Abstract
Background and Objectives
The serum galactomannan test (GM test) and the (1,3)-β-D-glucan test (G test) are utilized in diagnosing invasive fungal sinusitis. However, their effectiveness in detecting paranasal sinus fungus balls (FBs) has not been established. This study aimed to explore their diagnostic value in patients with FBs.
Methods
We retrospectively reviewed the medical records of 105 patients (42 with FBs and 63 with chronic rhinosinusitis [CRS]) who underwent serum GM and G tests between June 2020 and May 2021. Olfactory test results and demographics were also analyzed.
Results
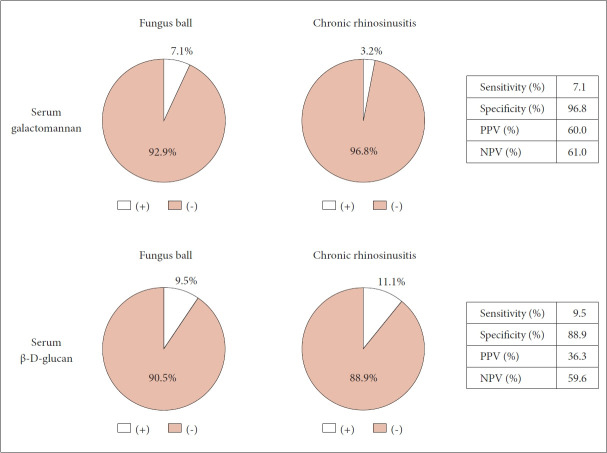
There were 42 FB patients (10 men, 32 women) and 63 CRS patients (27 men, 36 women). The positivity rates for serum GM (7.1% in the FB group vs. 3.2% in the CRS group, p=0.640) and G test (9.5% in the FB group vs. 11.1% in the CRS group, p=0.482) did not differ significantly between groups. The sensitivities of the GM and G tests were 7.1% and 9.5%, respectively, and their specificities were 96.8% and 88.9%, respectively. The positive predictive values were 60.0% for the GM test and 36.3% for the G test, and the negative predictive values were 61.0% for the GM test and 59.6% for the G test.
Conclusion
Serum GM and G tests demonstrated low sensitivity and high specificity, indicating limited effectiveness in differentiating between patients with FBs and those with CRS. Histological examination remains the gold standard for the definitive diagnosis of FBs.
Keywords: Paranasal sinus fungus ball, Galactomannan, β-D-glucan, Aspergillus
INTRODUCTION
Paranasal sinus fungus balls (FBs) are a noninvasive type of fungal rhinosinusitis, characterized by the presence of fungal hyphae without microscopic tissue invasion in the sinus [1]. Although FBs were once thought to be rare, their prevalence has significantly increased over the last four decades. This increase is likely attributable to the more frequent use of radiological evaluations, such as computed tomography (CT) scans, along with improvements in fungal culture techniques and surgical treatments [2].
Galactomannan (GM), a component of the cell wall in Aspergillus species, is measured using an enzyme-linked immunosorbent assay (ELISA) test. The serum GM test can be used to detect acute invasive fungal rhinosinusitis early [3]. The (1,3)-β-D-glucan (G test) detects wall polysaccharides present in most fungi and can assist in diagnosing invasive fungal infections, including invasive fungal rhinosinusitis [4].
Studies have been conducted on the effectiveness of the G test in analyzing sinus mucus from patients with FBs and chronic rhinosinusitis (CRS) with nasal polyps [5], as well as in FB specimens collected during surgery [6]. However, to our knowledge, studies have examined the sera of patients with FBs and CRS using both the GM and G tests. Therefore, this study aimed to evaluate the utility of the serum GM and G tests in diagnosing FBs.
METHODS
Subjects
We conducted a retrospective review of the medical records of 105 patients who underwent endoscopic sinus surgery and serum GM and G tests from June 2020 to May 2021. This group included 42 patients with FBs and 63 with CRS. The diagnoses of FBs and CRS were confirmed through postoperative biopsy results. Patients with FBs were identified as those with histologically confirmed Aspergillus species in the maxillary sinus, specifically excluding cases of allergic fungal sinusitis and acute/chronic invasive aspergillosis. CRS patients were defined as those without nasal polyps and with no evidence of maxillary sinusitis persisting for more than 12 weeks.
We analyzed demographic characteristics including sex, age, body mass index (kg/m2), smoking history, alcohol consumption, and medical conditions such as hypertension, diabetes, and asthma. Nasal discomfort was assessed using the Sino-Nasal Outcome Test-22 (SNOT-22). Additionally, olfactory function was evaluated with the YSK olfactory function test, which includes three subtests: threshold (T), discrimination (D), and identification (I). Anosmia was diagnosed with a TDI score of ≤14.5, hyposmia with a TDI score >14.5 and <21.0, and normosmia with a TDI score of ≥21.0. This study received approval from the Institutional Review Board of Severance Hospital, Seoul, Korea (IRB No. 4-2024-0507), and the requirement for informed consent was waived.
Serum GM and G tests
Serum GM antigen levels were measured in all patients using the Platelia Aspergillus ELISA kit (Bio-Rad, Hercules, CA, USA). The G test was conducted with the Fungus (1,3)-β-Dglucan test (chromogenic method; Beijing Gold Mountainriver Tech Development Co. Ltd., Beijing, China). A positive result for the serum GM test was defined as a value of ≥0.5, and for the G test, a positive result was defined as ≥80 pg/mL.
Statistical analysis
We extracted data on true positives, true negatives, false positives, and false negatives. Using this data, we calculated sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs). The chi-square test was employed for statistical analysis between the FB and CRS groups. All statistical analyses were conducted using SPSS version 28 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p<0.05.
RESULTS
The study included 42 patients with FBs (10 men and 32 women) and 63 with CRS (27 men and 36 women). There were no significant differences in the incidence of hypertension, diabetes mellitus, asthma, or allergic rhinitis between the two groups. However, the CRS group exhibited significantly higher SNOT-22 scores, averaging 26.0±20.2, compared to 17.3±15.9 in the FB group (p=0.023). In contrast, the FB group demonstrated a significantly higher olfactory function score, averaging 22.0±4.4, versus 18.9±6.7 in the CRS group (p=0.005). Additionally, the incidence of anosmia was greater in the CRS group at 23.8%, compared to 4.8% in the FB group (p=0.031).
However, there were no significant differences in the positivity rate between the two groups for either the serum GM test (7.1% for the FB group vs. 3.2% for the CRS group, respectively; p=0.640), or the G test (9.5% for the FB group vs. 11.1% for the CRS group, respectively; p=0.482) (Table 1). The serum GM test showed low sensitivity (7.1%) but high specificity (96.8%). The PPV was 60.0%, and the NPV was 61.0%. The serum G test results were similar, with a sensitivity of 9.5%, specificity of 88.9%, PPV of 36.3%, and NPV of 59.6% (Fig. 1).
Table 1.
Demographic data of the study population and positivity rate of serum galactomannan and β-D-glucan
| Fungus ball (n=42) | Chronic rhinosinusitis (n=63) | p | |
|---|---|---|---|
| Age (yr) | 64.0±12.2 | 60.8±11.8 | 0.177 |
| Sex | 0.073 | ||
| Male | 10 (23.8) | 27 (42.9) | |
| Female | 32 (76.2) | 36 (57.1) | |
| BMI (kg/m2) | 23.8±2.6 | 23.8±3.2 | 0.955 |
| HTN | 14 (33.3) | 26 (41.3) | 0.538 |
| DM | 8 (19.0) | 8 (19.0) | >0.999 |
| Asthma | 1 (2.4) | 4 (6.3) | 0.640 |
| Allergic rhinitis | 16 (38.1) | 29 (46.0) | 0.546 |
| Smoker | 3 (7.1) | 9 (14.3) | 0.416 |
| Alcohol consumption | 5 (11.9) | 15 (23.8) | 0.205 |
| SNOT-22 | 17.3±15.9 | 26.0±20.2 | 0.023* |
| Olfactory function test | 22.0±4.4 | 18.9±6.7 | 0.005* |
| Olfactory classification | 0.031* | ||
| Normosmia | 26 (61.9) | 29 (46.0) | |
| Hyposmia | 14 (33.3) | 19 (30.2) | |
| Anosmia | 2 (4.8) | 15 (23.8) | |
| Serum galactomannan | 0.640 | ||
| Positive | 3 (7.1) | 2 (3.2) | |
| Negative | 39 (92.9) | 61 (96.8) | |
| Serum β-D-glucan | 0.482 | ||
| Positive | 4 (9.5) | 7 (11.1) | |
| Negative | 38 (90.5) | 56 (88.9) |
Values are presented as mean±standard deviation or n (%).
statistical significance (p<0.05).
BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; SNOT-22, Sinonasal Outcome Test
Fig. 1.
The diagnostic performance of the serum galactomannan and β-D-glucan test for paranasal sinus fungus balls. PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
Our study revealed no significant differences in serum GM and G test results between FB and CRS patients. Additionally, these tests exhibited low sensitivity and PPV, underscoring their unsuitability for diagnosing FBs. In line with the findings of Kostamo et al. [5] regarding GM tests in FB patient mucus, our data also indicate that GM is unreliable for diagnosing FBs.
Paranasal sinus FBs are the most prevalent form of noninvasive fungal rhinosinusitis and are responsible for recurrent sinusitis in approximately 3.7% of CRS patients [1]. Its incidence has been on the rise worldwide, including in Korea [7]. The exact mechanisms behind FB formation are not well understood, but potential contributing factors include environmental influences, changes in sinus airflow resistance, and variations in nasal cavity anatomy [8]. The maxillary sinus, which is often affected by FBs, is commonly associated with dental procedures such as implant surgery. One study indicated that between 65% and 86.7% of FB patients had undergone previous dental treatments [9]. This link underscores the importance of meticulous implant placement in the maxillary sinus floor, as dental implants may pose a risk factor for FB development, a finding corroborated by other recent studies [10].
The GM test serves as a valuable initial screening tool for diagnosing acute invasive fungal sinusitis, which is characterized by elevated serum GM levels and high clinical suspicion [11]. In the study conducted by Melancon et al. [11], the GM test demonstrated a sensitivity of 44.8%, a specificity of 100%, a PPV of 100%, and an NPV of 36% for diagnosing acute invasive fungal sinusitis. Despite its relatively low sensitivity, the presence of acute invasive fungal sinusitis is confirmed when the GM test is positive and clinical suspicion is high. Conversely, the serum GM test is deemed ineffective for diagnosing FBs, as evidenced by the low sensitivity (7.1%) and PPV (60.0%) observed in our study. Invasive fungal sinusitis is characterized by the infiltration of surrounding tissues. In contrast, FBs involve the formation of dense clusters of fungal hyphae that do not invade the mucosa, blood vessels, or bones [2]. Therefore, unless there is an invasion into surrounding blood vessels, it is improbable that cell wall components of Aspergillus species will be detected in the serum. Consistent with this understanding, our study reported a low positivity rate for both GM and G tests in patients with FBs. Similarly, a study on pulmonary FBs indicated that the sensitivity of the GM test increased with the presence of hemoptysis [12], further underscoring the association between the test and blood vessel involvement.
The GM test sometimes shows false-positive results, which seem to be linked to the use of beta-lactam antibiotics. Components of these beta-lactam antibiotics, being semisynthetic drugs derived from fungi or higher bacteria, may exhibit cross-reactivity with Aspergillus GM molecules [13]. Consequently, varying concentrations of these cross-reactive GM molecules in different moieties of beta-lactam antibiotics can lead to positive results in the GM test
A limitation of our study is that we did not investigate dental treatment in CRS patients, which is a potential risk factor for FBs. However, our histopathological approach to distinguishing between FBs and CRS reduces the likelihood of overlooking FBs in the CRS group. Beta-lactam antibiotics can influence GM levels, which may lead to false positives [13]. Nonetheless, this is unlikely to significantly affect the overall sensitivity of the GM test in our study. Another limitation is that when the GM test yielded positive results in patients with FBs, we could not confirm the presence of infections in other areas. We plan to address this by conducting additional tests on the patient.
Despite these limitations, this is the first study to evaluate the diagnostic utility of the serum GM and G tests for FBs. Our findings confirm that the GM and G tests are not useful for diagnosing FBs, as indicated by their sensitivity, specificity, PPV, and NPV. Additionally, we observed a low probability of fungal invasion into the blood vessels of paranasal sinus FBs. This observation is further supported by the higher presence of fungal components in pulmonary FBs with hemoptysis, which may be attributed to anatomical differences between the paranasal sinuses and the lungs.
In conclusion, the serum GM and G tests for FBs demonstrate limited clinical diagnostic value. Although these tests exhibit high specificity, their low NPV indicates that a negative result does not exclude the presence of FBs. Consequently, clinicians should base their diagnosis of FBs on a comprehensive assessment that includes clinical symptoms, imaging studies such as CT scans, and, ultimately, histopathological examination during surgery.
Acknowledgments
None
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Suk Won Chang, Hyung-Ju Cho. Data curation: Suk Won Chang, Yeonsu Jeong, Ju Wan Kang. Formal analysis: Suk Won Chang, Yeonsu Jeong, Ju Wan Kang. Funding acquisition: Hyung-Ju Cho. Methodology: Yeonsu Jeong, Chang-Hoon Kim. Project administration: Suk Won Chang, Ju Wan Kang. Visualization: Suk Won Chang, Yeonsu Jeong. Writing—original draft: Suk Won Chang. Writing—review & editing: Chang-Hoon Kim, Hyung-Ju Cho.
Funding Statement
This study was supported by the “Team Science Award” of Yonsei University College of Medicine (6-2021-0005).
References
- 1.Kim DW, Kim YM, Min JY, Kim JW, Kim JK, Mo JH, et al. Clinicopathologic characteristics of paranasal sinus fungus ball: retrospective, multicenter study in Korea. Eur Arch Otorhinolaryngol. 2020;277(3):761–5. doi: 10.1007/s00405-019-05738-5. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Liu C, Wei H, He S, Dong S, Zhou B, et al. A retrospective analysis of 1,717 paranasal sinus fungus ball cases from 2008 to 2017. Laryngoscope. 2020;130(1):75–9. doi: 10.1002/lary.27869. [DOI] [PubMed] [Google Scholar]
- 3.Cho HJ, Hong SD, Kim HY, Chung SK, Dhong HJ. Clinical implications of serum galactomannan measurement in patients with acute invasive fungal rhinosinusitis. Rhinology. 2016;54(4):336–41. doi: 10.4193/Rhino15.186. [DOI] [PubMed] [Google Scholar]
- 4.Theel ES, Doern CD. β-D-glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol. 2013;51(11):3478–83. doi: 10.1128/JCM.01737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostamo K, Richardson M, Eerola E, Rantakokko-Jalava K, Meri T, Malmberg H, et al. Negative impact of Aspergillus galactomannan and DNA detection in the diagnosis of fungal rhinosinusitis. J Med Microbiol. 2007;56(Pt 10):1322–7. doi: 10.1099/jmm.0.47101-0. [DOI] [PubMed] [Google Scholar]
- 6.Kauffmann-Lacroix C, Rodier MH, Jacquemin JL, Goujon JM, Klossek JM. Detection of galactomannan for diagnosis of fungal rhinosinusitis. J Clin Microbiol. 2001;39(12):4593–4. doi: 10.1128/JCM.39.12.4593-4594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HW, Kang SH, Jang KH, Kim DS, Shin SH, Ye MK. [Changes in etiologies and clinical characteristics of operated unilateral sinus diseases: comparison study between 2005 and 2015] J Rhinol. 2017;24(1):26–30. Korean. [Google Scholar]
- 8.Klossek JM, Serrano E, Péloquin L, Percodani J, Fontanel JP, Pessey JJ. Functional endoscopic sinus surgery and 109 mycetomas of paranasal sinuses. Laryngoscope. 1997;107(1):112–7. doi: 10.1097/00005537-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Ledderose GJ, Braun T, Betz CS, Stelter K, Leunig A. Functional endoscopic surgery of paranasal fungus ball: clinical outcome, patient benefit and health-related quality of life. Eur Arch Otorhinolaryngol. 2012;269(10):2203–8. doi: 10.1007/s00405-012-1925-7. [DOI] [PubMed] [Google Scholar]
- 10.Han SA, Kim S, Seo Y, Yang SK, Rhee CS, Han DH. Dental implant as a potential risk factor for maxillary sinus fungus ball. Sci Rep. 2024;14(1):2483. doi: 10.1038/s41598-024-52661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melancon CC, Lindsey J, Russell GB, Clinger JD. The role of galactomannan Aspergillus antigen in diagnosing acute invasive fungal sinusitis. Int Forum Allergy Rhinol. 2019;9(1):60–6. doi: 10.1002/alr.22225. [DOI] [PubMed] [Google Scholar]
- 12.Park SY, Lee SO, Choi SH, Jeong JY, Sung H, Kim MN, et al. Serum and bronchoalveolar lavage fluid galactomannan assays in patients with pulmonary aspergilloma. Clin Infect Dis. 2011;52(7):e149–52. doi: 10.1093/cid/cir027. [DOI] [PubMed] [Google Scholar]
- 13.Boonsarngsuk V, Niyompattama A, Teosirimongkol C, Sriwanichrak K. False-positive serum and bronchoalveolar lavage Aspergillus galactomannan assays caused by different antibiotics. Scand J Infect Dis. 2010;42(6-7):461–8. doi: 10.3109/00365541003602064. [DOI] [PubMed] [Google Scholar]