Abstract
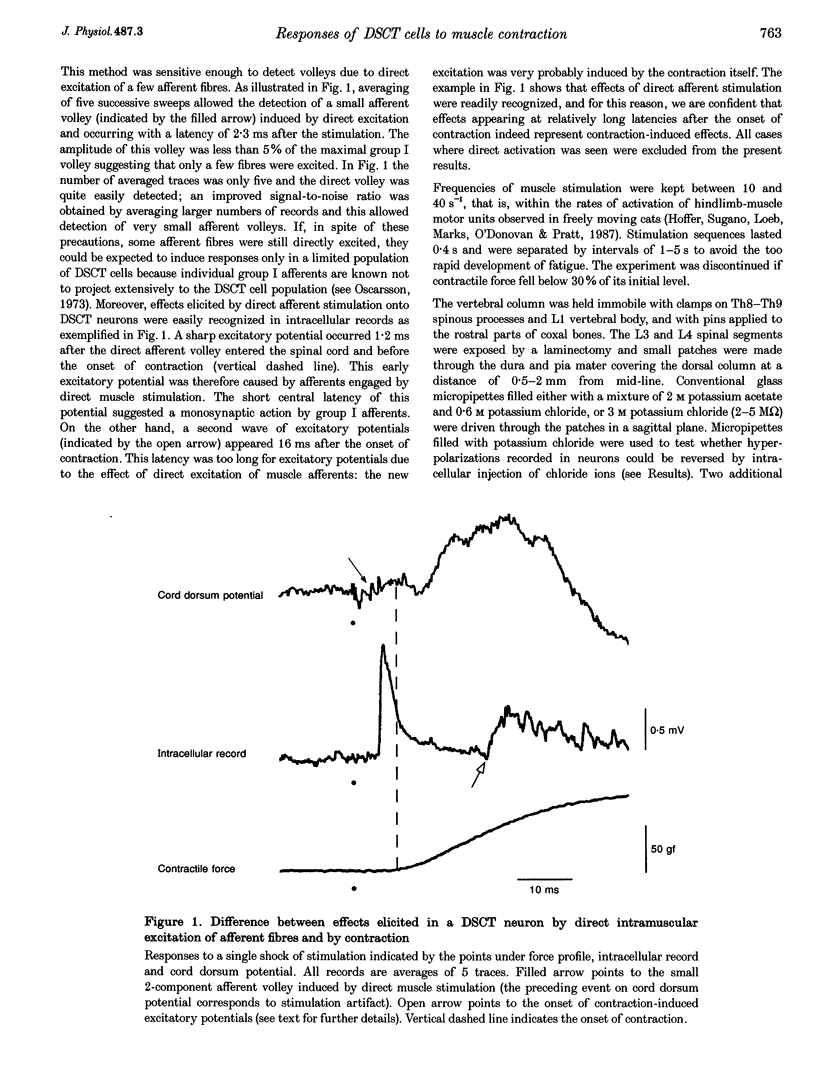
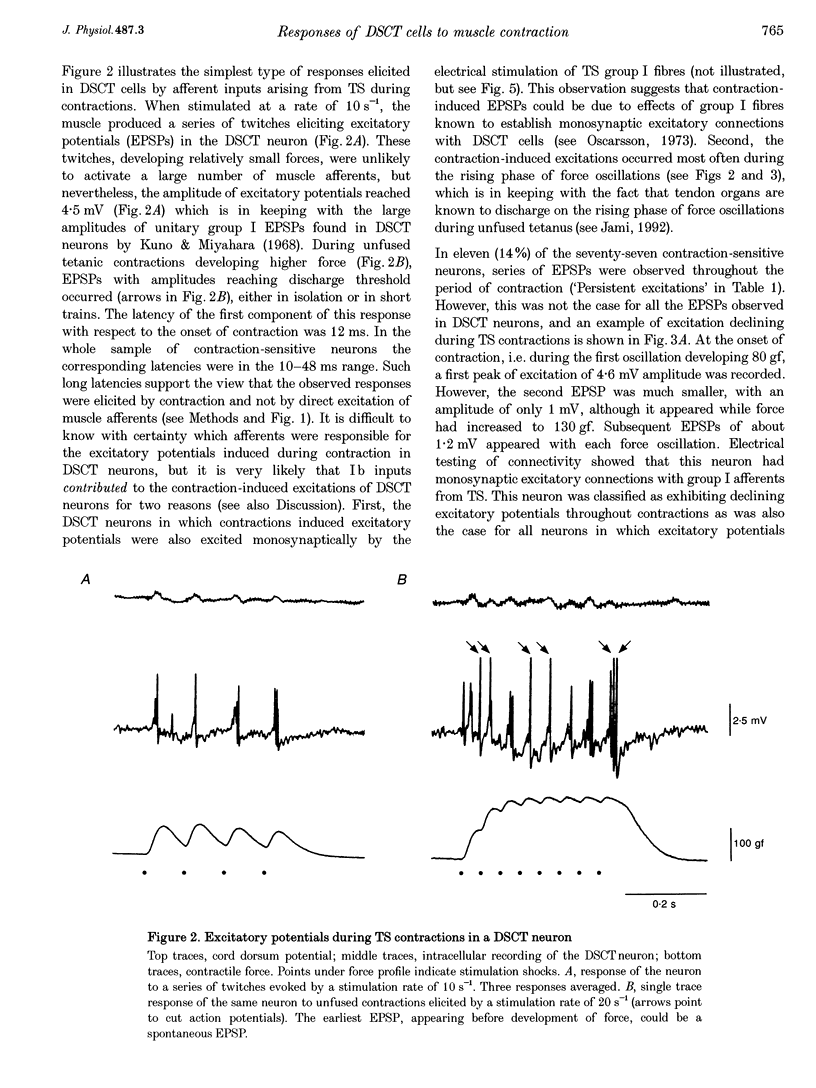
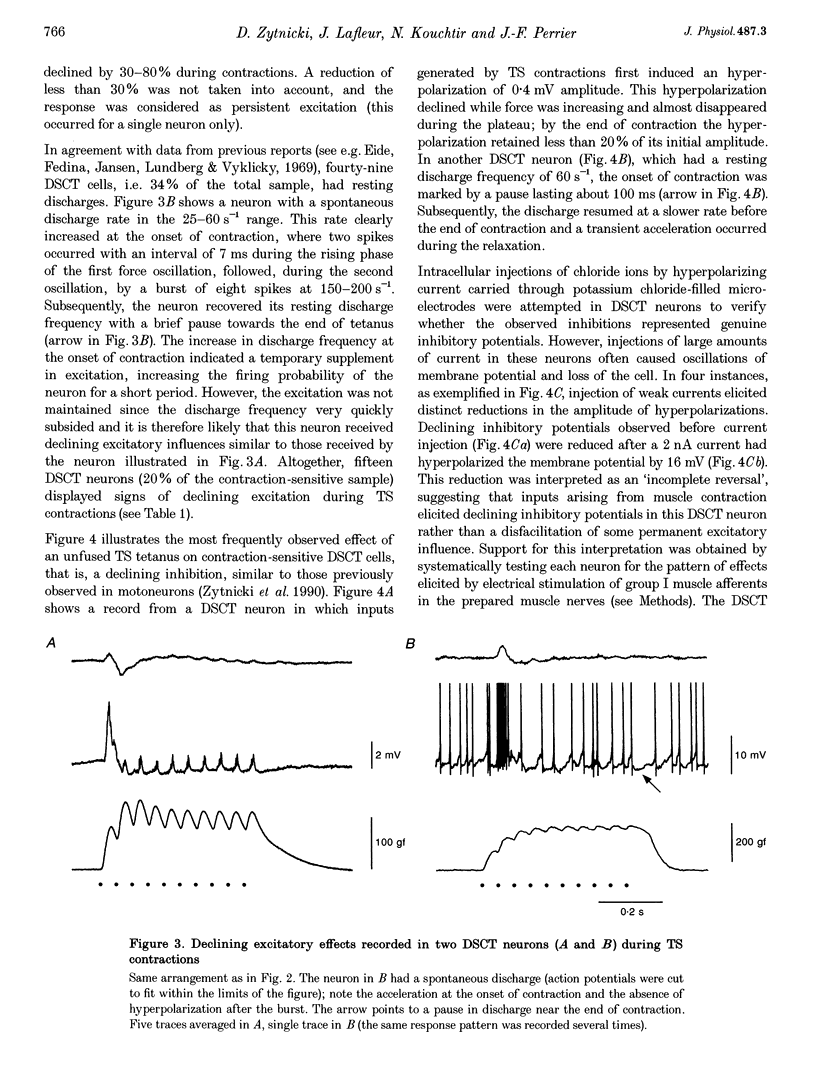
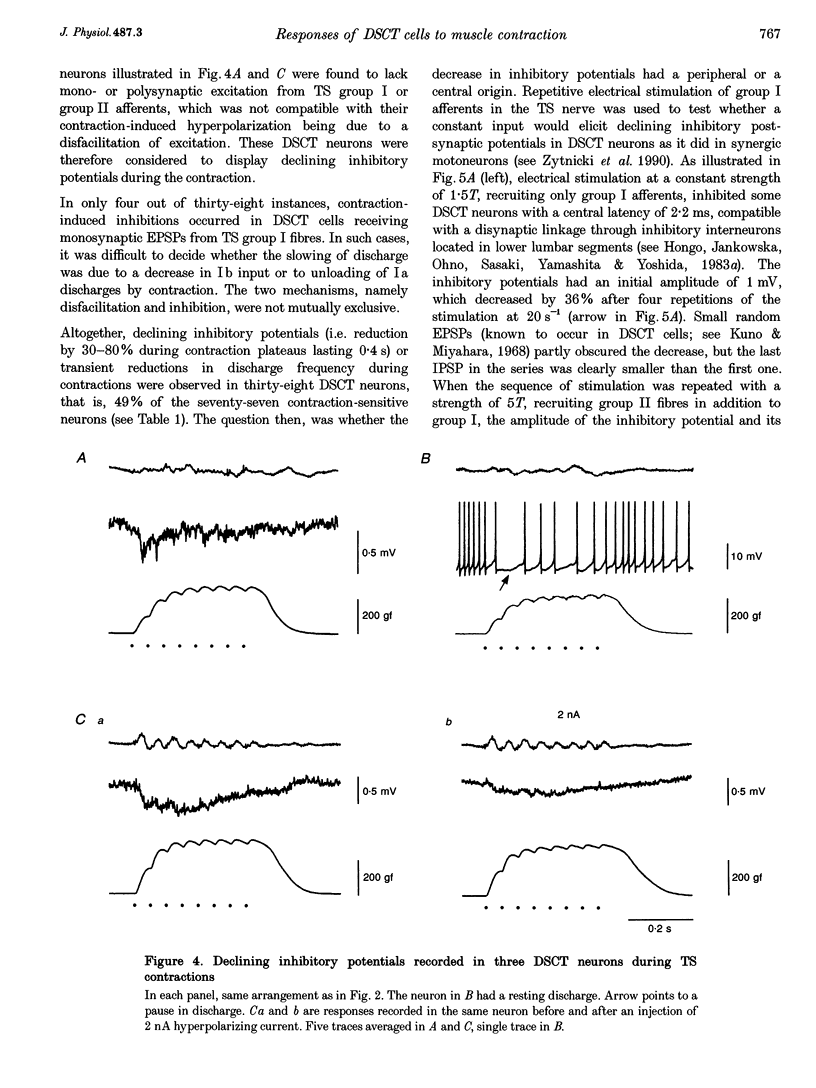
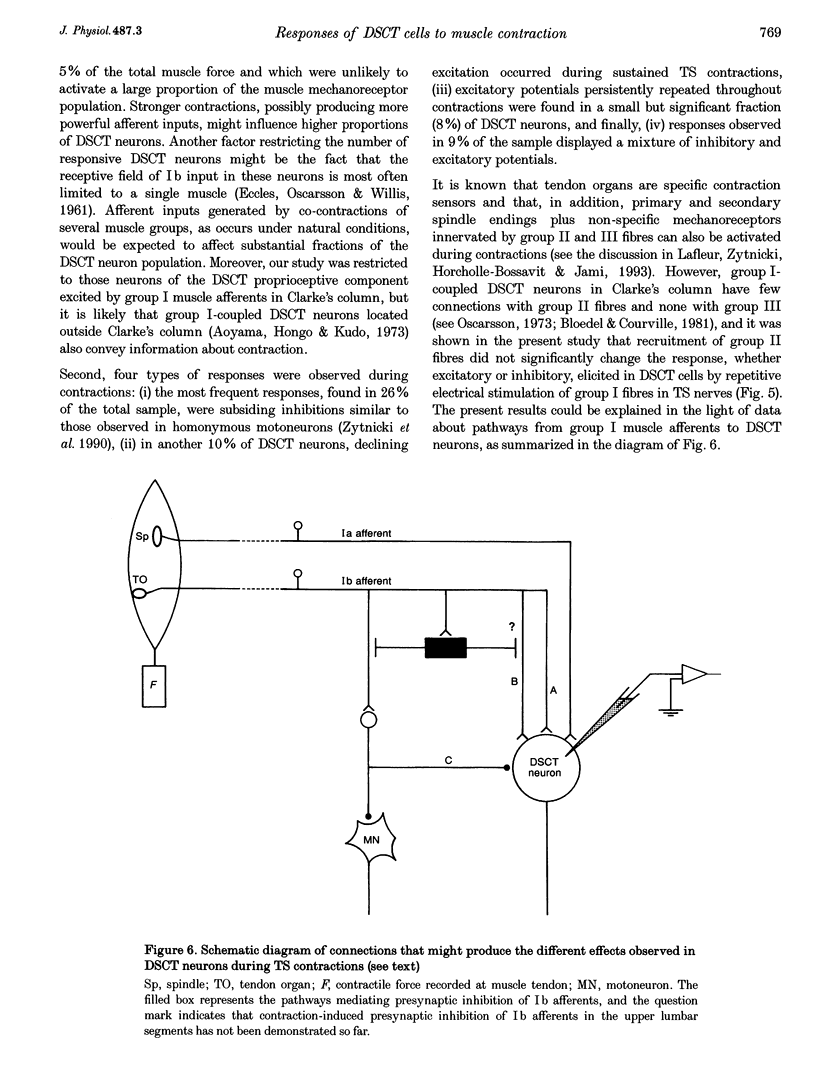
1. Clarke's column neurons of the dorsal spinocerebellar tract (DSCT) were recorded intracellularly in anaesthetized cats during weak sustained contractions of triceps surae (TS) produced by direct electrical stimulation of the muscle. 2. Of 145 DSCT neurons, 77 (53%) were contraction sensitive suggesting that information about weak contraction of a limited number of muscles is widely distributed among DSCT neurons. Four types of effects were observed in individual neurons during TS contractions. 3. In the first group of 11 DSCT neurons (14% of the contraction-sensitive cells), the effect was excitation persisting throughout the duration of contractions. These responses were ascribed to actions of afferents from contraction-activated tendon organs. 4. In a second group of 15 neurons (20% of the contraction-sensitive cells), quickly declining excitatory potentials were recorded during sustained TS contractions. By analogy with previous observations of contraction-induced effects in motoneurons, the decline of excitation might be explained by contraction-induced presynaptic inhibition of group I afferents in Clarke's column. 5. Declining inhibitions, resembling those previously observed in homonymous and synergic motoneurons, were recorded in 49% of contraction-sensitive DSCT neurons. This appears in keeping with the fact that interneurons mediating Ib inhibition to motoneurons project axon collaterals to DSCT neurons. Presynaptic inhibition of Ib fibres might therefore cause parallel reductions of inhibitory potentials in motoneurons and in DSCT neurons. 6. In a final group of 13 neurons, mixed excitatory and inhibitory effects were observed during TS contractions. Such DSCT neurons might monitor the excitability of Ib interneurons by integration of information about input to and output from these neurons. 7. The non-uniform patterns of DSCT responses to TS contractions suggest complex processing of information on ankle extensor activity in cerebellum. Phasic signalling of contraction onset is observed in many DSCT neurons while others carry messages about duration and strength of contraction.
Full text
PDF


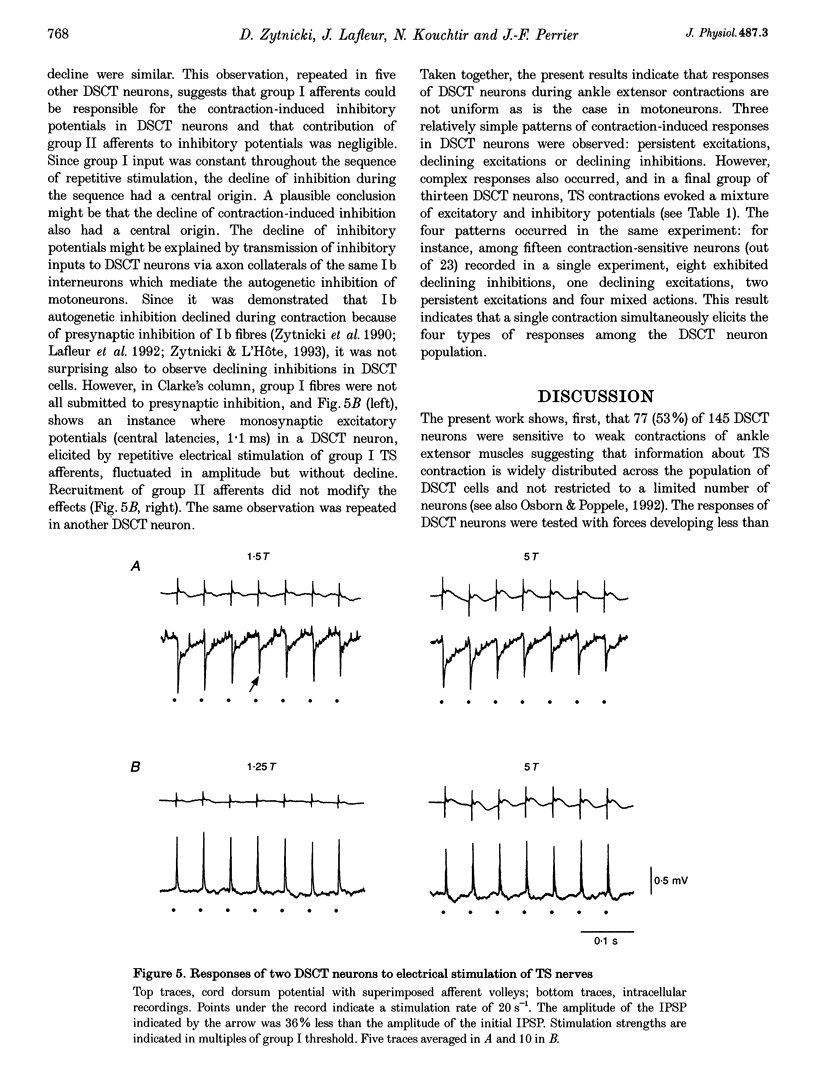



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama M., Hongo T., Kudo N. An uncrossed ascending tract originating from below Clarke's column and conveying group I impulses from the hindlimb muscles in the cat. Brain Res. 1973 Nov 9;62(1):237–241. doi: 10.1016/0006-8993(73)90634-3. [DOI] [PubMed] [Google Scholar]
- Asif M., Edgley S. A. Projections of group II-activated midlumbar spinocerebellar tract neurones to the region of nucleus Z in the cat. J Physiol. 1992 Mar;448:565–578. doi: 10.1113/jphysiol.1992.sp019058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley S. A., Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987 Apr;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Properties of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):125–144. doi: 10.1111/j.1748-1716.1969.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Sugano N., Loeb G. E., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J Neurophysiol. 1987 Feb;57(2):530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol. 1983 Sep;342:145–159. doi: 10.1113/jphysiol.1983.sp014844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983 Sep;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev. 1992 Jul;72(3):623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Factors responsible for multiple discharge of neurons in Clarke's column. J Neurophysiol. 1968 Jul;31(4):624–638. doi: 10.1152/jn.1968.31.4.624. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. VII. Identification of units by antidromic activation from the cerebellar cortex with recognition of five functional subdivisions. Acta Physiol Scand. 1960 Dec 30;50:356–374. doi: 10.1111/j.1748-1716.1960.tb00189.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spinocerebellar tract in the cat. IV. Synaptic connections of afferents from Golgi tendon organs and muscle spindles. Acta Physiol Scand. 1956 Dec 29;38(1):53–75. doi: 10.1111/j.1748-1716.1957.tb00173.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Functional organization of the dorsal spino-cerebellar tract in the cat. VI. Further experiments on excitation from tendon organ and muscle spindle afferents. Acta Physiol Scand. 1960 Jul 15;49:165–170. doi: 10.1111/j.1748-1716.1960.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Lafleur J., Zytnicki D., Horcholle-Bossavit G., Jami L. Declining inhibition in ipsi- and contralateral lumbar motoneurons during contractions of an ankle extensor muscle in the cat. J Neurophysiol. 1993 Nov;70(5):1797–1804. doi: 10.1152/jn.1993.70.5.1797. [DOI] [PubMed] [Google Scholar]
- Lafleur J., Zytnicki D., Horcholle-Bossavit G., Jami L. Depolarization of Ib afferent axons in the cat spinal cord during homonymous muscle contraction. J Physiol. 1992 Jan;445:345–354. doi: 10.1113/jphysiol.1992.sp018927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S., Silfvenius H. Nucleus Z, the medullary relay in the projection path to the cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol. 1971 Nov;218(3):551–571. doi: 10.1113/jphysiol.1971.sp009633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. S., Mantle-St John L. A., Tracey D. J. Nucleus z in the rat: spinal afferents from collaterals of dorsal spinocerebellar tract neurons. J Comp Neurol. 1986 Jan 22;243(4):510–526. doi: 10.1002/cne.902430406. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res. 1971;12(3):317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Mackel R., Miyashita E. Nucleus Z: a somatosensory relay to motor thalamus. J Neurophysiol. 1993 May;69(5):1607–1620. doi: 10.1152/jn.1993.69.5.1607. [DOI] [PubMed] [Google Scholar]
- McIntyre A. K., Proske U., Rawson J. A. Pathway to the cerebral cortex for impulses from tendon organs in the cat's hind limb. J Physiol. 1985 Dec;369:115–126. doi: 10.1113/jphysiol.1985.sp015891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn C. E., Poppele R. E. Components of responses of a population of DSCT neurons to muscle stretch and contraction. J Neurophysiol. 1989 Feb;61(2):456–465. doi: 10.1152/jn.1989.61.2.456. [DOI] [PubMed] [Google Scholar]
- Osborn C. E., Poppele R. E. Cross-correlation analysis of the response of units in the dorsal spinocerebellar tract (DSCT) to muscle stretch and contraction. Brain Res. 1983 Dec 5;280(2):339–342. doi: 10.1016/0006-8993(83)90064-1. [DOI] [PubMed] [Google Scholar]
- Osborn C. E., Poppele R. E. Parallel distributed network characteristics of the DSCT. J Neurophysiol. 1992 Oct;68(4):1100–1112. doi: 10.1152/jn.1992.68.4.1100. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Wieniawa-Narkiewicz E., Nicol M. J. Ultrastructural evidence related to presynaptic inhibition of primary muscle afferents in Clarke's column of the cat. J Neurosci. 1987 Jan;7(1):236–243. doi: 10.1523/JNEUROSCI.07-01-00236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zytnicki D., L'Hôte G. Neuromimetic model of a neuronal filter. Biol Cybern. 1993;70(2):115–121. doi: 10.1007/BF00200825. [DOI] [PubMed] [Google Scholar]
- Zytnicki D., Lafleur J., Horcholle-Bossavit G., Lamy F., Jami L. Reduction of Ib autogenetic inhibition in motoneurons during contractions of an ankle extensor muscle in the cat. J Neurophysiol. 1990 Nov;64(5):1380–1389. doi: 10.1152/jn.1990.64.5.1380. [DOI] [PubMed] [Google Scholar]


