Abstract
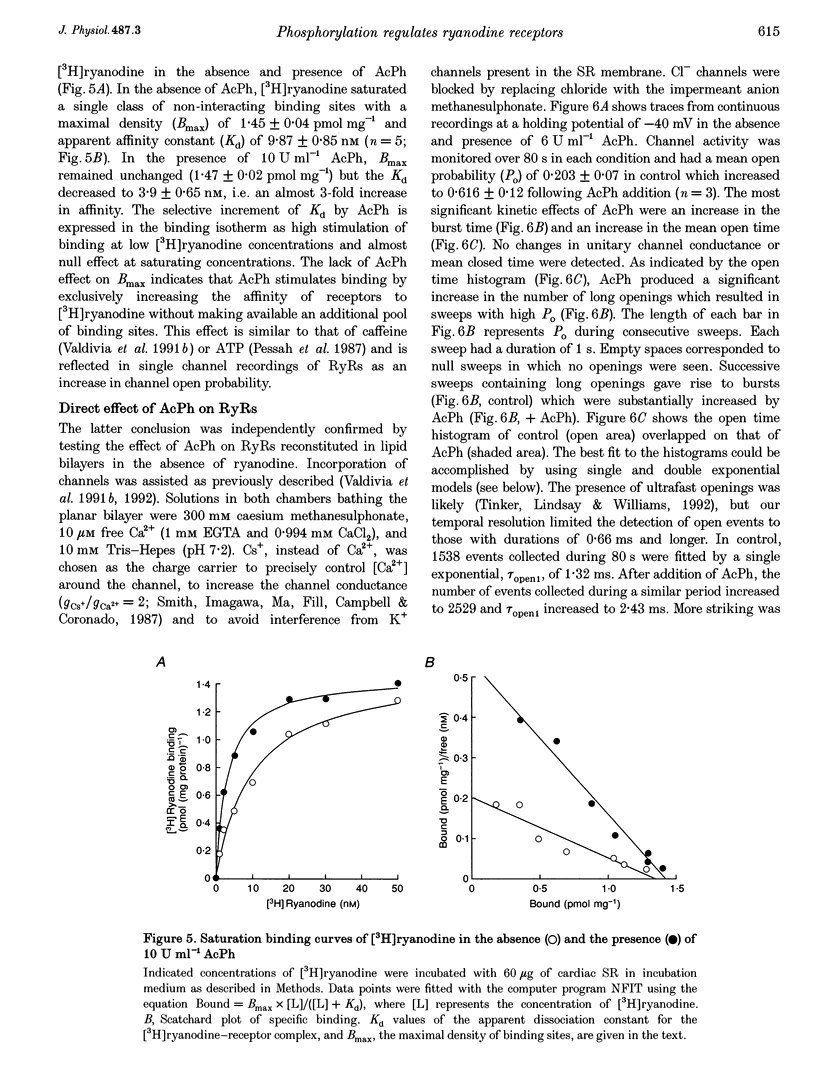
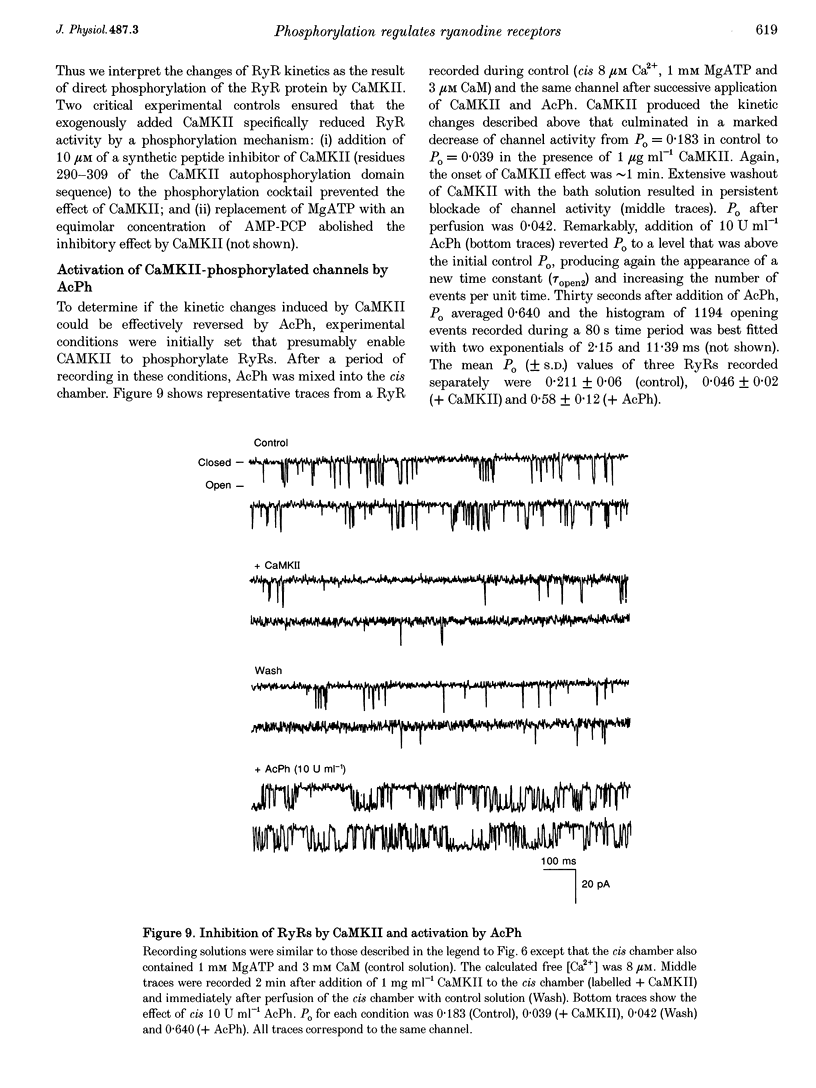
1. The regulation of the cardiac Ca2+ release channel-ryanodine receptor (RyR) by exogenous acid phosphatase (AcPh) and purified Ca(2+)-calmodulin-dependent protein kinase II (CaMKII) was studied in swine and rabbit sarcoplasmic reticulum (SR) vesicles using [3H]ryanodine binding and planar bilayer reconstitution experiments. 2. Addition of AcPh (1-20 U ml-1) to a standard incubation medium increased [3H]ryanodine binding in a Ca(2+)-dependent manner. Stimulation was only readily apparent in media containing micromolar Ca2+ concentrations. 3. Scatchard analysis of [3H]ryanodine binding curves revealed that AcPh enhanced binding by increasing the affinity of the receptor for [3H]ryanodine without recruiting additional receptor sites (Kd, 9.8 +/- 0.85 and 3.9 +/- 0.65 nM; Bmax (the maximal receptor density), 1.45 +/- 0.14 and 1.47 +/- 0.12 pmol mg-1 for control and AcPh, respectively). The failure of AcPh to increase Bmax suggested that the number of receptors that were 'dormant' due to phosphorylation in the SR preparation was very small. 4. At the single channel level, AcPh increased the open probability (Po) of RyR channels by increasing the opening rate and inducing the appearance of a longer open state while having no effect on single channel conductance. Thus AcPh acted directly on RyR channels or a closely associated regulatory protein. 5. CaMKII decreased both [3H]ryanodine binding and Po of RyRs when added to medium supplemented with micromolar levels of Ca2+ and calmodulin (CaM). Addition of a synthetic peptide inhibitor of CaMKII, or replacement of ATP with the non-hydrolysable ATP analogue adenylyl[beta, gamma-methylene]-diphosphate (AMP-PCP), prevented CaMKII inhibition of RyRs, suggesting that CaMKII acted specifically through a phosphorylation mechanism. 6. The inhibition of RyR channel activity by CaMKII was reversed by the addition of AcPh. Thus we showed that an in vitro phosphorylation-dephosphorylation mechanism effectively regulates RyRs. 7. The results suggest that intracellular signalling pathways that lead to activation of CaMKII may reduce efflux of Ca2+ from the SR by inhibition of RyR channel activity. The Ca2+ dependence of CaMKII inhibition suggests that the role of the phosphorylation mechanism is to modulate the RyR response to Ca2+.
Full text
PDF


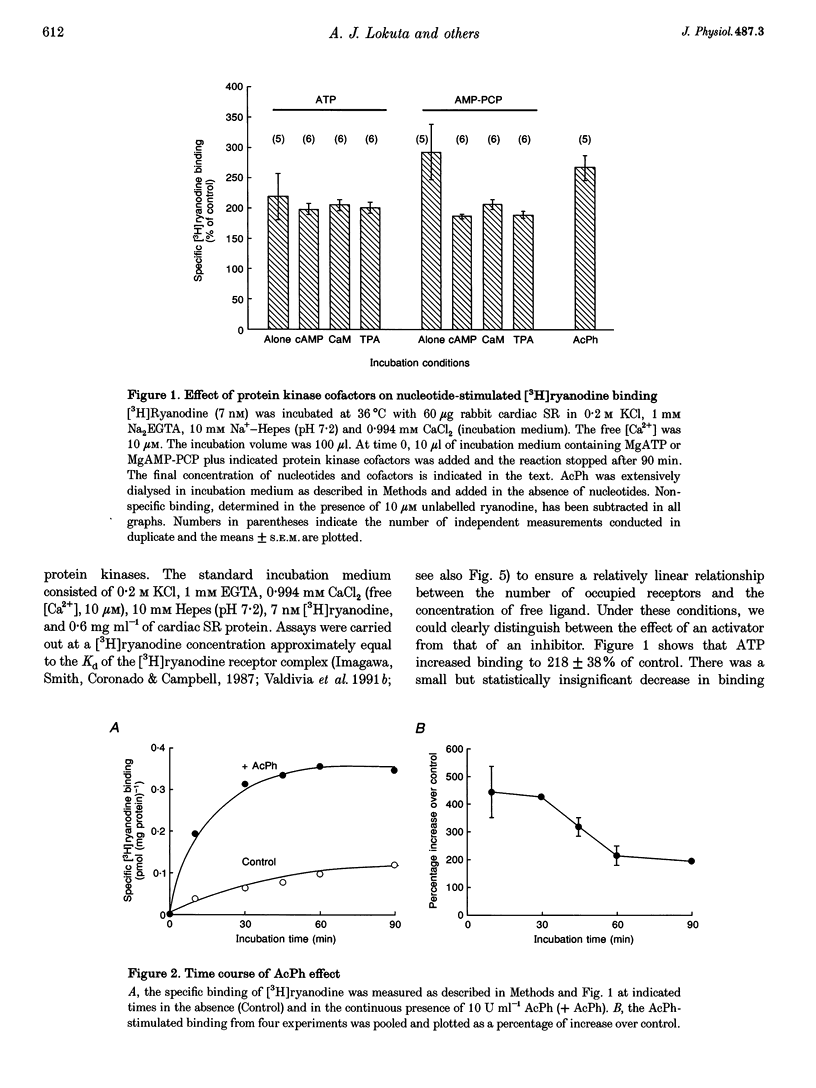
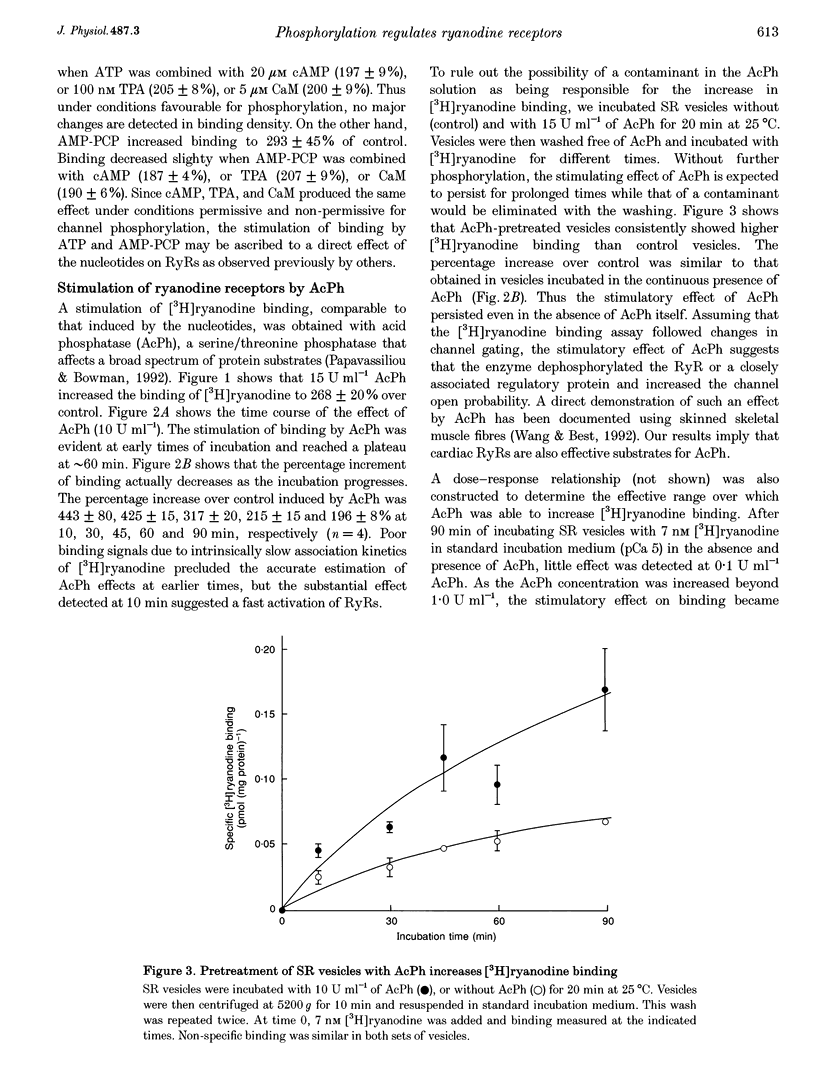
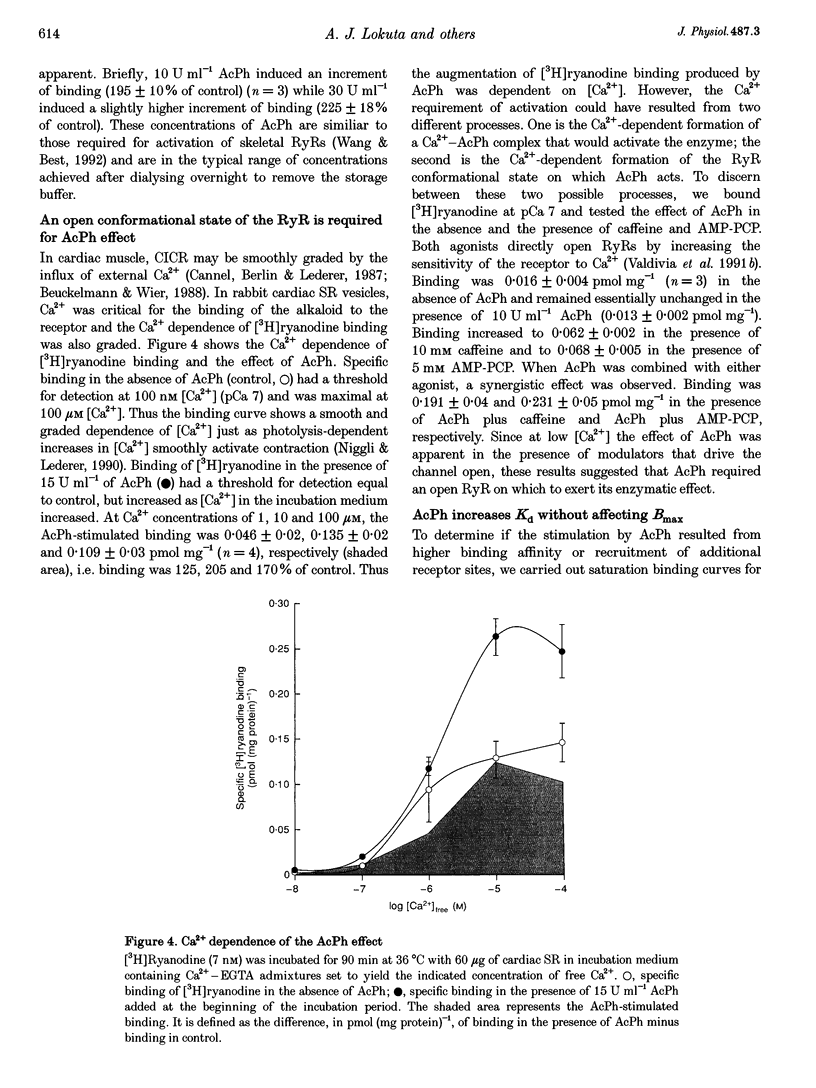
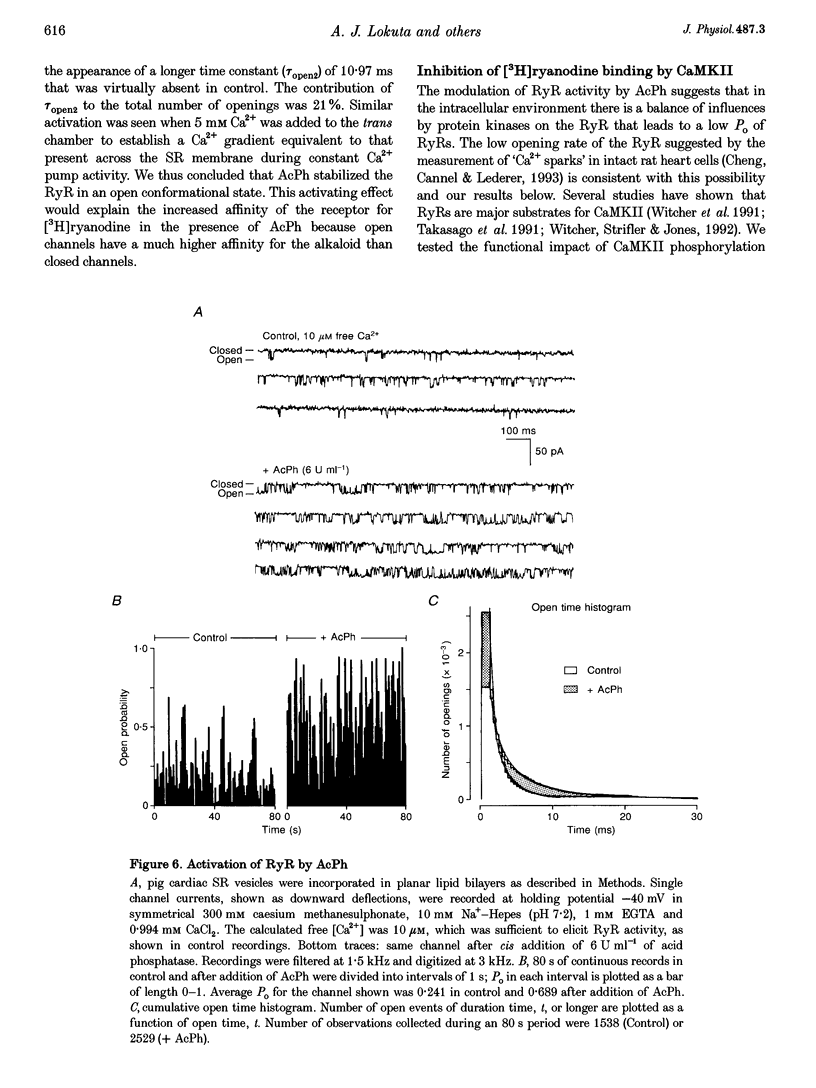



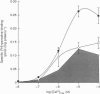
Images in this article
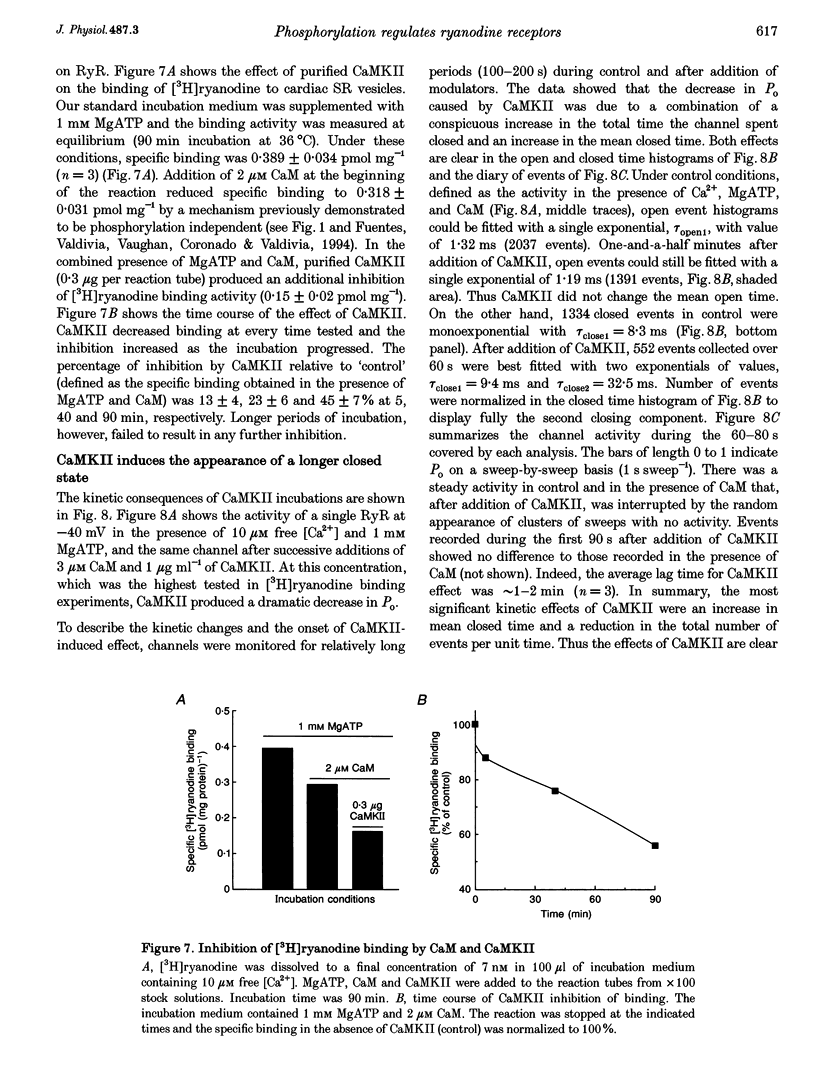
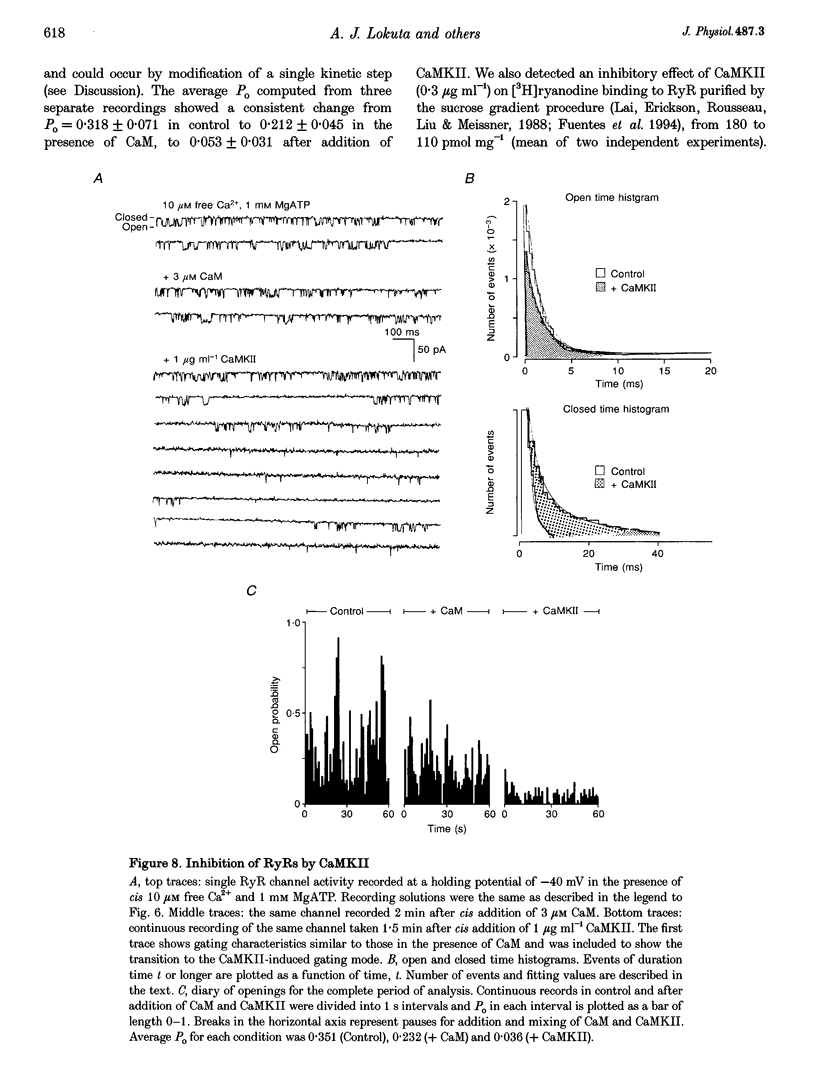
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beuckelmann D. J., Wier W. G. Mechanism of release of calcium from sarcoplasmic reticulum of guinea-pig cardiac cells. J Physiol. 1988 Nov;405:233–255. doi: 10.1113/jphysiol.1988.sp017331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck E., Zimanyi I., Abramson J. J., Pessah I. N. Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. J Biol Chem. 1992 Nov 25;267(33):23560–23567. [PubMed] [Google Scholar]
- Cannell M. B., Berlin J. R., Lederer W. J. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987 Dec 4;238(4832):1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Chu A., Sumbilla C., Inesi G., Jay S. D., Campbell K. P. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry. 1990 Jun 26;29(25):5899–5905. doi: 10.1021/bi00477a003. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes O., Valdivia C., Vaughan D., Coronado R., Valdivia H. H. Calcium-dependent block of ryanodine receptor channel of swine skeletal muscle by direct binding of calmodulin. Cell Calcium. 1994 Apr;15(4):305–316. doi: 10.1016/0143-4160(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Hain J., Nath S., Mayrleitner M., Fleischer S., Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from skeletal muscle. Biophys J. 1994 Nov;67(5):1823–1833. doi: 10.1016/S0006-3495(94)80664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenegger M., Suko J. Phosphorylation of the purified cardiac ryanodine receptor by exogenous and endogenous protein kinases. Biochem J. 1993 Dec 1;296(Pt 2):303–308. doi: 10.1042/bj2960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Schlatterer R. G., Nicar M., Campbell K. P., Sutko J. L. The effects of ryanodine on passive calcium fluxes across sarcoplasmic reticulum membranes. J Biol Chem. 1987 Feb 25;262(6):2711–2718. [PubMed] [Google Scholar]
- McPherson P. S., Campbell K. P. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993 Jul 5;268(19):13765–13768. [PubMed] [Google Scholar]
- Meissner G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986 Jan 14;25(1):244–251. doi: 10.1021/bi00349a034. [DOI] [PubMed] [Google Scholar]
- Meissner G., el-Hashem A. Ryanodine as a functional probe of the skeletal muscle sarcoplasmic reticulum Ca2+ release channel. Mol Cell Biochem. 1992 Sep 8;114(1-2):119–123. doi: 10.1007/BF00240306. [DOI] [PubMed] [Google Scholar]
- Michalak M., Dupraz P., Shoshan-Barmatz V. Ryanodine binding to sarcoplasmic reticulum membrane; comparison between cardiac and skeletal muscle. Biochim Biophys Acta. 1988 Apr 22;939(3):587–594. doi: 10.1016/0005-2736(88)90106-x. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Otsu K., Willard H. F., Khanna V. K., Zorzato F., Green N. M., MacLennan D. H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem. 1990 Aug 15;265(23):13472–13483. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Seiler S., Wegener A. D., Whang D. D., Hathaway D. R., Jones L. R. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease. J Biol Chem. 1984 Jul 10;259(13):8550–8557. [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Inui M. Regulation of calcium transport by the ATPase-phospholamban system. J Mol Cell Cardiol. 1983 Sep;15(9):565–575. doi: 10.1016/0022-2828(83)90267-5. [DOI] [PubMed] [Google Scholar]
- Takasago T., Imagawa T., Furukawa K., Ogurusu T., Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991 Jan;109(1):163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Tate C. A., Bick R. J., Chu A., Van Winkle W. B., Entman M. L. Nucleotide specificity of cardiac sarcoplasmic reticulum. GTP-induced calcium accumulation and GTPase activity. J Biol Chem. 1985 Aug 15;260(17):9618–9623. [PubMed] [Google Scholar]
- Tinker A., Lindsay A. R., Williams A. J. A model for ionic conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J Gen Physiol. 1992 Sep;100(3):495–517. doi: 10.1085/jgp.100.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Fuentes O., el-Hayek R., Morrissette J., Coronado R. Activation of the ryanodine receptor Ca2+ release channel of sarcoplasmic reticulum by a novel scorpion venom. J Biol Chem. 1991 Oct 15;266(29):19135–19138. [PubMed] [Google Scholar]
- Valdivia H. H., Hogan K., Coronado R. Altered binding site for Ca2+ in the ryanodine receptor of human malignant hyperthermia. Am J Physiol. 1991 Aug;261(2 Pt 1):C237–C245. doi: 10.1152/ajpcell.1991.261.2.C237. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Kirby M. S., Lederer W. J., Coronado R. Scorpion toxins targeted against the sarcoplasmic reticulum Ca(2+)-release channel of skeletal and cardiac muscle. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12185–12189. doi: 10.1073/pnas.89.24.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Witcher D. R., Kovacs R. J., Schulman H., Cefali D. C., Jones L. R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991 Jun 15;266(17):11144–11152. [PubMed] [Google Scholar]
- Witcher D. R., Strifler B. A., Jones L. R. Cardiac-specific phosphorylation site for multifunctional Ca2+/calmodulin-dependent protein kinase is conserved in the brain ryanodine receptor. J Biol Chem. 1992 Mar 5;267(7):4963–4967. [PubMed] [Google Scholar]
- Yoshida A., Takahashi M., Imagawa T., Shigekawa M., Takisawa H., Nakamura T. Phosphorylation of ryanodine receptors in rat myocytes during beta-adrenergic stimulation. J Biochem. 1992 Feb;111(2):186–190. doi: 10.1093/oxfordjournals.jbchem.a123735. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]