Abstract
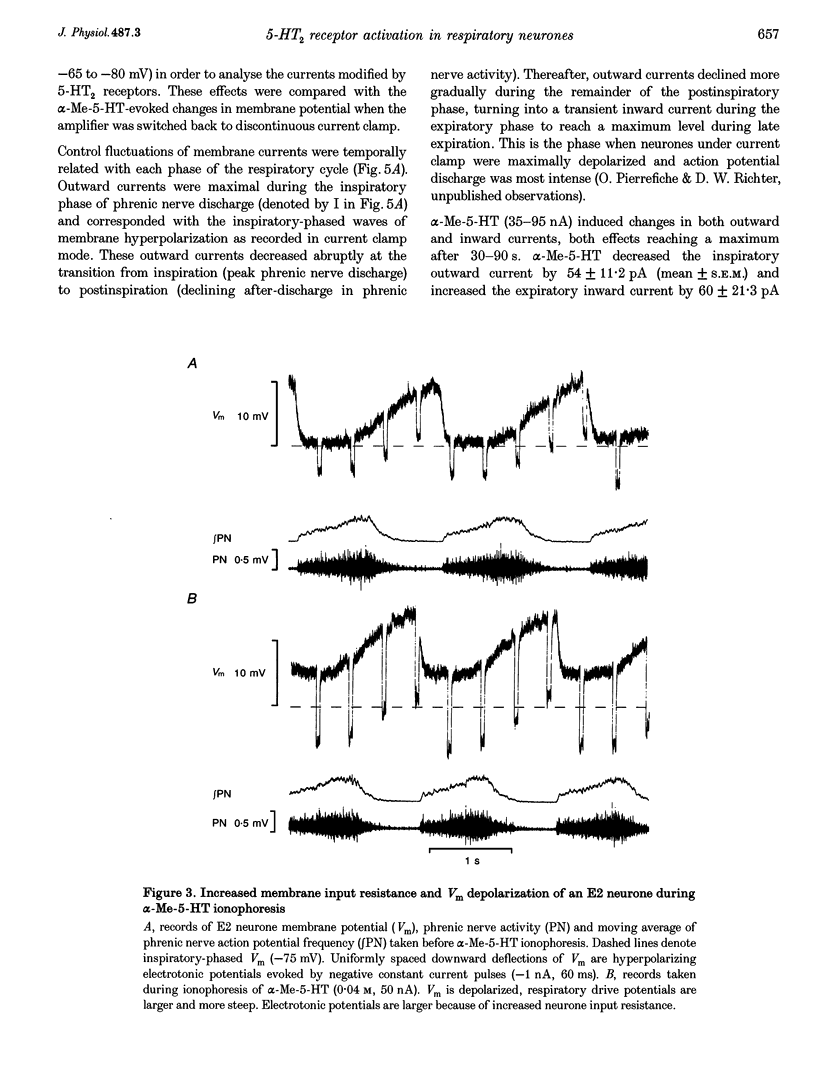
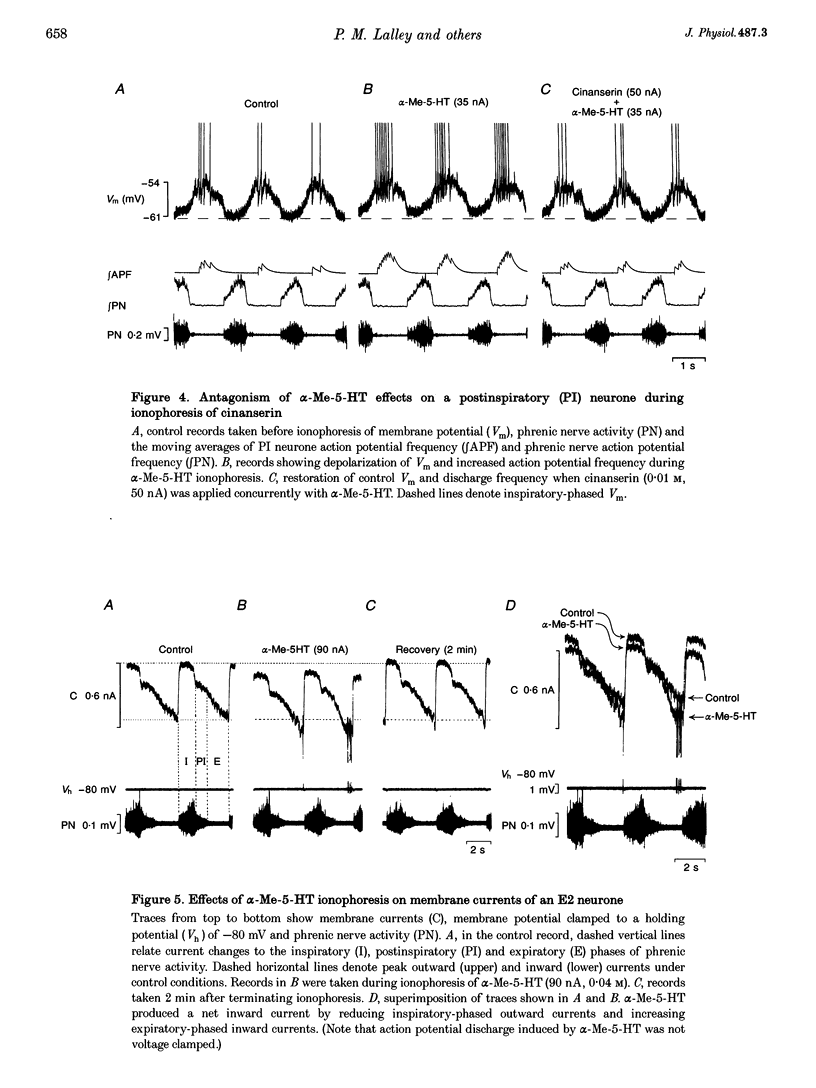
1. The effects of the 5-HT2 receptor agonist alpha-methyl-5-HT were studied on the membrane of expiratory (E2) and post-inspiratory (PI) neurones, by intracellular recordings in the caudal medulla of anaesthetized cats. 2. Ionophoresis of alpha-Me-5-HT depolarized membrane potential and increased action potential frequency in a majority of neurones tested. Depolarization of neurones by alpha-Me-5-HT was accompanied by increased input resistance throughout all phases of the respiratory cycle. These effects were antagonized by ionophoresis of cinanserin, a receptor-blocking agent with high affinity for 5-HT2 receptors. 3. E2 neurones were voltage clamped to measure membrane current changes induced by alpha-Me-5-HT ionophoresis. alpha-Me-5-HT induced a net inward current by reducing inspiratory-phase outward currents and increasing expiratory-phase inward currents. These changes were equivalent with steady membrane depolarization, decreased inspiratory phase membrane hyperpolarization and increased expiratory drive potential recorded from the same neurones in current clamp. 4. The effects of alpha-Me-5-HT are consistent with activation of 5-HT2 receptors on E2 and PI neurones leading to blockade of synaptically activated and persistent conductances to potassium ions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita H., Ochiishi M. Opposing effects of 5-hydroxytryptamine on two types of medullary inspiratory neurons with distinct firing patterns. J Neurophysiol. 1991 Jul;66(1):285–292. doi: 10.1152/jn.1991.66.1.285. [DOI] [PubMed] [Google Scholar]
- Armijo J. A., Mediavilla A., Flórez J. Inhibition of the activity of the respiratory and vasomotor centers by centrally administered 5-hydroxytryptamine in cats. Rev Esp Fisiol. 1979 Jun;35(2):219–227. [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker D. H. A slow excitatory postsynaptic potential mediated by 5-HT2 receptors in nucleus prepositus hypoglossi. J Neurosci. 1994 Apr;14(4):2428–2434. doi: 10.1523/JNEUROSCI.14-04-02428.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Ion conductances affected by 5-HT receptor subtypes in mammalian neurons. Trends Neurosci. 1990 May;13(5):169–173. doi: 10.1016/0166-2236(90)90042-9. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Denavit-Saubié M., Henry J. L., Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979 Jan 5;160(1):57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Richter D. W. Second messenger-induced modulation of the excitability of respiratory neurones. Neuroreport. 1993 Jul;4(7):861–863. doi: 10.1097/00001756-199307000-00005. [DOI] [PubMed] [Google Scholar]
- Davies M. F., Deisz R. A., Prince D. A., Peroutka S. J. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987 Oct 13;423(1-2):347–352. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. Theory and operation of a single microelectrode voltage clamp. J Neurosci Methods. 1984 Jun;11(2):101–127. doi: 10.1016/0165-0270(84)90029-3. [DOI] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. Involvement of N-methyl-D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Res. 1989 Oct 23;500(1-2):199–208. doi: 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- Glennon R. A. Central serotonin receptors as targets for drug research. J Med Chem. 1987 Jan;30(1):1–12. doi: 10.1021/jm00384a001. [DOI] [PubMed] [Google Scholar]
- Haji A., Remmers J. E., Connelly C., Takeda R. Effects of glycine and GABA on bulbar respiratory neurons of cat. J Neurophysiol. 1990 May;63(5):955–965. doi: 10.1152/jn.1990.63.5.955. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Dick T. E., Berger A. J. Serotonin-mediated excitation of recurrent laryngeal and phrenic motoneurons evoked by stimulation of the raphe obscurus. Brain Res. 1987 Aug 4;417(1):12–20. doi: 10.1016/0006-8993(87)90174-0. [DOI] [PubMed] [Google Scholar]
- Ismaiel A. M., Titeler M., Miller K. J., Smith T. S., Glennon R. A. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin. J Med Chem. 1990 Feb;33(2):755–758. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- Lalley P. M., Bischoff A. M., Richter D. W. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994 Apr 1;476(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M. Effects of baclofen and gamma-aminobutyric acid on different types of medullary respiratory neurons. Brain Res. 1986 Jun 25;376(2):392–395. doi: 10.1016/0006-8993(86)90206-4. [DOI] [PubMed] [Google Scholar]
- Lalley P. M. Serotoninergic and non-serotoninergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986 Nov;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M. The excitability and rhythm of medullary respiratory neurons in the cat are altered by the serotonin receptor agonist 5-methoxy-N,N, dimethyltryptamine. Brain Res. 1994 Jun 13;648(1):87–98. doi: 10.1016/0006-8993(94)91909-7. [DOI] [PubMed] [Google Scholar]
- Lindsay A. D., Feldman J. L. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993 Feb;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon D. R., Lalley P. M. Inhibition of respiratory neural discharges by clonidine and 5-hydroxytryptophan. J Pharmacol Exp Ther. 1982 Sep;222(3):771–777. [PubMed] [Google Scholar]
- Morin D., Hennequin S., Monteau R., Hilaire G. Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res. 1990 Dec 10;535(2):281–287. doi: 10.1016/0006-8993(90)91611-j. [DOI] [PubMed] [Google Scholar]
- Morin D., Monteau R., Hilaire G. Compared effects of serotonin on cervical and hypoglossal inspiratory activities: an in vitro study in the newborn rat. J Physiol. 1992;451:605–629. doi: 10.1113/jphysiol.1992.sp019181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- North R. A., Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurones. J Physiol. 1989 Oct;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A. E., Spanswick D., Logan S. D. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994 Oct 1;480(Pt 1):109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O., Schmid K., Foutz A. S., Denavit-Saubie M. Endogenous activation of NMDA and non-NMDA glutamate receptors on respiratory neurones in cat medulla. Neuropharmacology. 1991 May;30(5):429–440. doi: 10.1016/0028-3908(91)90003-t. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Ballanyi K., Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1992 Dec;2(6):788–793. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Champagnat J., Jacquin T., Benacka R. Calcium currents and calcium-dependent potassium currents in mammalian medullary respiratory neurones. J Physiol. 1993 Oct;470:23–33. doi: 10.1113/jphysiol.1993.sp019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Roberts M. H., Straughan D. W. Excitation and depression of cortical neurones by 5-hydroxytryptamine. J Physiol. 1967 Nov;193(2):269–294. doi: 10.1113/jphysiol.1967.sp008357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Zifa E., Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992 Sep;44(3):401–458. [PubMed] [Google Scholar]



