Abstract
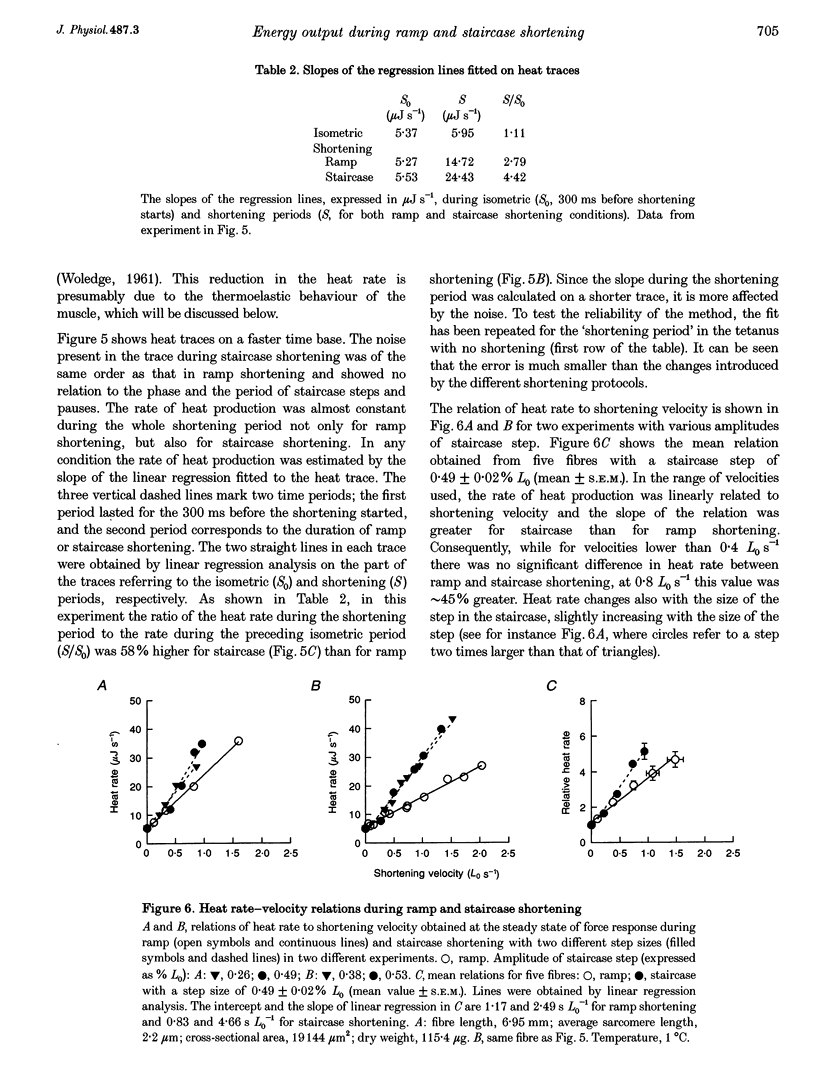
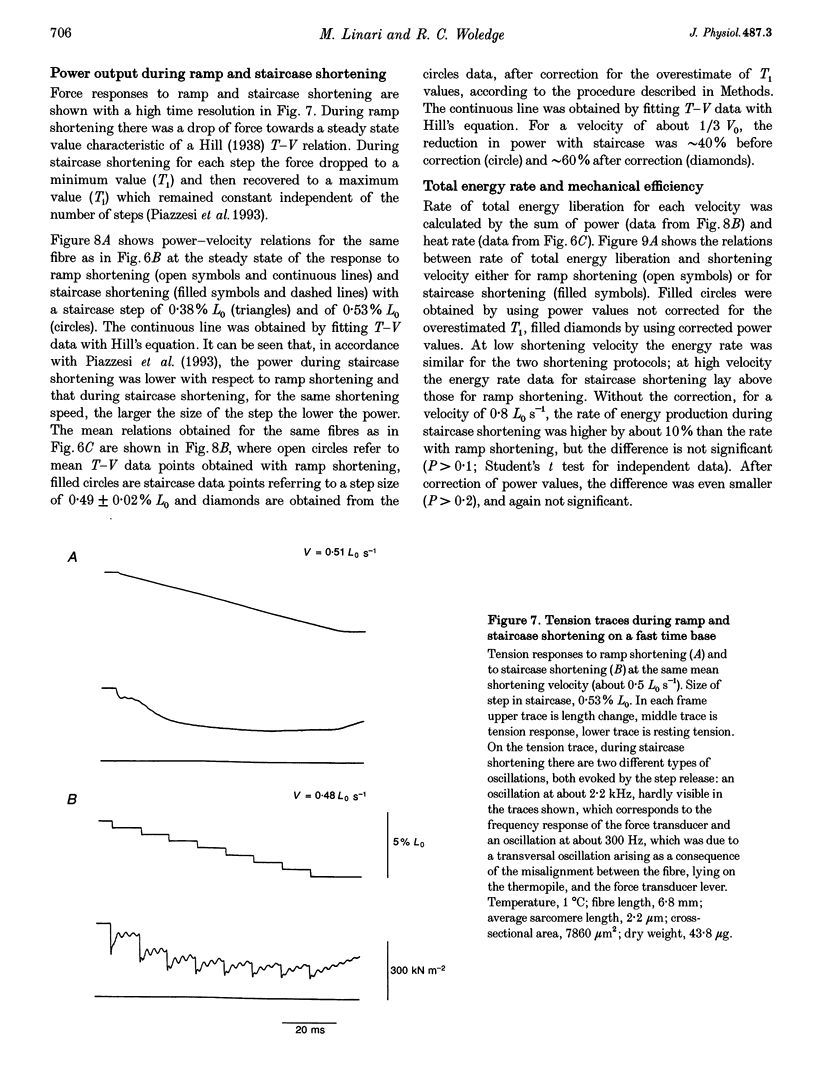
1. We compared the rates of work and heat production during ramp shortening with those during staircase shortening (sequence of step releases of the same amplitude, separated by regular time intervals). Ramp or staircase shortening was applied to isolated muscle fibres (sarcomere length, 2.2 microns; temperature, approximately 1 degree C) at the plateau of an isometric tetanus. The total amount of shortening was no greater than 6% of the fibre length. 2. During ramp shortening the power output showed a maximum at about 0.8 fibre lengths per second (Lo s-1), which corresponds to 1/3 the maximum shortening velocity (Vo). For the same average shortening velocity during staircase shortening (step size, approximately 0.5% Lo) the power output was 40-60% lower. The rate of heat production for the same average shortening velocity was approximately 45% higher during staircase shortening than during ramp shortening. 3. The relation between rate of total energy output and shortening velocity was well described by a second order regression line in the range of velocities used (0.1-2.3 Lo s-1). For any shortening velocity the rate of total energy output (power plus heat rate) was not statistically different for staircase (step size, approximately 0.5% Lo) and ramp shortening. 4. The mechanical efficiency (the ratio of the power over the total energy rate) during ramp shortening had a maximum value of 0.36 at 1/5 Vo; during staircase shortening, for any given shortening velocity, the mechanical efficiency was reduced compared with ramp shortening: with a staircase step of about 0.5% Lo at 1/5 Vo the efficiency was approximately 0.2. 5. The results indicate that a cross-bridge is able to convert different quantities of energy into work depending on the different shortening protocol used. The fraction of energy dissipated as heat is larger during staircase shortening than during ramp shortening.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrogi-Lorenzini C., Colomo F., Lombardi V. Development of force-velocity relation, stiffness and isometric tension in frog single muscle fibres. J Muscle Res Cell Motil. 1983 Apr;4(2):177–189. doi: 10.1007/BF00712029. [DOI] [PubMed] [Google Scholar]
- Barclay C. J., Constable J. K., Gibbs C. L. Energetics of fast- and slow-twitch muscles of the mouse. J Physiol. 1993 Dec;472:61–80. doi: 10.1113/jphysiol.1993.sp019937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Colomo F., Lombardi V., Piazzesi G. Stiffness of frog muscle fibres during rise of tension and relaxation in fixed-end or length-clamped tetani. Pflugers Arch. 1987 Jun;409(1-2):39–46. doi: 10.1007/BF00584747. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Rall J. A., Wilson M. G., Woledge R. C. Simultaneous heat and tension measurements from single muscle cells. Adv Exp Med Biol. 1984;170:887–899. doi: 10.1007/978-1-4684-4703-3_87. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Howarth J. V., Woledge R. C. Heat production by single fibres of frog muscle. J Muscle Res Cell Motil. 1983 Apr;4(2):207–222. doi: 10.1007/BF00712031. [DOI] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Active and rigor muscle stiffness [proceedings]. J Physiol. 1977 Jul;269(1):55P–57P. [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Rapid 'give' and the tension 'shoulder' in the relaxation of frog muscle fibres. J Physiol. 1970 Sep;210(1):32P–33P. [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Doi T., Sakurada K., Yanagida T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature. 1991 Jul 25;352(6333):301–306. doi: 10.1038/352301a0. [DOI] [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. A new method for absolute heat measurement, utilizing the Peltier effect. J Physiol. 1972 Jul;224(1):18P–21P. [PubMed] [Google Scholar]
- Kretzschmar K. M., Wilkie D. R. The use of the Peltier effect for simple and accurate calibration of thermoelectric devices. Proc R Soc Lond B Biol Sci. 1975 Aug 19;190(1100):315–321. doi: 10.1098/rspb.1975.0095. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Lombardi V., Piazzesi G., Linari M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature. 1992 Feb 13;355(6361):638–641. doi: 10.1038/355638a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Mulieri L. A., Luhr G., Trefry J., Alpert N. R. Metal-film thermopiles for use with rabbit right ventricular papillary muscles. Am J Physiol. 1977 Nov;233(5):C146–C156. doi: 10.1152/ajpcell.1977.233.5.C146. [DOI] [PubMed] [Google Scholar]
- WOLEDGE R. C. The thermoelastic effect of change of tension in active muscle. J Physiol. 1961 Jan;155:187–208. doi: 10.1113/jphysiol.1961.sp006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge R. C., Reilly P. J. Molar enthalpy change for hydrolysis of phosphorylcreatine under conditions in muscle cells. Biophys J. 1988 Jul;54(1):97–104. doi: 10.1016/S0006-3495(88)82934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge R. C., Wilson M. G., Howarth J. V., Elzinga G., Kometani K. The energetics of work and heat production by single muscle fibres from the frog. Adv Exp Med Biol. 1988;226:677–688. [PubMed] [Google Scholar]
- Yanagida T., Arata T., Oosawa F. Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature. 1985 Jul 25;316(6026):366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]


