Abstract
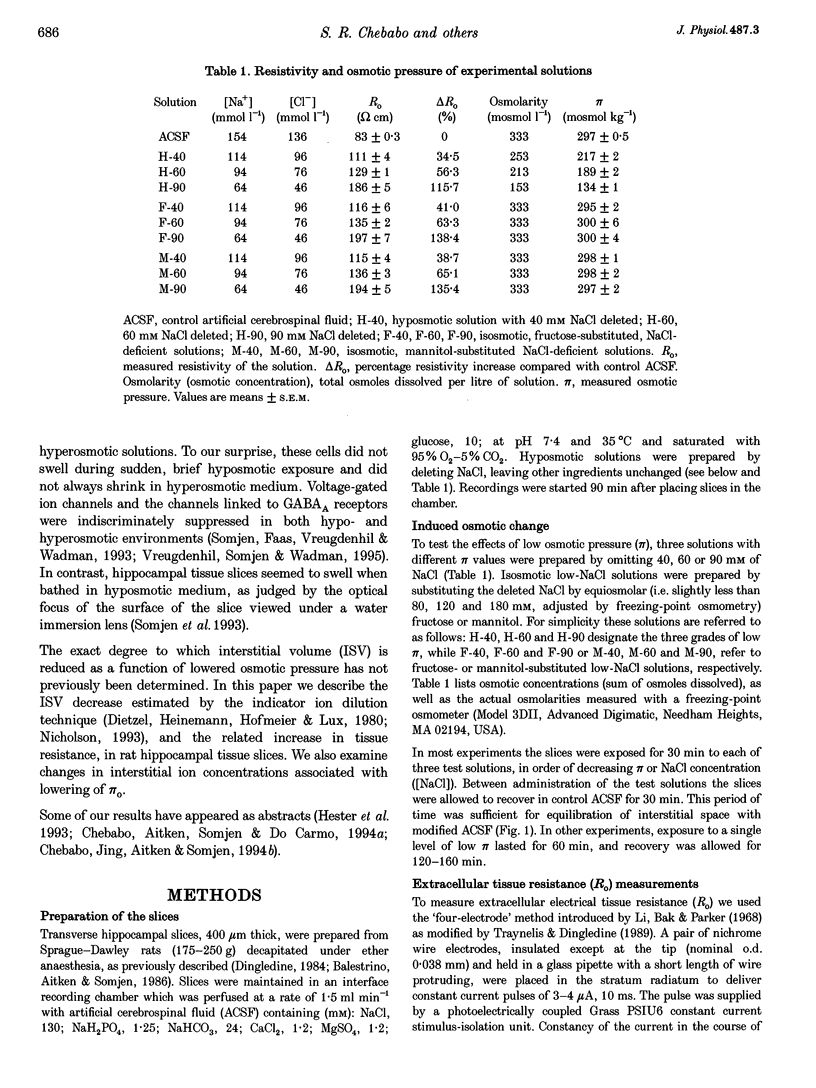
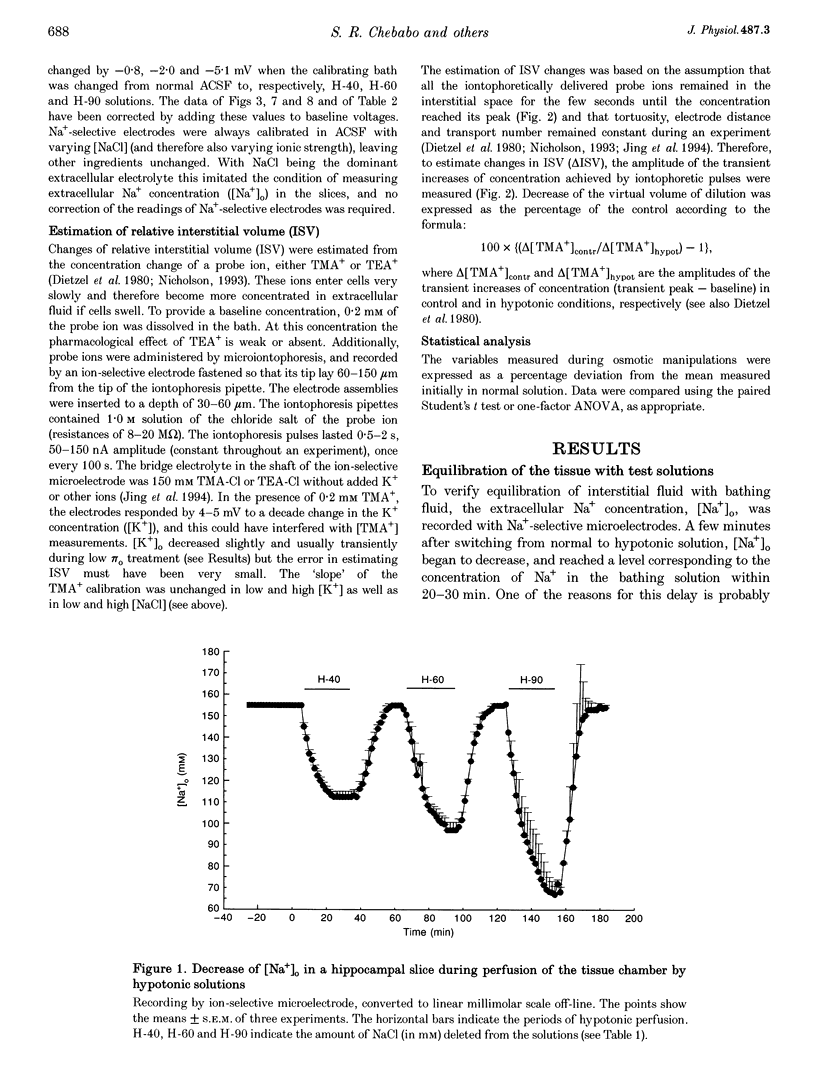
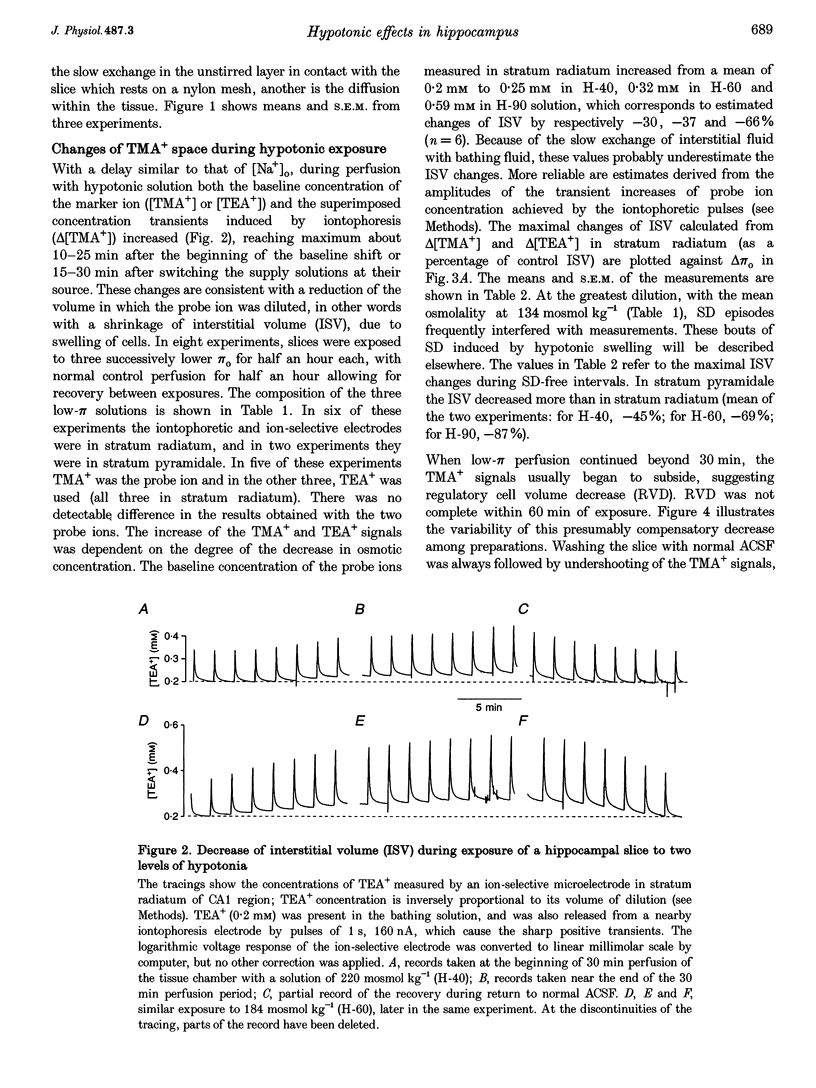
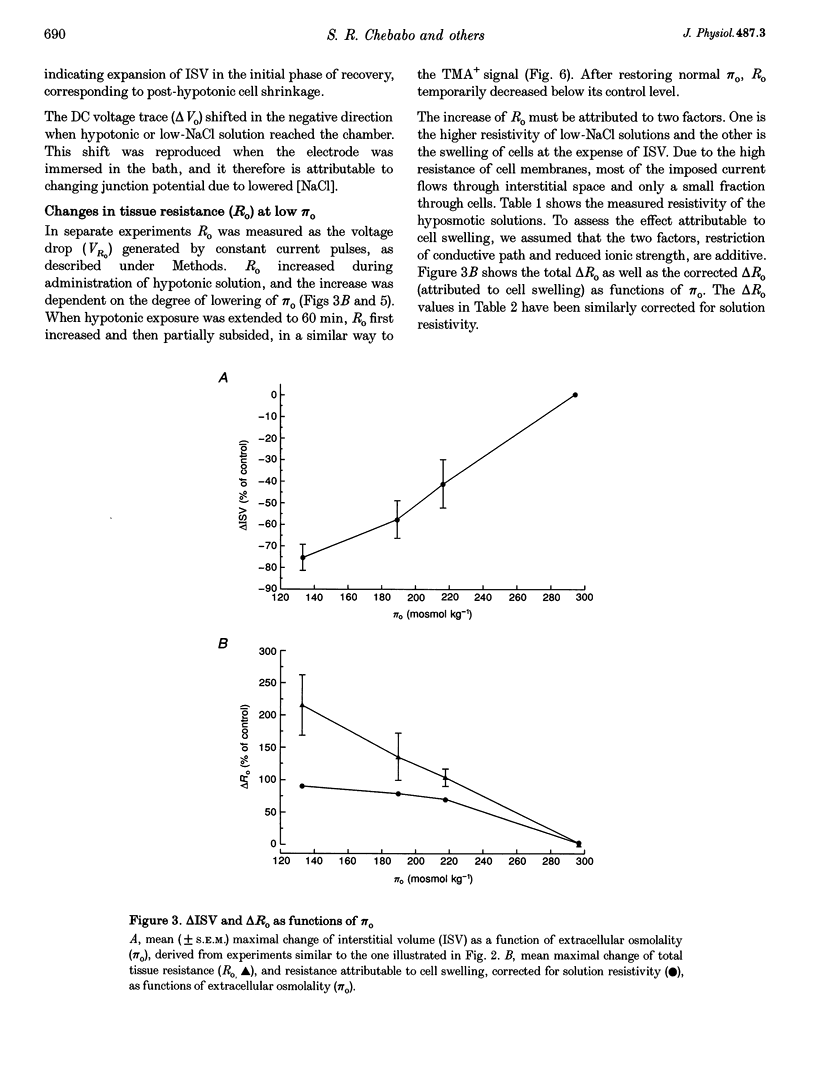
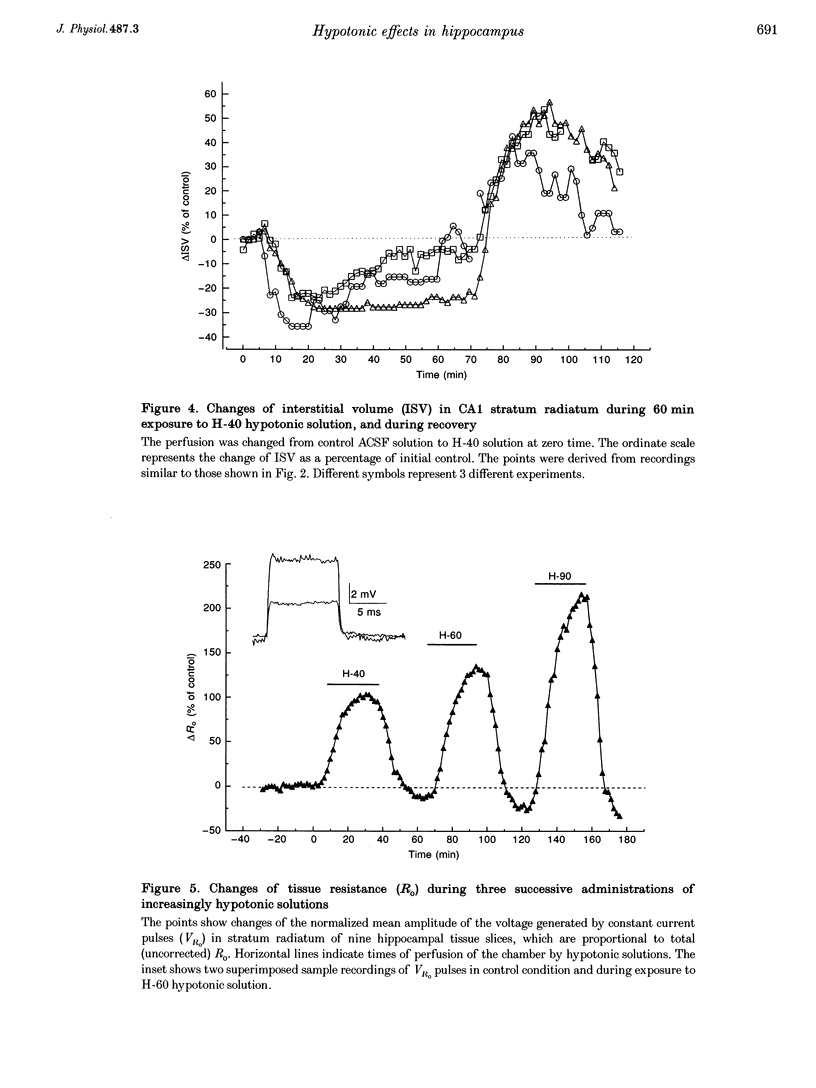
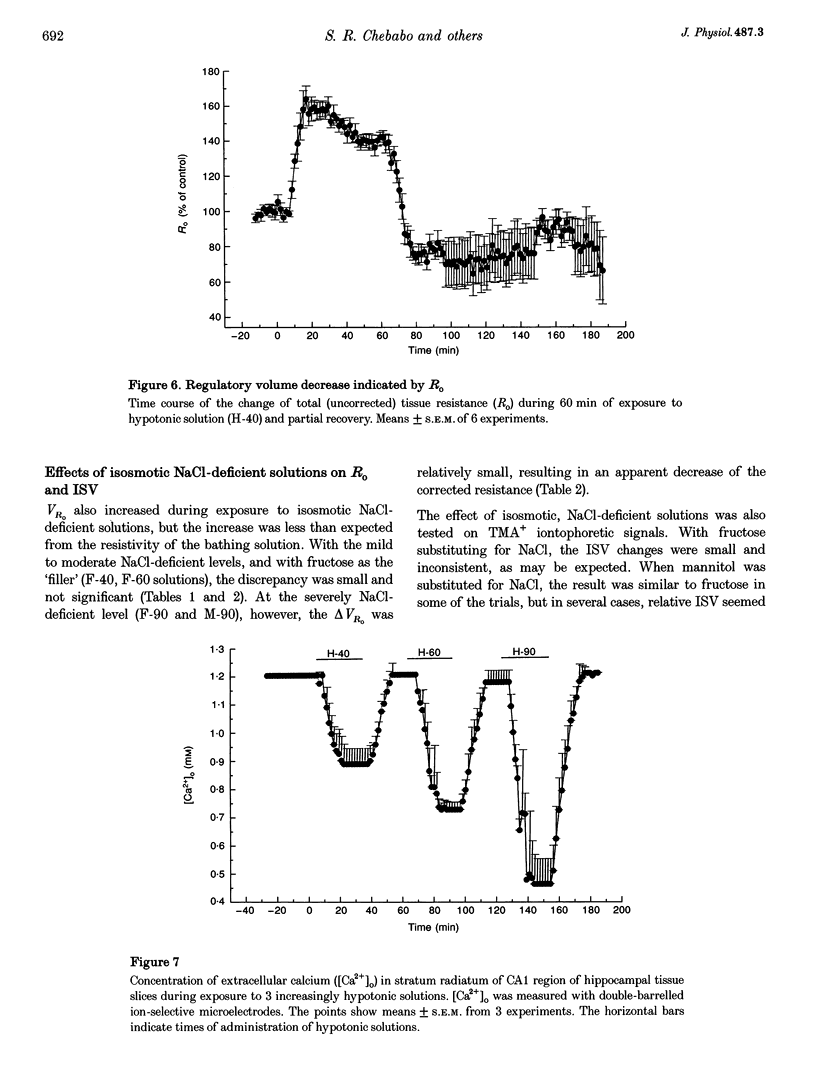
1. The degree to which mammalian brain cells swell in hypotonic environments has not previously been determined. We exposed hippocampal tissue slices prepared from anaesthetized rats to artificial cerebrospinal fluid from which varying amounts of NaCl had been deleted. Interstitial volume (ISV) change was determined from the volume of dilution of the marker ions tetramethylammonium (TMA+) or tetraethylammonium (TEA+). Tissue electrical resistance was measured as the voltage generated by constant current pulses. 2. ISV decreased as a function of lowered extracellular osmolality (osmotic pressure, pi o), indicating cell swelling. After reaching a minimum, ISV recovered partially, suggesting regulatory volume decrease of cells. After restoring normal pi o the ISV expanded, indicating post-hypotonic cell shrinkage. The electrical resistance of the tissue (Ro) increased when pi o was lowered, due to the reduced ionic strength, as well as restricted ISV. 3. To control for low NaCl concentration, reduced NaCl was replaced by mannitol or fructose. In isosmotic, NaCl-deficient solution, ISV showed inconsistent change, and Ro corrected for ionic strength tended to decrease. 4. Extracellular K+ concentration decreased slightly in low pi o except when spreading depression caused it to increase. Extracellular Ca2+ concentration decreased substantially, consistently and reversibly. Administration of isosmotic low-NaCl concentration solutions caused a similar decrease in extracellular Ca2+ concentrations. We propose that low Na+ concentration in extracellular fluid impaired the extrusion of Ca2+. 5. In severely hypotonic solution, ISV was reduced to 25% of its control volume, corresponding to a mean cell volume increase of at least 11%, probably more. From plotting relative changes in ISV against osmolarity we concluded that, within the range tested, hypotonic cell swelling was not opposed by the close approach of plasma membranes of neighbouring cells.
Full text
PDF

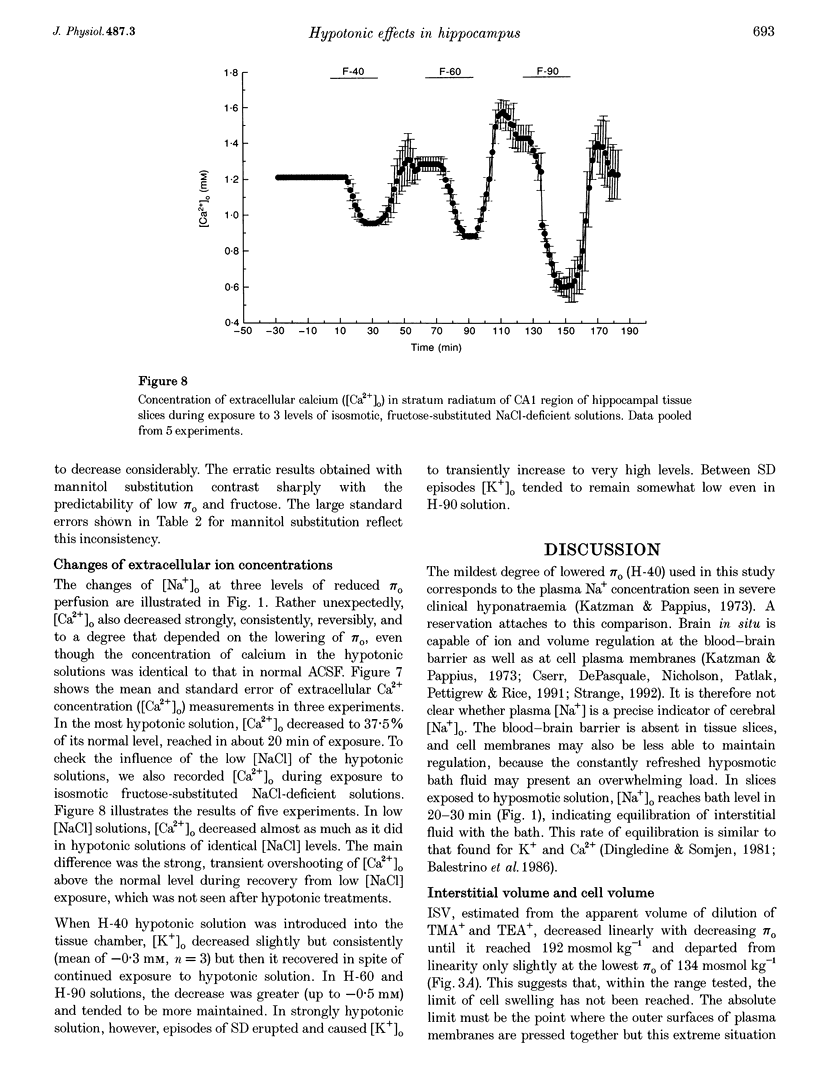




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew R. D., MacVicar B. A. Imaging cell volume changes and neuronal excitation in the hippocampal slice. Neuroscience. 1994 Sep;62(2):371–383. doi: 10.1016/0306-4522(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Andrew R. D. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci. 1991 Jan;101(1):7–18. doi: 10.1016/0022-510x(91)90013-w. [DOI] [PubMed] [Google Scholar]
- Balestrino M., Aitken P. G., Somjen G. G. The effects of moderate changes of extracellular K+ and Ca2+ on synaptic and neural function in the CA1 region of the hippocampal slice. Brain Res. 1986 Jul 9;377(2):229–239. doi: 10.1016/0006-8993(86)90863-2. [DOI] [PubMed] [Google Scholar]
- Ballyk B. A., Quackenbush S. J., Andrew R. D. Osmotic effects on the CA1 neuronal population in hippocampal slices with special reference to glucose. J Neurophysiol. 1991 May;65(5):1055–1066. doi: 10.1152/jn.1991.65.5.1055. [DOI] [PubMed] [Google Scholar]
- Bowman C. L., Ding J. P., Sachs F., Sokabe M. Mechanotransducing ion channels in astrocytes. Brain Res. 1992 Jul 3;584(1-2):272–286. doi: 10.1016/0006-8993(92)90906-p. [DOI] [PubMed] [Google Scholar]
- Cserr H. F., DePasquale M., Nicholson C., Patlak C. S., Pettigrew K. D., Rice M. E. Extracellular volume decreases while cell volume is maintained by ion uptake in rat brain during acute hypernatremia. J Physiol. 1991 Oct;442:277–295. doi: 10.1113/jphysiol.1991.sp018793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I., Heinemann U., Hofmeier G., Lux H. D. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res. 1980;40(4):432–439. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Somjen G. Calcium dependence of synaptic transmission in the hippocampal slice. Brain Res. 1981 Feb 23;207(1):218–222. doi: 10.1016/0006-8993(81)90697-1. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Obenaus A., Tasker J. G. Osmolality-induced changes in extracellular volume alter epileptiform bursts independent of chemical synapses in the rat: importance of non-synaptic mechanisms in hippocampal epileptogenesis. Neurosci Lett. 1990 Dec 11;120(2):267–270. doi: 10.1016/0304-3940(90)90056-f. [DOI] [PubMed] [Google Scholar]
- Gullans S. R., Verbalis J. G. Control of brain volume during hyperosmolar and hypoosmolar conditions. Annu Rev Med. 1993;44:289–301. doi: 10.1146/annurev.me.44.020193.001445. [DOI] [PubMed] [Google Scholar]
- Hansen A. J., Olsen C. E. Brain extracellular space during spreading depression and ischemia. Acta Physiol Scand. 1980 Apr;108(4):355–365. doi: 10.1111/j.1748-1716.1980.tb06544.x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Electrical conductivity of the hippocampal CA1 layers and application to current-source-density analysis. Exp Brain Res. 1987;67(2):402–410. doi: 10.1007/BF00248560. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G., Haas H. L. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982 Dec 2;300(5891):448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- Jing J., Aitken P. G., Somjen G. G. Interstitial volume changes during spreading depression (SD) and SD-like hypoxic depolarization in hippocampal tissue slices. J Neurophysiol. 1994 Jun;71(6):2548–2551. doi: 10.1152/jn.1994.71.6.2548. [DOI] [PubMed] [Google Scholar]
- Kempski O., von Rosen S., Weigt H., Staub F., Peters J., Baethmann A. Glial ion transport and volume control. Ann N Y Acad Sci. 1991;633:306–317. doi: 10.1111/j.1749-6632.1991.tb15622.x. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Sankar P., O'Connor E. R., Jalonen T., Goderie S. K. Functional consequences of astrocytic swelling. Prog Brain Res. 1992;94:57–68. doi: 10.1016/s0079-6123(08)61739-2. [DOI] [PubMed] [Google Scholar]
- Li C. L., Bak A. F., Parker L. O. Specific resistivity of the cerebral cortex and white matter. Exp Neurol. 1968 Apr;20(4):544–557. doi: 10.1016/0014-4886(68)90108-8. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Traynelis S. F., Dingledine R. Regional variation of extracellular space in the hippocampus. Science. 1990 Aug 10;249(4969):674–677. doi: 10.1126/science.2382142. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991 Mar 8;251(4998):1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Comparative neurophysiology of spreading depression in the cerebellum. An Acad Bras Cienc. 1984 Dec;56(4):481–494. [PubMed] [Google Scholar]
- Nicholson C. Ion-selective microelectrodes and diffusion measurements as tools to explore the brain cell microenvironment. J Neurosci Methods. 1993 Jul;48(3):199–213. doi: 10.1016/0165-0270(93)90092-6. [DOI] [PubMed] [Google Scholar]
- Okada Y. C., Huang J. C., Rice M. E., Tranchina D., Nicholson C. Origin of the apparent tissue conductivity in the molecular and granular layers of the in vitro turtle cerebellum and the interpretation of current source-density analysis. J Neurophysiol. 1994 Aug;72(2):742–753. doi: 10.1152/jn.1994.72.2.742. [DOI] [PubMed] [Google Scholar]
- RANCK J. B., Jr Analysis of specific impedance of rabbit cerebral cortex. Exp Neurol. 1963 Feb;7:153–174. doi: 10.1016/s0014-4886(63)80006-0. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985 Jun;5(6):1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Somjen G. G., Faas G. C., Vreugdenhil M., Wadman W. J. Channel shutdown: a response of hippocampal neurons to adverse environments. Brain Res. 1993 Dec 31;632(1-2):180–194. doi: 10.1016/0006-8993(93)91153-j. [DOI] [PubMed] [Google Scholar]
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992 Jul;3(1):12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]
- Traynelis S. F., Dingledine R. Modification of potassium-induced interictal bursts and electrographic seizures by divalent cations. Neurosci Lett. 1989 Mar 27;98(2):194–199. doi: 10.1016/0304-3940(89)90509-0. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Khattab F. I. Changes in cortical extracellular space during spreading depression investigated with the electron microscope. J Neurophysiol. 1967 Jul;30(4):911–929. doi: 10.1152/jn.1967.30.4.911. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M., Somjen G. G., Wadman W. J. Effects of strongly anisosmotic and NaCl deficient solutions on muscimol- and glutamate evoked whole-cell currents in freshly dissociated hippocampal neurons. Brain Res. 1995 Jan 23;670(1):89–96. doi: 10.1016/0006-8993(94)01283-n. [DOI] [PubMed] [Google Scholar]


