distribution of age and the respective AP severity of the study patients are shown in Fig. S1. Only 36.3% of the enrolled patients were known to have confirmed HTG before the index presentation with AP.
Fig. 1.
Flow chart of patient enrolment. AP, acute pancreatitis; PE, plasma exchange; HF, haemofiltration; HP, haemoperfusion
Table 1.
Demographic and baseline characteristics
| Variables | The overall study cohort (n = 300) |
|---|---|
| Age, mean (SD) | 38.1 (8.9) |
| Sex, n (%) | |
| Female | 91 (30.3) |
| Male | 209 (69.7) |
| BMI, median (IQR) | 27.4 (24.8–29.7) |
| Pre-existing hyperlipidaemia, n (%) | |
| Yes | 109 (36.3) |
| No or unknown | 191 (63.7) |
| Familial hyperlipidaemia, n (%) | |
| Yes | 5 (1.7) |
| No or unknown | 295 (98.3) |
| Pre-existing biliary tract disease | |
| Yes | 17 (5.7) |
| No or unknown | 283 (94.3) |
| History of smoking | |
| Yes | 120 (40.0) |
| No or unknown | 180 (60.0) |
| History of drinking | |
| Yes | 114 (38.0) |
| No or unknown | 186 (62.0) |
| Amylase, median (IQR), U/L | 308.0 (124.0–529.5) |
| Lipase, median (IQR), U/L | 531.3 (185.2–1478.3) |
| Triglyceride, median (IQR), mmol/l | 21.3 (16.2–32.9) |
| ALT, median (IQR), U/L | 29.0 (18.0–49.4) |
| AST, median (IQR), U/L | 29.0 (19.0–46.0) |
| High density lipoprotein, median (IQR), mmol/L | 0.78 (0.48–1.67) |
| Low density lipoprotein, median (IQR), mmol/L | 2.57 (1.56–3.55) |
| C-reacting protein, median (IQR), mg/L | 56.8 (10.4–163.6) |
| Procalcitonin, median (IQR), μg/L | 0.47 (0.12–3.13) |
| Creatinine, median (IQR), μmol/l | 69.0 (52.0–93.5) |
| Lactate dehydrogenase, median (IQR), U/L | 339.0 (215.8–592.5) |
| Severity score, median (IQR) | |
| APACHE.II | 5.0 (3.0–9.0) |
| SOFA | 1 (0–2) |
TG triglyceride, SD standard deviation, IQR interquartile range, BMI body mass index, ALT alanine aminotransferase, AST aspartate aminotransferase, APACHE II Acute Physiology and Chronic Health Evaluation II, SOFA sequential organ failure assessment
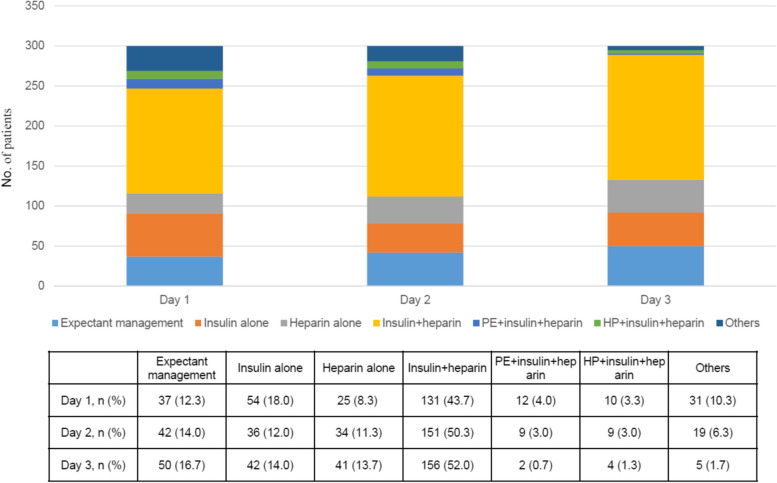
During the first 3 days of enrolment, most study patients (253/300, 84.3%) received no oral intake (nil per os, NPO). For triglyceride-lowering therapies, 211 (70.3%) of 300 patients received exclusive medical treatment, including expectant management (10 patients, 3.3%), insulin alone (20 patients, 6.6%), heparin/low molecular weight heparin (LMWH) alone (19 patients, 6.4%), or a combination of insulin and heparin/LMWH (157 patients, 52.3%). The remaining 89 patients (29.7%) had various modes of blood purification therapies, including plasma exchange (18.7%), haemoperfusion (9.3%), and haemofiltration (19.3%), either alone or in combination. The distribution of different treatment strategies over the first 3 days was shown in Fig. 2. In patients undergoing blood purification, 81 patients (91.0%) were also treated with heparin and/or insulin (Table S2).
Fig. 2.
Distribution of treatment strategies over the first three days of enrolment. PE, plasma exchange; HP, haemoperfusion
Association between target-reaching and clinical outcomes
Triglyceride levels on day 3 were available in 230 of the 300 patients, among whom 122 (53.0%) reached the target (≤ 5.65 mmol/l) on day 3. Between patients reaching the target or not, there were no significant differences in age, hyperlipidaemia history, lipoprotein, C-reactive protein, procalcitonin, and disease severity scores at enrolment (Table S3). Patients in the target-reaching group were more frequently male (75.4% vs. 63.0%, p = 0.045) and had lower BMI [26.9 (24.5–29.6) vs. 27.8 (26.2–29.8), p = 0.036] compared with patients that did not reach the target.
The crude and adjusted analysis of the primary outcome and adjusted analyses of the secondary outcomes are shown in Table 2. The OFFD to study day 14 were similar between patients reaching the target or not [median (IQR): 13 (10–14) vs. 14 (10–14), p = 0.46]. The results remain unchanged after adjustment for potential confounders. For the secondary outcomes, patients in the target-reaching group had a higher need for ICU admission (50.8%[62/122] vs. 38.9% [42/108]) with an OR of 1.99 (95% CI, 1.06 to 3.74) in the adjusted GLM (p = 0.032). All the other secondary outcomes were comparable between groups. Subgroup analyses of the effect of Day3-TG ≤ 5.65 mmol/L on organ failure-free days to day 14 of enrollment were shown in Fig. S2.
Table 2.
Crude analysis of primary outcome and adjusted analyses of the primary and secondary outcomes in patients with Day3-triglyceride above 5.65 mmol/L or not
| Variables | Day3-TG ≤ 5.65 (n = 122) | Day3-TG > 5.65 (n = 108) | Difference or OR (95% CI) | p |
|---|---|---|---|---|
| Primary outcome | ||||
| OFFD, median (IQR), days* | 13.0 (10.0, 14.0) | 14.0 (10.0, 14.0) | 0 (0, 0) | 0.46 |
| OFFD, median (IQR), days# | 13.0 (10.0, 14.0) | 14.0 (10.0, 14.0) | 0.21 (− 0.66, 1.09) | 0.63 |
| Secondary outcomes# | ||||
| Composite outcome, n (%) | 16 (13.1) | 13 (12.0) | 1.13 (0.45, 2.85) | 0.80 |
| ICU-free day to day 14, median (IQR), days | 12.0 (7.0, 14.0) | 14.0 (9.0, 14.0) | − 0.57 (− 1.62, 0.48) | 0.29 |
| Hospital-free day to day 14, median (IQR), days | 5.0 (0, 7.1) | 5.0 (0.1, 7.0) | 0.32 (− 0.55, 1.20) | 0.47 |
| Hospital-free day to day 28, median (IQR), days | 19.0 (14.0, 21.0) | 19.0 (14.1, 21.0) | 0.50 (− 1.03, 2.02) | 0.52 |
| Hospital-free day to day 60, median (IQR), days | 51.0 (46.0, 53.0) | 51.0 (46.1, 53.0) | 1.72 (− 0.98, 4.41) | 0.21 |
| SOFA rank to day 14, median (IQR) | − 10.0 (− 26.0, 1.0) | − 5.0 (− 21.8, 2.50) | − 4.13 (− 9.82, 1.55) | 0.16 |
| ΔSOFA max to day 14, median (IQR) | 1.0 (0, 3.0) | 1.0 (0, 3.0) | − 0.24 (− 1.08, 0.60) | 0.58 |
| ΔSOFA14 to day 14, median (IQR) | − 1.0 (− 3.0, 0) | − 1.0 (− 2.0, 0) | − 0.39 (− 0.84, 0.06) | 0.088 |
| 60-day mortality, n (%) | 4 (3.3) | 5 (4.6) | 0.37 (0.05, 2.58) | 0.32 |
| 60-day IPN, n (%) | 2 (1.6) | 5 (4.6) | 0.33 (0.06, 2.01) | 0.23 |
| Blood purification requirement during index admission, n (%) | 47 (38.5) | 31 (28.7) | 1.80 (0.92, 3.51) | 0.087 |
| ICU requirement during index admission, n (%) | 62 (50.8) | 42 (38.9) | 1.99 (1.06, 3.74) | 0.032 |
| New-onset organ failure to day 14, n (%) | ||||
| Respiratory | 34 (27.9) | 28 (25.9) | 0.95 (0.51, 1.76) | 0.86 |
| Renal | 11 (9.0) | 13 (12.0) | 0.63 (0.26, 1.53) | 0.31 |
| Cardiovascular | 5 (4.1) | 9 (8.3) | 0.40 (0.13, 1.29) | 0.13 |
TG triglyceride, OFFD organ failure-free days to day 14, Composite outcome a composite of death from any cause by day 28 and the presence of at least one organ failure at day 7, ICU intensive care unit; SOFA, sequential organ failure assessment, IPN infected pancreatic necrosis
*Wilcoxon rank-sum test
#Adjusted for sex, age, BMI, APACHE II, and baseline TG level
Death from any cause by day 28 and the presence of at least one organ failure at day 7
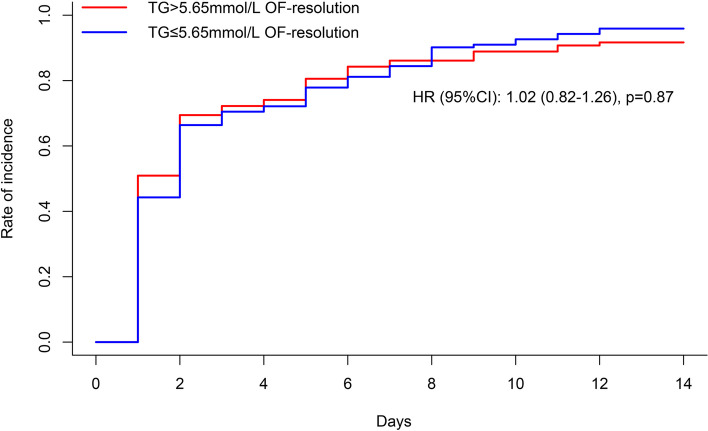
In the Fine and Grey analysis, the probability of organ failure resolution during the first 14 days was similar between those who did and did not reach the target [HR 0.98, 95% CI, 0.79 to 1.22, p = 0.86] (Fig. 3).
Fig. 3.
Organ failure resolution analysed using Fine-Gray competing risk analysis. OF, organ failure; TG, triglyceride; HR, hazard ratio
Association between triglyceride decline rate and clinical outcomes
The same cohort of 230 patients was involved in this analysis. The median (IQR) triglyceride decline slope in the cohort was − 7.94 (− 14.15, − 5.10). The correlations between baseline characteristics and triglyceride decline slope were shown in Table S4. In the adjusted GLM model, the OFFD did not change significantly per increase of the triglyceride decline slope [RD-0.088, 95% CI, − 0.334 to 0.158, p = 0.48] (Table S5).
The effect of blood purification therapies
The change in triglyceride levels during the first 5 days was presented in Fig. S3. Triglyceride levels did not differ between those who received blood purification and those who received medical treatment during the first 5 days.
After PSM, 63 matched pairs were created for comparison. The baseline characteristics of the PSM cohort and the IPTW cohort are presented in Table S6. The imbalance in the baseline characteristics was significantly reduced after PSM or IPTW (Fig. S4). In the matched PSM cohort, patients undergoing blood purification had fewer OFFD to day 14 than those who did not [median difference − 1.00, 95% CI, − 2.00 to 0; p = 0.0040]. For the secondary outcomes, the blood purification group had fewer days out of ICU to day 14, fewer hospital-free days to day 14, day 28, and day 60, higher ΔSOFArank and ΔSOFAmax to day 14, higher need for ICU admission during the index admission, and higher risk of death or presence of organ failure on study day 7 (all p < 0.05). All the other secondary outcomes were comparable between groups. The IPTW cohort yielded similar results (Table 3).
Table 3.
Clinical outcomes of patients with blood purification or medical treatment after propensity score matching or inverse probability weighting
| Clinical outcomes | After PSM | After IPTW | ||||||
|---|---|---|---|---|---|---|---|---|
| Blood purification (n = 63) | Conventional treatment (n = 63) | Difference (95% CI) | p | Blood purification (n = 282) | Conventional treatment (n = 310) | Difference (95% CI) | p | |
| Primary outcome | ||||||||
| OFFD, median (IQR), days | 12 (7, 14) | 13 (11, 14) | − 1 (− 2, 0) | 0·0040 | 13 (8, 14) | 14 (11, 14) | − 1 (− 1, 0) | < 0·001 |
| Secondary outcomes | ||||||||
| Composite outcome, n (%) | 13 (20·6) | 2 (3·2) | 0·174 (0·065, 0·283) | 0·0070 | 56 (19·9) | 9 (2·9) | 0·170 (0·120, 0·220) | < 0·001 |
| ICU-free day to day 14, median (IQR), days | 9 (3, 11) | 14·0 (9, 14) | − 4 (− 5, − 3) | < 0·001 | 9 (4, 11) | 14 (10, 14) | − 4 (− 4, − 3) | < 0·001 |
| Hospital-free day to day 14, median (IQR), days | 0 (0, 6) | 5 (2, 7) | − 2 (− 4, − 0·5) | 0·0010 | 3 (0, 7) | 6 (2, 7) | − 1 (− 2, − 0·5) | < 0·001 |
| Hospital-free day to day 28, median (IQR), days | 14 (7, 20) | 19 (16, 21) | − 4 (− 7, − 2) | < 0·001 | 17 (9, 21) | 20 (16, 21) | − 2·5 (− 4, − 1) | < 0·001 |
| Hospital-free day to day 60, median (IQR), days | 46 (39, 52) | 51 (48, 53) | − 4 (− 7, − 2) | < 0·001 | 49 (41, 53) | 52 (48, 53) | − 3 (− 4, − 2) | < 0·001 |
| SOFA rank to day 14, median (IQR) | − 11 (− 26, 2) | − 19 (− 36, 0) | 7·0 (0, 15) | 0·022 | − 10 (− 22, 2) | − 12 (− 28, 1) | 4 (1, 8) | 0·0020 |
| ΔSOFA max to day 14, median (IQR) | 2 (0, 3) | 0 (− 1, 2) | 1 (0, 2) | 0·020 | 1 (0, 3) | 1 (0, 2) | 1 (1, 1) | < 0·001 |
| ΔSOFA14 to day 14, median (IQR) | − 2 (− 3, 0) | − 2 (− 3, 0) | 0 (0, 1) | 0·30 | − 2 (− 2, 0) | − 1 (− 3, 0) | 0 | 0·39 |
| 60-day mortality, n (%) | 2 (3·2) | 0 | 0·032 (− 0·011, 0·075) | 0·50 | 16 (5·7) | 0 | 0·057 (0·030, 0·084) | < 0·001 |
| 60-day IPN, n (%) | 4 (6·3) | 0 | 0·063 (0, 0·12) | 0·13 | 18 (6·4) | 4 (1·3) | 0·051 (0·020, 0·082) | 0·0020 |
| ICU requirement during index admission, n (%) | 59 (93·7) | 24 (38·1) | 0·556 (0·422, 0·690) | < 0·001 | 258 (91·8) | 80 (25·8) | 0·660 (0·602, 0·718) | < 0·001 |
| New-onset organ failure to day 14, n (%) | ||||||||
| Respiratory | 20 (31·7) | 13 (20·6) | 0·111 (− 0·041, 0·263) | 0·25 | 84 (29·8) | 80 (25·8) | 0·040 (− 0·032, 0·112) | 0·31 |
| Renal | 5 (7·9) | 5 (7·9) | 0 | 1·0 | 16 (5·7) | 30 (9·6) | − 0·039 (− 0·082, 0·004) | 0·090 |
| Cardiovascular | 6 (9·5) | 4 (6·3) | 0·032 (− 0·062, 0·126) | 0·73 | 31 (11·0) | 14 (4·5) | 0·065 (0·022, 0·108) | 0·0030 |
TG triglyceride, OFFD organ failure-free days to day 14, Composite outcome a composite of death from any cause by day 28 and the presence of at least one organ failure at day 7, ICU intensive care unit, SOFA sequential organ failure assessment, IPN infected pancreatic necrosis
Discussion
To our knowledge, the PERFORM study is hitherto the largest multicentre, prospective study exploring triglyceride-lowering therapies in HTG-AP patients. The main finding of the study is that there is substantial variation in triglyceride-lowering therapies for early HTG-AP patients. Moreover, the results showed that more rapid triglyceride decline was not associated with improving incidence and duration of organ failure. Though our results were not the first in the literature, the multicentre and prospective design, and the relatively large sample size added more credit to our findings.
Elevated triglyceride level is an established aetiology for AP, but the relationship between early plasma triglyceride and the prognosis of AP is controversial [29]. It is generally believed that triglyceride can cause pancreatic injury through its hydrolysate and generation of free fatty acids (FFA). However, most studies only assessed the relationship between initial triglyceride levels and clinical outcomes while ignoring the importance of dynamic changes. In a retrospective cohort study, Lu et al. found that rapid reduction of triglyceride levels was associated with decreased incidence of persistent organ failure in a retrospective study [17]. Differently, in a randomised trial performed by He et al., though high-volume haemofiltration reduced triglyceride levels more efficiently when compared with medical therapy (insulin and heparin), no improvement was found in mortality, hospital duration, or incidence of infected pancreatic necrosis [16].
A possible explanation for the futility of pursuing rapid triglyceride decline might be that FFA, produced by the hydrolysis of triglyceride, plays a key role in the pathophysiology of HTG-AP [30]. In this regard, triglyceride-lowering therapy by enhancing lipoprotein lipase activity (heparin and insulin) may increase short-term blood FFA, thereby negating the beneficial effect of reduced triglyceride. On the other hand, early fasting and continuous use of insulin are common practices when treating HTG-AP, which may increase the risk of hypoglycaemia in these patients [31]. Hypoglycaemia is known to be associated with a series of adverse clinical outcomes, including prolonged mechanical ventilation, more cardiovascular events, and increased risk of death [32–34]. Therefore, potential hypoglycaemia events associated with the wide use of insulin may counteract the benefits brought by rapid TG decline, though we did not have data on that. The incidence of hypoglycaemia should be of concern in future studies on the management of HTG-AP.
As an alternative to medical therapy, blood purification therapy like plasmapheresis or haemoperfusion can remove triglycerides without excessive FFA production [35]. However, our results showed blood purification therapies were not associated with improving organ failure. Besides, we combined varied blood purification modes for analysis in this study, which may introduce some bias. The potential impact of the most commonly used mode, plasmapheresis, was analysed in a separate study [36], and the results showed that it was not associated with clinical benefits. These results are consistent with Berberich et al.’s study, showing that conservative management was effective and safe [37]. Overall, these findings from observational data do not support the use of blood purification therapies in the management of HTG-AP, a randomised trial is needed to clarify this.
Notably, our study showed that patients reaching the triglyceride target on day 3 had significantly higher ICU requirements. Procedure-related ICU admission may partly explain the results. In our study, 47 of 122 (38.5%) patients in the target-reaching group received blood purification, while in those who did not reach the target on day 3, it was 28.7% (31/108) (p = 0.13). The increasing ICU need in the target-reaching group and worsening outcomes in patients undergoing blood purification therapies indicate that these invasive procedures are not without harm—they require central venous access, have the potential for infections or allergic reactions, and may not be widely available.
The study has several limitations. The first is that the observational nature precludes causal relationship analysis, and the sample size estimation was flawed with an unrealistic assumption that the patients reaching or not reaching the target would be numerically equal. The second is that the treatment was at the treating physician’s discretion, which represents the potential for significant selection bias. The third is that patients with baseline triglyceride levels less than 11.3 mmol/L were excluded from the study. The conclusion of this study may not be applicable to patients who present with triglyceride levels less than 11.3 mmol/L, and the effect of triglyceride-lowering for these patients still needs to be investigated in future studies. Moreover, some patients may have other aetiologies like alcohol/biliary/others, which were not accounted for. Last, we did not measure FFA in this study because of the difficulty in multiple lab quality control.
Conclusions
In conclusion, triglyceride-lowering therapies vary greatly among centres in the management of HTG-AP. More rapid triglyceride decline was not associated with improving the incidence and duration of organ failure in HTG-AP patients but may be associated with more ICU requirements. In light of our results, pursuing rapid TG decline with aggressive TG-lowering therapies may be ineffective and bear unnecessary risks, though a confirmatory trial is needed before formal recommendations can be made.
Supplementary Information
Additional file 1. Table S1. Numbers of cases from each site. Table S2. Distribution of medical treatment in patients undergoing blood purification. Table S3. Demographic and baseline characteristics in patients with Day3-triglyceride above 5.65 mmol/L or not. Table S4. The correlations between baseline characteristics and triglyceride slope. Table S5. Adjusted generalised linear analyses of the associations between TG slope and the primary outcome. Table S6. Demographic and baseline characteristics in patients with blood purification or medical treatment before and after propensity score matching or inverse probability weighting. Fig. S1. The distribution of age and AP severity. Fig. S2. Subgroup analyses of the effect of Day3-TG ≤ 5.65 mmol/L on organ failure-free days to day 14 of enrollment. Fig. S3. Change of plasma triglyceride levels during the first five days in patients undergoing blood purification or medical treatment. Fig. S4. Standardised mean difference (SMD) of variables before and after propensity score matching and weighting.
Additional file 2. Protocol and statistic analysis plan of the study.
Acknowledgements
We would like to acknowledge all the patients and health staffs who participated in this trial. The full list of members of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG) is as follows: Lu Ke, Jing Zhou, Wenjian Mao, Jiajia Lin, Longxiang Cao, Mingfeng Huang, Mengjie Lu, Yan Chen, Gang Li, Bo Ye, Baiqiang Li, Zhihui Tong, Yuxiu Liu, Weiqin Li, Jinling Hospital. Tao Chen, Liverpool University. Fang Shao, Nanjing Medical University. Nonghua Lv, Yin Zhu, Liang Xia, Wenhua He, Zhenping Chen, The First Affiliated Hospital of Nanchang University. Xinting Pan, Qingyun Zhu, Youdong Wan, The Affiliated Hospital of Qingdao University. Hong Mei, Kang Li, Miao Chen, The Affiliated Hospital of Zunyi Medical University. Chengjian He, Hongyi Yao, Zigui Zhu, Nanhua Hospital. Weili Gu, Affiliated Hospital 2 of Nantong University. Weihua Lu, Jingyi Wu, Feng Zhou, The First Affiliated Hospital of Wannan Medical College. Shumin Tu, Long Fu, Bing Xue, Shangqiu First People’s Hospital. Haibin Ni, Xiaofei Huang, Dandan Zhou, Jiangsu Provincial Hospital of Integrated Chinese and Western Medicine. Guoxiu Zhang, Lening Ren, Dahuan Li, The First Affiliated Hospital and College of Clinical Medicine of Henan University of Science and Technology. Xiangyang Zhao, Wei Zhao, Xiaomei Chen, Qilu Hospital of Shandong University. Junli Sun, Keke Xin, Luoyang Central Hospital. Weiwei Chen, Qingcheng Xu, Clinical Medical College of Yangzhou University. Jingchun Song, Qingbo Zeng, 94th Hospital of PLA. Min Shao, Dongsheng Zhao, The First Affiliated Hospital of Anhui Medical University. Jianfeng Tu, Hongguo Yang, Zhejiang Provincial People’s Hospital. Bin Wu, Huaguang Ye, The Third Hospital of Xiamen City. Mingzhi Chen, Yingjie Chen, Jinjiang Hospital of Traditional Chinese Medicine. Mei Yang, Hong Gao, The Qujing NO.1 People’s Hospital. Qiang Li, The First Affiliated Hospital of Nanjing Medical University. Lijuan Zhao, Guobing Chen, Yafei Li, First People’s Hospital of Yunnan Province. Honghai Xia, Dongliang Yang, Shusheng Zhou, The First Affiliated Hospital of the University of Science and Technology of China. Jiyan Lin, Siyao Liu, The First Affiliated Hospital of Xiamen University. Donghuang Hong, Songjing Shi, Fujian Province Hospital. Zuozheng Wang, Weijie Yao, General Hospital of Ningxia Medical University. Yi Sun, Suining Central Hospital. Kaixiu Qin, Shan Xu, Lei Yu, The Second Affiliated Hospital of Chongqing Medical University. Feng Guo, Yongjun Lin, Sir Run Run Shaw Hospital of Zhejiang University. Yun Zhou, Pingxiang People’s Hospital. Qinghai Jiao, The First Hospital of HanDan. Quanxing Feng, The Fourth Military Medical University. Zhiyong Liu, Xiangya Hospital.
Abbreviations
- AP
Acute pancreatitis
- HTG
Hypertriglyceridemia
- HTG-AP
Hypertriglyceridemia-associated acute pancreatitis
- TG
Triglyceride
- OFFD
Organ failure-free day
- SOFA
Sequential organ failure assessment, which ranges from 0 to 24, with higher scores indicating more severe organ failure
- ICU
Intensive care units
- IPN
Infected pancreatic necrosis
- SD
Standard deviation
- IQR
Interquartile range
- PSM
Propensity score matching
- GLM
Generalised linear model
- BMI
Body mass index
- APACHE II
Acute physiology and chronic health evaluation II
- HR
Hazard ratio
- CI
Confidence interval
- IPTW
Inverse probability of treatment weighting
- SIRS
Systemic inflammatory response syndrome
- CRP
C-reactive protein
- FFA
Free fatty acids
- LMWH
Low molecular weight heparin
Authors’ contributions
Guarantor of article: LK. Study design: LK, ZHT, XCZ, DW, WH, and XFL. Data collection: JZ, ZZW, QHL, LXC, YJC, SYL, DHH, KXQ, HBN,YS, YL, FG, GXZ, KZ, YZC, QHJ, XL, GL, BY, LTW, and SL. Data analysis: CL and LXC. Drafting of the article: JZ, LK, YS, ZGZ, XSZ1, XSZ2, EM, GC, CS, JW, YXL and WQL. Data interpretation, review and/or revision of the manuscript: all authors. Study concept and study supervision: WQL, YXL, ZHT, and LK. All authors read and approved the final manuscript.
Authors’ twitter handles
Twitter handles: @CAPCTG2023.
Funding
The study was funded partly by Key Research and Development Programme Foundation of Jiangsu Province of China (No. BE 2016749). This study was funded partly by the National Science Foundation of China (No. 81900592).
Data availability
Deidentified individual participant data are available indefinitely in the electronic database. Data can be accessed through capctg.medbit.cn with the approval of the authors. Request for data can be made to the corresponding author (ctgchina@medbit.cn) and will be discussed during a meeting of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG).
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Jinling Hospital (ethical number: 2020NZKY-016–01) and registered with the Chinese Clinical Trial Registry (ChiCTR2000039541). The local hospital ethics committees of all the participating sites also approved the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zhou, Zuozheng Wang and Qinghong Liu contributed equally to this work.
Contributor Information
Weiqin Li, Email: ctgchina@medbit.cn.
Lu Ke, Email: ctgkelu@nju.edu.cn.
for the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG):
Jing Zhou, Zuozheng Wang, Longxiang Cao, Yingjie Chen, Siyao Liu, Donghuang Hong, Kaixiu Qin, Haibin Ni, Yi Sun, Feng Guo, Guoxiu Zhang, Qinghai Jiao, Gang Li, Bo Ye, Yuxiu Liu, Zhihui Tong, Weiqin Li, Lu Ke, Wenjian Mao, Jiajia Lin, Mingfeng Huang, Mengjie Lu, Yan Chen, Baiqiang Li, Tao Chen, Fang Shao, Nonghua Lv, Yin Zhu, Liang Xia, Wenhua He, Zhenping Chen, Xinting Pan, Qingyun Zhu, Youdong Wan, Hong Mei, Kang Li, Miao Chen, Chengjian He, Hongyi Yao, Zigui Zhu, Weili Gu, Weihua Lu, Jingyi Wu, Feng Zhou, Shumin Tu, Long Fu, Bing Xue, Xiaofei Huang, Dandan Zhou, Lening Ren, Dahuan Li, Xiangyang Zhao, Wei Zhao, Xiaomei Chen, Junli Sun, Keke Xin, Weiwei Chen, Qingcheng Xu, Jingchun Song, Qingbo Zeng, Min Shao, Dongsheng Zhao, Jianfeng Tu, Hongguo Yang, Bin Wu, Huaguang Ye, Mingzhi Chen, Mei Yang, Hong Gao, Qiang Li, Lijuan Zhao, Guobing Chen, Yafei Li, Honghai Xia, Dongliang Yang, Shusheng Zhou, Jiyan Lin, Songjing Shi, Weijie Yao, Shan Xu, Lei Yu, Yongjun Lin, Yun Zhou, Quanxing Feng, and Zhiyong Liu
References
- 1.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33(4):323–30. [DOI] [PubMed] [Google Scholar]
- 2.Matta B, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18(7):1567-1575 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen SS, et al. Hypertriglyceridemia is often under recognized as an aetiologic risk factor for acute pancreatitis: a population-based cohort study. Pancreatology. 2021;21(2):334–41. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8-year period. Pancreas. 2017;46(4):504–9. [DOI] [PubMed] [Google Scholar]
- 5.He W, et al. Elevated hypertriglyceridemia and decreased gallstones in the etiological composition ratio of acute pancreatitis as affected by seasons and festivals: A Two-center real-world study from China. Front Cell Infect Microbiol. 2022;12: 976816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian, X., Y. Huang, and H. Wang, Deviation of Chinese adults’ diet from the Chinese Food Pagoda 2016 and its association with adiposity. Nutrients, 2017. 9(9):995. [DOI] [PMC free article] [PubMed]
- 7.Li XY, et al. Identification of a novel LPL nonsense variant and further insights into the complex etiology and expression of hypertriglyceridemia-induced acute pancreatitis. Lipids Health Dis. 2020;19(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baranyai T, et al. Hypertriglyceridemia causes more severe course of acute pancreatitis. Clinical Lipidology. 2012;7(6):731–6. [Google Scholar]
- 9.Li X, et al. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center. BMC Gastroenterol. 2018;18(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pothoulakis I, et al. Clinical features of hypertriglyceridemia-induced acute pancreatitis in an international, multicenter, prospective cohort (APPRENTICE consortium). Pancreatology. 2020;20:325. [DOI] [PubMed] [Google Scholar]
- 11.Criddle DN, et al. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci U S A. 2004;101(29):10738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandhu S, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adiamah A, et al. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin Nutr. 2018;37(6 Pt A):1810–22. [DOI] [PubMed] [Google Scholar]
- 14.Tamez-Perez HE, et al. Insulin therapy in patients with severe hypertriglyceridemia. Rev Med Inst Mex Seguro Soc. 2006;44(3):235–7. [PubMed] [Google Scholar]
- 15.Chen X, et al. Effectiveness of continuous veno-venous hemofiltration in the treatment of severe acute pancreatitis. Exp Ther Med. 2019;17(4):2720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He WH, et al. Emergent triglyceride-lowering therapy with early high-volume hemofiltration against low-molecular-weight heparin combined with insulin in hypertriglyceridemic pancreatitis: a prospective randomized controlled trial. J Clin Gastroenterol. 2016;50(9):772–8. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, et al. Timely reduction of triglyceride levels is associated with decreased persistent organ failure in hypertriglyceridemic pancreatitis. Pancreas. 2020;49(1):105–10. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, et al. The effect of plasma triglyceride-lowering therapy on the evolution of organ function in early hypertriglyceridemia-induced acute pancreatitis patients with worrisome features (PERFORM study): rationale and design of a multicenter, prospective, observational, cohort study. Front Med (Lausanne). 2021;8: 756337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks PA, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 20.Gelrud A, Whitcomb DC UpToDate. Hypertriglyceridemia-induced acute pancreatitis. 2021. Available online at: https://www.uptodate.com/contents/hypertriglyceridemia-induced-acute-pancreatitis. Accessed 30 June 2021.
- 21.Dellinger EP, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256(6):875–80. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–800. [DOI] [PubMed] [Google Scholar]
- 23.Gelissen H, et al. Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA. 2021;326(10):940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Grooth HJ, et al. SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care. 2017;21(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, et al. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas. 2015;44(3):409–14. [DOI] [PubMed] [Google Scholar]
- 26.Nawaz H, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110(10):1497–503. [DOI] [PubMed] [Google Scholar]
- 27.Cho JH, et al. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21(8):2387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chesnaye NC, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanutti C, Labbadia G, Morozzi C. Severe hypertriglyceridemia-related acute pancreatitis. Ther Apher Dial. 2013;17(2):130–7. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira C, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest. 2020;130(4):1931–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacherade J-C, Jacqueminet S, Preiser J-C. An overview of hypoglycemia in the critically ill. J Diabetes Sci Technol. 2009;3(6):1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35(10):2262–7. [DOI] [PubMed] [Google Scholar]
- 33.Egi M, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85(3):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egi M, et al. Pre-morbid glycemic control modifies the interaction between acute hypoglycemia and mortality. Intensive Care Med. 2016;42(4):562–71. [DOI] [PubMed] [Google Scholar]
- 35.Gubensek J. Potential differences between double-filtration plasmapheresis and therapeutic plasma exchange in the treatment of acute hypertriglyceridemic pancreatitis. J Clin Apher. 2021;36(1):223–4. [DOI] [PubMed] [Google Scholar]
- 36.Cao L, et al. Early plasmapheresis among patients with hypertriglyceridemia-associated acute pancreatitis. JAMA Netw Open. 2023;6(6): e2320802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berberich AJ, et al. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med. 2019;286(6):644–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Numbers of cases from each site. Table S2. Distribution of medical treatment in patients undergoing blood purification. Table S3. Demographic and baseline characteristics in patients with Day3-triglyceride above 5.65 mmol/L or not. Table S4. The correlations between baseline characteristics and triglyceride slope. Table S5. Adjusted generalised linear analyses of the associations between TG slope and the primary outcome. Table S6. Demographic and baseline characteristics in patients with blood purification or medical treatment before and after propensity score matching or inverse probability weighting. Fig. S1. The distribution of age and AP severity. Fig. S2. Subgroup analyses of the effect of Day3-TG ≤ 5.65 mmol/L on organ failure-free days to day 14 of enrollment. Fig. S3. Change of plasma triglyceride levels during the first five days in patients undergoing blood purification or medical treatment. Fig. S4. Standardised mean difference (SMD) of variables before and after propensity score matching and weighting.
Additional file 2. Protocol and statistic analysis plan of the study.
Data Availability Statement
Deidentified individual participant data are available indefinitely in the electronic database. Data can be accessed through capctg.medbit.cn with the approval of the authors. Request for data can be made to the corresponding author (ctgchina@medbit.cn) and will be discussed during a meeting of the Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG).