Abstract
Nanotechnology holds immense promise in revolutionising healthcare, offering unprecedented opportunities in diagnostics, drug delivery, cancer therapy, and combating infectious diseases. This review explores the multifaceted landscape of nanotechnology in healthcare while addressing the critical aspects of safety and environmental risks associated with its widespread application. Beginning with an introduction to the integration of nanotechnology in healthcare, we first delved into its categorisation and various materials employed, setting the stage for a comprehensive understanding of its potential. We then proceeded to elucidate the diverse healthcare applications of nanotechnology, spanning medical diagnostics, tissue engineering, targeted drug delivery, gene delivery, cancer therapy, and the development of antimicrobial agents. The discussion extended to the current situation surrounding the clinical translation and commercialisation of these cutting-edge technologies, focusing on the nanotechnology-based healthcare products that have been approved globally to date. We also discussed the safety considerations of nanomaterials, both in terms of human health and environmental impact. We presented the in vivo health risks associated with nanomaterial exposure, in relation with transport mechanisms, oxidative stress, and physical interactions. Moreover, we highlighted the environmental risks, acknowledging the potential implications on ecosystems and biodiversity. Lastly, we strived to offer insights into the current regulatory landscape governing nanotechnology in healthcare across different regions globally. By synthesising these diverse perspectives, we underscore the imperative of balancing innovation with safety and environmental stewardship, while charting a path forward for the responsible integration of nanotechnology in healthcare.
Graphical abstract
Keywords: Nanotechnology, Healthcare, Safety, Environmental risks, Regulatory policies
Introduction
Nanomaterials are defined as materials that possess one or more peripheral nanoscale dimensions (in the range 1–100 nm). Nanotechnology, the field dedicated to the science and applications of these nanomaterials, is experiencing rapid and continuous growth. At this scale, the properties of materials undergo significant alterations. Characteristics such as solubility, reactivity, spectroscopy, electrical and magnetic attributes, as well as membrane transport, typically diverge from those exhibited by the same materials at larger scales [1]. The unique properties exhibited by nanomaterials open up avenues for diverse applications and hold great promise for transformative advancements in various scientific and technological domains.
In recent years, nanotechnology has emerged as a transformative force in the field of healthcare, offering continued innovation in medicine development and unprecedented possibilities for enhancing the performance and applications of medical devices. The ability to manipulate matter at the nanoscale has paved the way for groundbreaking innovations, promising revolutionary breakthroughs in diagnostics, treatment modalities, and overall patient care. Specific areas demonstrating the potential of nanotechnology in healthcare include medical diagnostics, where nanomaterials are used to improve the sensitivity and accuracy of imaging and biosensing techniques, enabling earlier and more precise detection of diseases [2, 3]. In tissue engineering, nanomaterials enhance cell interactions and tissue regeneration, offering promising advancements in areas like neural, dental, bone, and skin repair [4]. Furthermore, nanomaterials are being developed for drug and gene delivery systems, where their ability to target specific cells or tissues can significantly enhance the efficacy of treatments while minimising side effects [5]. In cancer therapies, nanomaterials can enhance the efficacy of traditional treatments such as surgery, radiotherapy, and chemotherapy, while also enabling the development of novel therapeutic approaches including biotherapy, photothermal therapy, and photodynamic therapy [6]. Additionally, nanomaterials are being employed in antimicrobial and antiviral applications, including the creation of coatings and materials that can prevent infections and combat resistant pathogens [7].
Despite the immense potential, however, the current application of nanotechnology in medicines and medical devices faces substantial challenges, both of a technical nature and within the complex realm of regulatory policies [8, 9]. This review aims to provide a comprehensive overview of the present status of nanotechnology in the medical domain, focusing on three critical dimensions: clinical applications, safety and environmental considerations, and the global regulatory landscape. By delving into the intricacies of these key aspects, we seek to elucidate the current achievements, gaps, and hurdles in the integration of nanotechnology into medicines and medical devices. Our exploration will shed light on the diverse facets of this dynamic field, addressing the promising clinical advancements, potential safety concerns, and the evolving regulatory frameworks that shape the landscape of nanotechnology in healthcare.
Advanced nanotechnology in healthcare
Categories and classification of nanotechnology materials
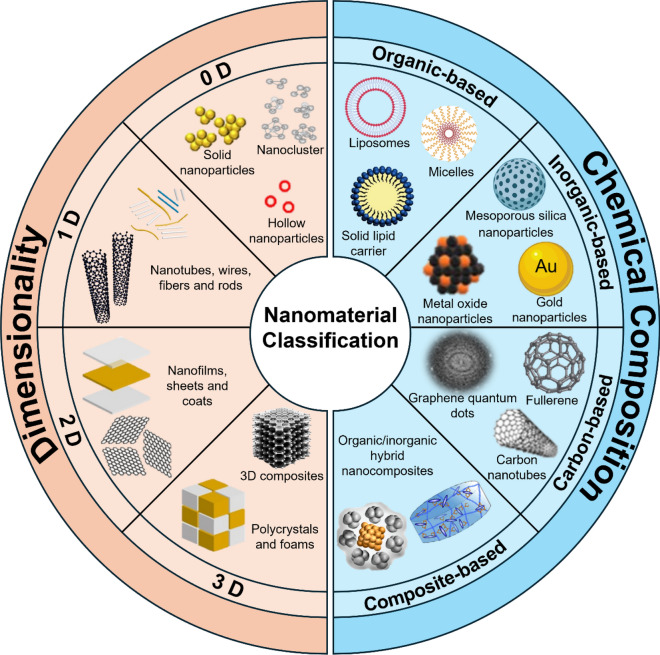
Nanomaterials can be categorised into four primary types based on the degree of spatial confinement [10] (Fig. 1). These include (i) zero-dimensional nanomaterials, where all dimensions are on the nanometer scale (e.g., nanoparticles), (ii) one-dimensional nanomaterials, where any one of the three dimensions is in the nanometer range (e.g., nanorods, nanowires, etc.), (iii) two-dimensional nanomaterials, where any two of the three dimensions are nanometer-sized (e.g., nanosheets, nanoplates, and nanocoatings), and (iv) three-dimensional nanomaterials, with each dimension in nanometer scale, thus allowing electrons to move freely without being confined in any direction. Examples include nanoflowers, nanocubes, nanocages, nanowire bundles, and various self-assemblies of lower-dimensional nanomaterials.
Fig. 1.
Classifications of nanomaterials based on dimensionality and chemical composition
In addition to spatial confinement, nanomaterials can also be classified based on origin, porosity, phase and dispersion [11]. The multitude of classifications reflects the abundance of nanotechnology materials, which are playing an increasingly important role in healthcare applications. However, a more common method of classifying nanomaterials is based on their chemical composition [12]. According to this, they can be classified into four material-based categories: organic-based, inorganic-based, carbon-based and composite-based nanomaterials (Fig. 1).
Organic-based nanomaterials
Organic-based nanomaterials, which are formed via covalent or non-covalent assemblies of organic molecules, encompass a wide range of types used in healthcare applications, mainly including micelles, dendrimers, liposomes, nanogels, polymeric nanoparticles (NPs), extracellular vesicles, and nanoscale covalent-organic frameworks (COFs) [13, 14]. The majority of them, with the exception of a limited number of modernist molecular machines, are polymeric in nature [15]. These materials are highly regarded for their biocompatibility, making them ideal for in vivo applications, with certain types, such as aliphatic polyesters, being particularly renowned for their exceptional biodegradability [16]. Additionally, organic-based nanomaterials can be easily functionalised, allowing for precise control over their chemical compositions, shape, size, and surface properties, making them highly adaptable to a variety of biomedical applications, such as bioimaging, drug delivery, and therapy (Fig. 2) [17, 18]. For instance, semiconducting polymeric NPs are prized for their high extinction coefficients, photostability, and tunable emission profiles in the near-infrared (NIR) spectrum, making them ideal for deep-tissue imaging, including NIR-II fluorescence and photoacoustic imaging [19, 20]. Additionally, COFs have emerged as promising candidates in drug delivery and phototherapeutics due to their large porosity and superior photoelectric properties [21, 22]. Liposomes, vesicles, and micelles, which are extensively used for drug encapsulation, play a crucial role in minimising the off-target toxicity of potent therapeutic agents [23–26]. The core composition, dimensions, and surface characteristics of these organic NPs are critical factors that determine their biocompatibility and functional efficacy in vivo.
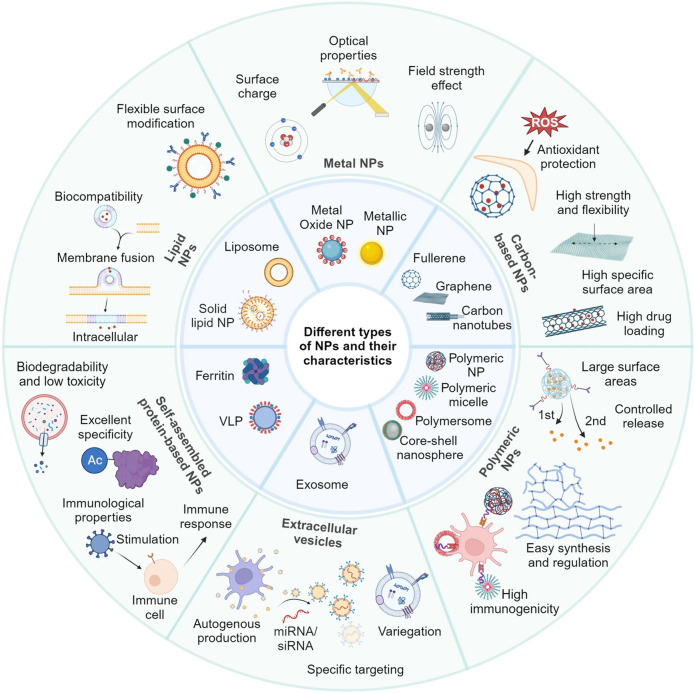
Fig. 2.
Characteristics of six common nanomaterials. Lipid NPs, composed of lipids like phospholipids, offer excellent biocompatibility and flexible surface modification capabilities. Metal NPs, including metals such as gold, silver, and copper, as well as their oxides, exhibit outstanding optical, electronic, and magnetic properties, making them ideal for biological imaging, photothermal therapy (PTT), and sensing applications. Carbon-based nanomaterials, such as CNTs, graphene, and fullerenes, feature a large surface area, high drug-loading capacity, and chemical stability, providing resistance to oxidative environments. Polymeric NPs, made from various polymers, display diverse structures and properties suitable for multiple biomedical applications. Self-assembled NPs, including ferritin family proteins and virus-like particles (VLPs), offer good biodegradability in the case of ferritin proteins and the ability to mimic viral stimuli to trigger immune responses in the case of VLPs. Exosomes, small vesicles secreted by cells, are rich in proteins, nucleic acids, and signaling molecules, playing vital roles in cellular communication and regulation. These nanomaterials have broad applications in drug delivery, molecular imaging, biosensing, tissue engineering, and disease diagnosis. Adapted with permission from Shen et al. [37]
However, despite their advantages, organic-based nanomaterials also present certain limitations, including lower mechanical strength and stability compared to inorganic counterparts, which can restrict their use in applications requiring high structural integrity and thermal stability [27]. Additionally, batch-to-batch variability in synthesis can affect reproducibility and scalability [28]. Nonetheless, ongoing research aims to address these challenges, further enhancing the potential of organic nanomaterials in healthcare.
Inorganic-based nanomaterials
Inorganic-based nanomaterials not only exhibit better chemical and mechanical stability compared to their organic counterparts, but also possess unique physicochemical advantages, such as optical, electrical, magnetic, ultrasonic, and catalytic properties [29, 30]. These advantages make them highly promising for biomedical applications, particularly in cancer imaging and therapy. However, their potential toxicity and poor biocompatibility, which can trigger adverse immune responses, induce inflammation, immunogenicity, and long-term toxicity, complicating their clinical translation [31]. In addition, inorganic-based nanomaterials often suffer from limited biodegradability, leading to accumulation in tissues and organs, which can pose significant risks over extended periods [32]. Nonetheless, progress in design and surface modification is steadily enhancing the safety and clinical potential of inorganic-based nanomaterials.
Generally, inorganic-based nanomaterials in healthcare applications include different metal and metal oxide nanomaterials. Examples of metal-based inorganic nanomaterials are silver (Ag), gold (Au), aluminum (Al), cadmium (Cd), copper (Cu), iron (Fe), zinc (Zn), and lead (Pb) nanomaterials, whereas examples of metal oxide-based inorganic nanomaterials are zinc oxide (ZnO), copper oxide (CuO), magnesium aluminum oxide (MgAl2O4), TiO2, cerium oxide (CeO2), and iron oxide (Fe3O4), etc. Figure 2 highlights the advantages of metal and metal oxide NPs in healthcare applications. In addition, silicon-based nanomaterials, including porous silicon nanoparticles (pSiNPs), mesoporous silica nanoparticles (MSNs) and periodic mesoporous silica nanoparticles (PMONPs), have been intensively investigated for therapeutic and diagnostic applications [33]. Moreover, layered double hydroxides (LDHs), metal carbides and nitrides (MXenes), and 2D transition metal borides (MBenes) are emerging as promising inorganic-based two-dimensional nanomaterials due to their exceptional surface area, tunable chemistry, and potential for drug delivery, biosensing, and theranostics [34, 35].
Carbon-based nanomaterials
Carbon-based nanomaterials are considered as a separate class of nanomaterial with features such as diversity in their structures and facile functionalisation [36]. Due to the unique properties, carbon can form covalent bonds with other carbons in different hybridisation states such as sp, sp2, and sp3 in order to form a variety of structures of small molecules and longer chains [12]. Graphene (Gr), graphene oxide (GO), carbon nanotubes (CNTs), fullerenes (C60), carbon dots (CDs), graphene quantum dots (GQDs), carbon nanofibers (CFs), carbon onions, and carbon black are the different categories of carbon-based nanomaterials. These materials are highly valued in healthcare for their exceptional surface area, electrical conductivity, and mechanical strength. However, like inorganic nanomaterials, they face challenges in biocompatibility that need to be addressed. Their potential applications span drug delivery, bioimaging, tissue engineering, and diagnostic sensing.
Composite-based nanomaterials
Composite-based nanomaterials, also known as hybrid nanomaterials, are any combination of metal-based, metal oxide-based, carbon-based, and/or organic-based nanomaterials, which often have complicated structures. Although many biomaterials use organic and inorganic materials individually, the increased demand for highly functionalised biomaterials has necessitated the development of organic/inorganic composite materials that not only integrate the advantages of each component, but also provide synergistic properties to meet new demands [38–41]. For example, water non-dispersible inorganic NPs, such as gold nanoparticles (Au NPs), Fe3O4 NPs, semiconductor QDs, CDs, etc., can be rendered dispersible in water through surface modification with organic polymers like polyethylene glycol (PEG) or polydopamine (PDA) [42]. This modification enables precise control over their size, surface charge, and functionalisation, while maintaining desirable physical properties of the inorganic core such as magnetic, optical or catalytic activity. As a result, the presence of a organic polymer shell not only improves the dispersion of inorganic nanomaterials in biological fluids, but can also significantly enhance the biocompatibility of the inorganic core, acting as anchor sites for molecular linkages or protecting them from oxidation [43]. Appropriately designed shell thicknesses can improve the chemical and thermal stability of NPs, while also regulating and controlling the release of molecules from the core [44].
Such core–shell NPs have been widely investigated for cancer therapy, specific delivery of therapeutics, drug delivery monitoring, biosensors, bioimaging and antimicrobial activity. For example, a biosensor for detecting influenza A viral HA proteins (H1 and H5) was developed by modifying the surface of a field effect transistor (FET) with an aminooxy-terminated silane coupling reagent, highlighting the utility of surface-modified inorganic nanomaterials in biosensing applications [45]. Moreover, A dual pH- and light-controlled drug delivery system was developed using surface-modified mesoporous silica NPs, demonstrating precise drug release and enhanced therapeutic efficacy in cancer treatment [46]. Beyond surface modification, organic–inorganic NPs can be engineered through the self-assembly of block copolymers. Furthermore, inorganic NPs can be incorporated into polymer matrices to create bulk composite nanomaterials, such as functional hydrogels. A previous review provided a systematic analysis of hybrid nanomaterials [42].
Another category of composite-based nanomaterials is metal–organic frameworks (MOFs), which are constructed from coordination bonds between metal-containing nodes and multidentate organic bridging ligands. MOFs offer customizable compositions and topologies, as well as high porosity and larger Brunauer–Emmett–Teller (BET) surface areas compared to single-component nanostructures. These properties make MOFs highly suitable for applications in cancer therapy, drug delivery, and bioimaging, positioning them as promising platforms in the field of biomedicine [47, 48]. For instance, a recently reported hierarchical and size-adaptable MOF nanovehicle was designed to effectively cross biological barriers at the tissue, cellular, and nuclear levels, enabling efficient nucleus-targeted delivery of doxorubicin (DOX) [49]. Additionally, a newly developed MOF/sulfonated hyaluronic acid composite coating was applied to the surface of magnesium alloy vascular stents, enhancing their biocompatibility and corrosion resistance, which is essential for improving the performance and longevity of vascular implants [48].
Given their unique physicochemical properties, composite-based nanomaterials have become essential in advancing pharmaceuticals, targeted drug delivery, biosensors, medical imaging, and next-generation medical devices, driving innovation in more effective and personalised therapies.
Healthcare applications of nanotechnology
Nanotechnology has emerged as a promising field with significant potential for advancements in various healthcare and biomedical applications. Considering the extensive research conducted in this field and the numerous comprehensive reviews available, our focus will primarily be on providing concise descriptions of several mainstream applications of nanotechnology in recent years (Fig. 3).
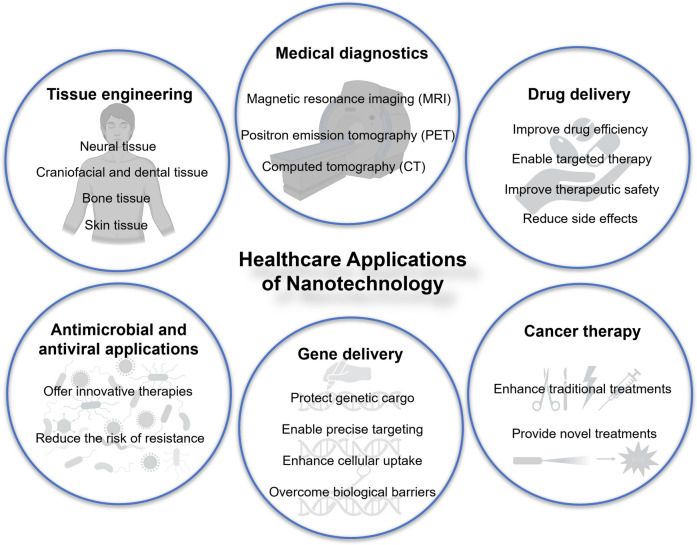
Fig. 3.
Applications of nanotechnology in healthcare. These applications involve a number of medical fields such as diagnostics, tissue engineering, drug delivery, gene delivery, cancer therapy, antimicrobial and antiviral applications
Medical diagnostics
The progress of nanotechnology has significantly influenced the improvement of imaging techniques, early detection, diagnosis, and prognosis of diseases, enhancing existing clinically relevant technologies [2]. The field of diagnostic sciences has incorporated nanodevices to achieve early and swift disease identification, leading to subsequent medical procedural recommendations [5]. The potential of nanotechnology to transform healthcare diagnostics lies in its ability to enhance the precision, sensitivity, and speed of medical tests. One of the profound applications includes nanoparticle-based diagnostic imaging. The unique biophysical properties of NPs enable their attachment to specific biomarkers, thereby improving imaging modalities like magnetic resonance imaging (MRI), computed tomography (CT) scans, and positron emission tomography (PET) scans [2].
Magnetic resonance imaging (MRI)
MRI uses strong magnetic fields and radio waves to create detailed images of soft tissues by exploiting the behaviour of hydrogen atoms in the body. However, traditional MRI has limitations in contrast resolution, making it challenging to distinguish between different soft tissue types and detect small or early-stage lesions [50]. Paramagnetic or superparamagnetic nanomaterials enhance MRI contrast by increasing interactions between water protons and paramagnetic centers, accelerating energy transfer and shortening spin–lattice (T1) or spin–spin (T2) relaxation times, thereby improving signal intensity and image clarity [51]. These nanomaterials encompass paramagnetic complexes based on gadolinium (Gd) ions or manganese (Mn) ions, as well as superparamagnetic iron oxide NPs (SPIONs), all of which are widely used as contrast agents in MRI [50]. Surface functionalisation can further improve their biocompatibility, prolongs circulation time, and enables targeted imaging, increasing both specificity and sensitivity [52]. Recent advancements include the development of pH-activatable manganese carbonate (MnCO3) NPs that enhance T1-weighted MRI specifically in acidic tumour environments, significantly improving the ability to distinguish malignant from benign tissues and enabling more accurate detection of tumour malignancy and metastasis [53]. Furthermore, a novel hypoxia-responsive tri-modal MRI nanoprobe (SPION@ND-IR780) encompassing T1, T2, and T2 mapping capabilities has been recently developed for enhanced imaging of hypoxic tumour regions in breast cancer [54]. This nanoprobe undergoes structural transformations under hypoxic conditions, thereby significantly improving imaging accuracy and resolution. The precise visualisation of hypoxic areas facilitated by this system enables more targeted and effective radiotherapy interventions, addressing issues of tumour radioresistance (Fig. 4).
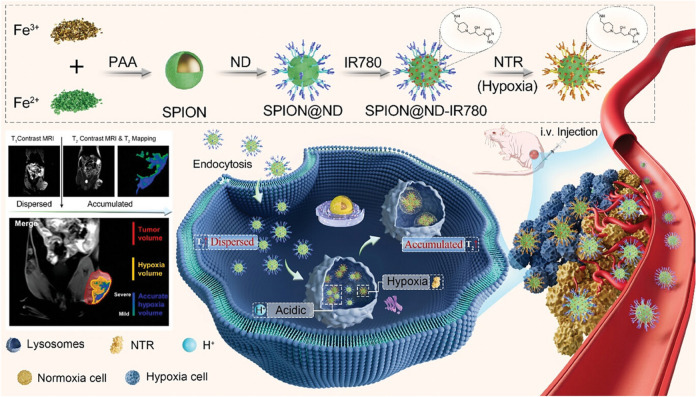
Fig. 4.
Schematic representation of SPION@ND-IR780, a tri-modal hypoxia imaging nanoprobe designed for precise tumour hypoxia imaging and the streamlined construction of hypoxia-guided biological target volumes (BTVs). The nanoprobe exhibits dual-stimuli responsive characteristics: hypoxia sensitivity, which facilitates nanoprobe accumulation, and acidic pH sensitivity, which induces nanoprobe aggregation. Adapted with permission from Wu et al. [54]
Computed tomography (CT)
CT imaging relies on X-ray attenuation to produce detailed images, making it crucial for diagnosing various medical conditions. Traditional small-molecule iodine-based contrast agents, such as iohexol and iodixanol, while effective, are constrained by rapid metabolic clearance, suboptimal targeting specificity, and the risk of adverse effects, including nephrotoxicity and allergic reactions, which limit their efficacy, especially in enhancing soft tissue contrast. Nanotechnology has introduced advanced contrast agents, including NPs like Au NPs, which offer improved stability, targeting, and biocompatibility. These nano-contrast agents can enhance imaging precision, reduce side effects, and improve the overall diagnostic capability of CT imaging by leveraging properties such as enhanced permeability and retention (EPR) and surface modifiability [55].
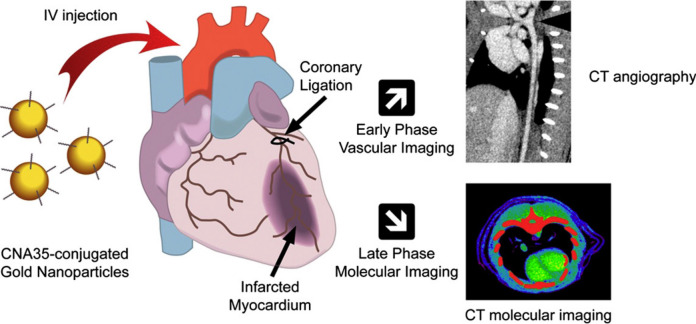
For example, Au NPs functionalised with collagen-binding adhesion protein 35 (CNA35) have shown significant potential in improving CT imaging of myocardial scars [56]. This approach allows for prolonged blood pool enhancement and specific targeting of collagen within scar tissue, providing clearer and more detailed images compared to conventional iodine-based agents. In a study involving a rat model of myocardial infarction, CNA35-conjugated AuNPs provided substantial signal enhancement in the scar tissue up to six hours post-injection, highlighting their potential for more precise cardiovascular diagnostics (Fig. 5).
Fig. 5.
CNA35-conjugated Au NPs were explored as CT contrast agents for vascular and molecular imaging. Early phase imaging, within the first hour post-injection, showed uniform and stable blood pool enhancement. By six hours, after clearance of circulating NPs, late phase imaging revealed specific localisation and enhancement of myocardial scars. This provides essential diagnostic information on vascular lesions and scar burden, aiding in risk stratification and management of coronary artery disease. Adapted with permission from Kee and Danila [56]
Additionally, a recent study introduced a novel self-assembled CT contrast agent, BioDHU-CT NPs, designed to leverage size aggregation for improved tumour imaging [57]. They are composed of a biotin polyethylene glycol (Biotin-PEG) segment for hydrophilic properties and active targeting, an ROS-responsive group for rapid reactivity to reactive oxygen species, and an iodine-containing component modified with tetraphenylethene (TPE) for enhanced imaging capabilities. These NPs remain small for effective tumour penetration but rapidly aggregate upon activation by ROS in the tumour microenvironment. This size transformation enhances retention and extends the imaging window, significantly improving the contrast and clarity of tumour images in CT scans. The study demonstrated that these NPs provide more sustained and targeted imaging, offering a promising alternative to conventional contrast agents for tumour diagnostics.
Positron emission tomography (PET)
PET imaging is a diagnostic tool that enables the visualisation of metabolic processes within the body. By detecting pairs of gamma rays emitted indirectly by a positron-emitting radiotracer, PET provides detailed images of the function of tissues and organs. Nevertheless, traditional PET imaging faces certain limitations, including low spatial resolution and sensitivity, which hinder its ability to detect small or early-stage tumours. Moreover, the radiotracers commonly used in conventional PET tend to accumulate non-specifically in tissues, resulting in background noise that can obscure critical details.
Similarly, PET imaging has seen significant advancements with the use of nanomaterials. NPs can be engineered to carry PET radiotracers, which accumulate in specific tissues, providing high-contrast images that are crucial for early detection of tumours and monitoring of metastatic spread [58]. By addressing the limitations of traditional PET, NPs enable more precise and sensitive imaging. For instance, nanoscale MOFs have been developed that are intrinsically radioactive, such as the UiO-66 MOF radiolabeled with zirconium-89 [59]. It combines PET isotope zirconium-89 with 1,4-benzenedicarboxylate (BDC) and benzoic acid (BA) linkers, forming a highly porous structure. This porosity allows effective drug loading, while surface functionalisation with PEG and tumour-targeting peptides enhances stability, biocompatibility, and enables targeted imaging and drug delivery. In addition, nano-coordination polymers (NCPs) offer another promising example in PET imaging. Zr-P1 NCPs, composed of zirconium ions and tetrakis (4-carboxyphenyl)ethylene ligands, are radiolabeled with zirconium-89. Functionalised with PEG and cyclo(Arg-Gly-Asp-d-Phe-Cys) peptides, these NCPs target integrin αvβ3 in tumours, enhancing biocompatibility and specificity [60]. This design improves PET imaging by reducing background noise and providing clearer, more precise tumour visualisation. Moreover, in the domain of single-cell tracking, radiolabeled NPs, such as 68Ga radioisotope labeled mesoporous silica NPs, have been used to track individual cancer cells though PET (Fig. 6). This technique helps study cell trafficking, immune cell movement in cancer immunotherapy, and cell distribution post-transplantation [61].
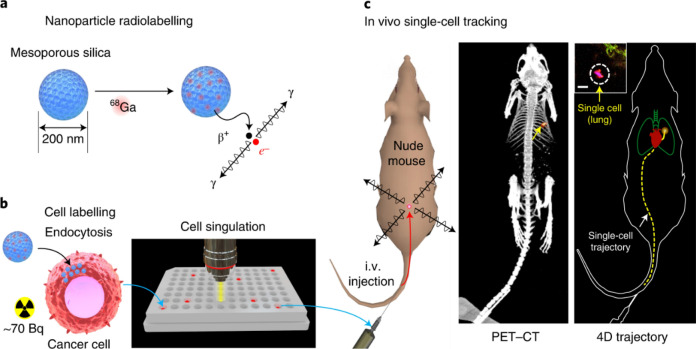
Fig. 6.
a Mesoporous silica nanoparticles (MSNs) concentrate ⁶⁸Ga from a clinical PET generator. b The NPs are then loaded into cells, achieving up to 100 Bq per cell, a fraction of a standard PET dose. c These isolated single cells are administered into mice, with gamma rays emitted from each cell detected by a small-animal PET scanner. The captured data is processed to estimate the cell's location in real time. In this example, single human breast cancer cells, administered intravenously, were detected in the lungs (yellow arrows), as confirmed by ex vivo analysis (inset; scale bar, 20 µm). Adapted with permission from Pratx et al. [61]
Beyond these key medical imaging techniques (MRI, CT, and PET), nanomaterials are also being increasingly used in other advanced imaging modalities such as fluorescence imaging, photoacoustic imaging (PAI), and ultrasound imaging. A recent review provides a systematic study of nanomaterials used in these technologies, highlighting their potential to significantly enhance diagnostic precision and expand the capabilities of medical imaging [62]. As research advances, these innovative nanomaterials are poised to revolutionise healthcare by enabling more accurate and personalised diagnostic solutions.
Moreover, the impact of nanotechnology extends beyond imaging, notably enhancing the performance of biosensors. By increasing sensitivity and dramatically lowering detection limits by several orders of magnitude, nanomaterials facilitate the detection of trace biomolecules in bodily fluids such as blood and urine, which is critical for early diagnosis and effective disease management. These materials are integral in the immobilisation of biomolecules, signal amplification, and serving as mediators, electroactive species, and detection nanoprobes. Diverse nanomaterials, including NPs, nanowires, nanofilms, QDs, nanocrystals, nanorods, nanobelts, nanotubes, embedded nanostructures, and self-assembled nanomaterials, have been successfully integrated into biosensors [3]. Their use is tailored to the specific conduction mechanisms of the biosensors, whether electrochemical, optical, or thermoelectric, further advancing the field of biomedical diagnostics and treatment strategies [63, 64]. The field is vast and continually evolving, with numerous studies and reviews offering more comprehensive insights into the latest developments [65–70].
Tissue engineering
Tissue engineering combines biology, engineering, and materials science to develop substitutes that restore or enhance tissue function. This involves using scaffolds, cells, and bioactive molecules to create functional tissues for medical applications, aiming to repair damaged tissues and reduce the need for organ transplants [71]. Key research areas include neural, dental, bone, and skin tissue engineering (Fig. 7) due to the high incidence of injuries and diseases affecting these tissues, as well as their complex structures and functions [72]. These areas have significant clinical demand and potential for improving patients' quality of life. Advancements in nanotechnology have significantly improved therapeutic outcomes in these fields by enhancing biomaterials properties, cell interactions, and tissue regeneration processes.
Fig. 7.
Applications of nanomaterials in tissue engineering
Neural tissue engineering
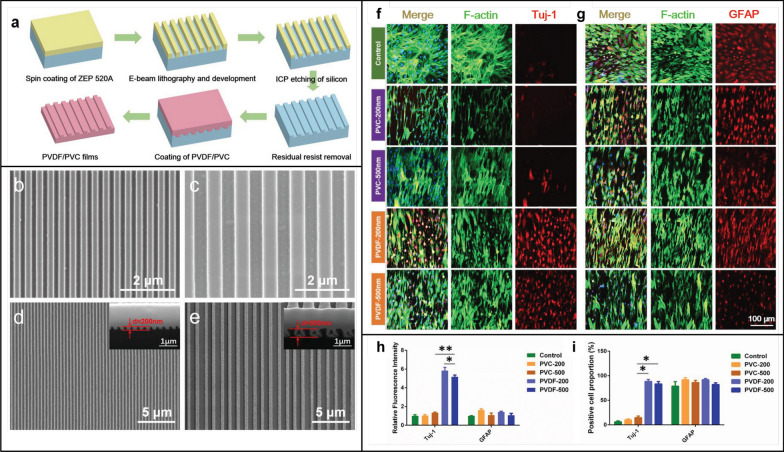
Neural engineering has benefited from the use of nanomaterials, demonstrating encouraging outcomes by facilitating cell adhesion and proliferation, and promoting neuronal cell differentiation, thus augmenting neuron regeneration. Currently, nanomaterials are expected to be used for the treatment of neurological injuries. Manipulation of the physicochemical properties of nanomaterials can prevent and/or treat neurodegeneration [73]. For example, biodegradable natural polymers, SPIONs, carbon-based nanomaterials, and silica nanostructures have demonstrated exceptional properties and superior efficacy in facilitating neural cell differentiation and outgrowth [73]. Composite-based nanomaterial of graphene and polyethylene terephthalate (PET) are employed in non-toxic, non-contact electrical stimulation to enhance cell-to-cell coupling in human neuroblastoma [74]. Magnetic nanomaterials exploit magneto-responsive effects in response to an external magnetic field, allowing for the modulation of neuronal cell activity to either activate or inhibit, aiming to treat neurological diseases such as Alzheimer's disease and Parkinson's disease [75–77]. In addition, studies have demonstrated the ability of metallic NPs like gold, iron, and cerium to ameliorate adverse pathological changes associated with spinal cord injury [78]. Furthermore, innovative approaches using nanomaterials combined with other bioactive substances have shown synergistic effects in neural tissue engineering. Zhang et al. fabricated two piezoelectric polyvinylidene fluoride (PVDF) nanostripe array structures, with ridge, groove, and height dimensions of either 200 or 500 nm, designed as scaffold surfaces for neural tissue engineering [79]. Their results revealed that the combination of piezoelectricity and nanogeometry favorably influenced neuron-like differentiation in both cell morphology and gene/protein expression, promising to promote nerve repair and regeneration (Fig. 8). In addition to the above, NPs can be functionalised to facilitate the crossing of the blood–brain barrier for targeted brain drug delivery [80].
Fig. 8.
a The schematic illustration of fabrication procedure of PVDF or non-piezoelectric polyvinyl chloride (PVC) films. SEM images of the silicon molds with repetition period of b 400 nm (ridge, groove, and height were all 200 nm) and c 1000 nm (ridge, groove, and height were all 500 nm). SEM images of d the PVDF-200 (ridge, groove, and height were all 200 nm) and e the PVDF-500 (ridge, groove, and height were all 500 nm). f Immunofluorescent staining of the neuron specific maker Tuj-1 and g a neurogliocyte specific maker GFAP after 7 days culture. The cell nuclei were stained with DAPI (blue) and F-actin was stained with phalloidin-Alexa Fluor 488 (green). Tuj-1 and GFAP were immunostained, respectively (red). h Statistical analysis of the fluorescence intensity of Tuj-1 and GFAP. i Statistical analysis of the percentage of Tuj-1 positive cells and GFAP positive cells. Adapted with permission from Zhang et al. [79]
In conclusion, nanotechnology holds significant potential for indispensable applications in neural tissue engineering.
Dental tissue engineering
Nanotechnology holds significant promise for advancing oral health in the field of craniofacial and dental tissue engineering, making it a prominent area for growth and potential enhancement of the quality of life of patients [81].
The involvement of periodontal diseases in the hard and soft tissues surrounding the teeth results in gum diseases, bone loss, and, in more severe cases, tooth loss. Nanomaterials are expected to offer innovative, nonsurgical alternatives for addressing periodontal disease, exhibiting minimal side effects yet high efficiency. The post-subgingival injection of biodegradable polydopamine NPs has been shown to be capable of efficiently remove excessive reactive oxygen species (ROS) in vivo and decrease local periodontal inflammation [82]. Au NPs with surface-anchored chiral amino acids (L-cysteine) have been reported to be able to regulate cellular behaviour through chiral effects and autophagy, thereby stimulating periodontal regeneration [83]. In addition, lithiated porous silicon nanowires (LipSiNs) have been recently shown to combine osteogenic, cementogenic and Wnt/β-catenin stimuli, leading to the regeneration of bone, cementum, and periodontal ligament fibers in a murine model of periodontal defect (Fig. 9). This regenerative potential proved to be significantly higher compared to previously studied materials, including lithium chloride, porous silicon nanowires, lithium-substituted bioglass, and a commercial guided tissue regeneration membrane (GTR, BioGide®) used for the treatment of periodontitis [84].
Fig. 9.
a Schematic of the metal assisted electrochemical etching (MACE) process used to generate porous silicon nanowires (pSiNs). b Schematic of the lithiation process for porous silicon nanowires. c Quantification of Li/Si ratio of LipSiNs of fully dissolved nanowires as a function lithium precursor, lithiation temperature, time and atmosphere. d The model of periodontal fenestration defect (standardised with 3 mm in length, 3 mm in height and < 1 mm in deep). e Micro-computed tomography (μCT) scans of rat mandibles at day 0. Analysis was performed on 5 animals. f μCT scans of rat mandibles showing regeneration of periodontal defects 2 weeks and 6-weeks post-operative with lithium chloride, commercial guided tissue regeneration membrane (GTR, BioGide®), pSi, and LipSi − 1.2%; serves as baseline comparison. The dotted yellow line outlines the newly formed bone. g μCT analysis for the quantification of bone volume over total volume (BV/TV) and bone mineral density (BMD). Adapted with permission from Kaasalainen et al. [84]
Oral and cranio-maxillofacial diseases are complications of the soft and hard tissues of the craniofacial, face and dental arches induced by physical, chemical, microbiological factors, and systemic disorders. These conditions include craniofacial defects and conditions such as head and neck tumours, craniosynostosis, dental implant failures, and osseous malunion. They often result in facial deformities and bone defects that require surgical intervention to correct normal facial features. Zirconium dioxide (ZrO2) NPs have been investigated for improving the mechanical properties and colour instability of maxillofacial prostheses such as silicone and silicone elastomers in order to enhance functionality and aesthetics [85, 86]. An electro-spun titanium oxide (TiO2)/hydroxyapatite (HA)/polyurethane (PU) nanocomposite fibre has been designed for use as an osseointegrated membrane to enhance new bone formation in oral and maxillofacial surgery [87].
Furthermore, incorporating nanotechnology, specifically antibacterial NPs, into endodontic materials can enhance their properties, preventing recurrent infections and improving the success rates of root canal treatments [88]. The incorporation of antimicrobial nano-coatings in orthodontic materials prevents the dental plaque formation around orthodontic appliances and prevents dental caries associated with orthodontic treatments [81]. Nanomaterials like TiO2 nanotubes, silver nanoparticles (Ag NPs), and graphene oxide have been employed to coat the surface of titanium dental implants, aiming to improve osseointegration, soft tissue integration, immunomodulation, antitumour and antibacterial properties [89].
Advances in nanomaterials, whether utilised independently or in conjunction with existing materials for oral medicine, offer innovative strategies for craniofacial and dental tissue engineering, thereby enhancing health, functionality, and aesthetics.
Bone tissue engineering
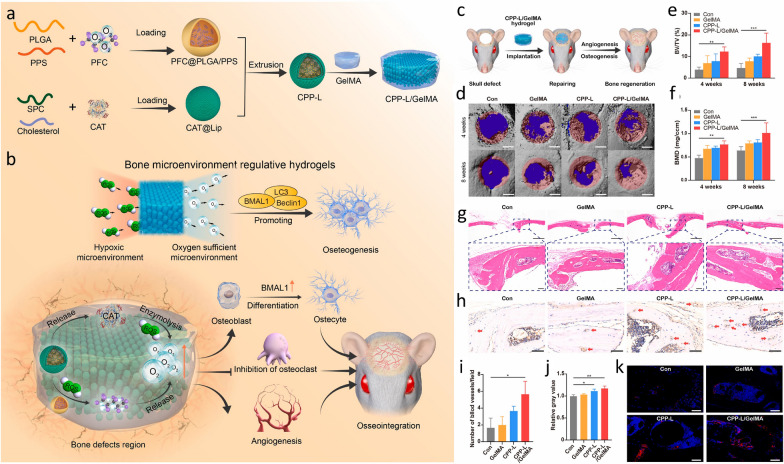
Bone defects are the result of the compromised structural integrity of bones, primarily attributed to trauma and bone disease [90]. The integration of nanotechnology into strategies for bone tissue engineering offers benefits by guiding cellular differentiation toward osteogenesis and aiding in the repair of substantial bone defects. NPs play a crucial role in advancing both scaffold-free and scaffold-based tissue engineering methods for promoting osteogenesis and bone regeneration. They modulate inflammatory responses and signaling pathways related to osteogenesis, angiogenesis, and osteoclast activity to create an osteogenic niche. Additionally, NPs interact with biomolecules, extending their half-life and enhancing bioavailability, making them highly promising materials for promoting osteogenesis [91]. In bone tissue engineering, commonly used nanomaterials include metallic/metallic oxide NPs such as silver (Ag NP), strontium (Sr NP), magnesium oxide (MgO NP), cobalt ferrite (CoFe2O4 NP), among others. Additionally, calcium phosphate NPs including hydroxyapatite (HA) and tricalcium phosphate (TCP) are prevalent, alongside silicate-based NPs like bioactive silica-based glass particles (SBA2) and mesoporous silica NPs. Furthermore, polymeric NPs made of polymers such as poly(lactic acid) (PLA), poly(lactic acid) (PLA) nanofibers, poly(glycolic acid) (PGA), and poly(lactic-co-glycolide acid) (PLGA), chitosan, gelatin (Gel), and silk fibroin (SF) are commonly employed [90, 91]. For example, Sun et al. developed Gelatin Methacrylate (GelMA) hydrogels (CPP-L/GelMA) incorporating polymeric NPs to regulate the bone microenvironment by scavenging ROS and generating prolonged oxygen release [92]. These NPs, serving as carriers of catalase (CAT) and oxygen, improved oxygen supply to the bone defect area, promoted angiogenesis and osteogenesis, inhibited osteoclastogenesis, and ultimately facilitated bone regeneration (Fig. 10).
Fig. 10.
a Perfluorocarbon (PFC) is loaded into PLGA/poly (propylene sulphide) (PPS) NPs to form PFC@PLGA/PPS, which, along with CAT, is encapsulated in liposomes and GelMA hydrogel to create CPP-L/GelMA. b CPP-L/GelMA hydrogel reverses hypoxia in bone defects by releasing CAT and oxygen, promoting osteogenesis and neovascularisation while inhibiting osteoclastogenesis. c Schematic of hydrogel implantation in a mouse skull defect. d 3D micro-CT images of treated skull defects at 4 and 8 weeks. e, f Bone volume fraction and bone mineral density measurements. g H&E staining of skull defects at 8 weeks. h, i CD31 staining and vessel density analysis. j, k Flk-1 immunofluorescence staining and analysis. Significance: *p < 0.05, **p < 0.01, ***p < 0.005. Adapted with permission from Sun et al. [92]
In addition, nano-surface modification of bone implants is widely adopted. Enhanced osteogenic capacity, modulation of macrophage-mediated inflammatory responses, and promotion of osteointegration are attainable through nano-surface modifications [93]. Various techniques, such as acid etching, sandblasting, laser modification, anodisation, micro-arc oxidation, hydrothermal treatment, chemical vapor deposition (CVD), atomic layer deposition (ALD), plasma-immersion ion implantation (PIII), and lithography, have also been employed to modify the surface topography of grafts [94–96].
These developments underscore the potential of nanomaterials to improve bone tissue engineering strategies, highlighting their promise in creating more effective bone repair solutions.
Skin tissue engineering
Nanotechnology has been extensively studied in skin tissue engineering. The objective of skin tissue engineering is to rebuild both the structural and functional elements of skin, with the goal of minimising scar formation and enhancing the overall quality of wound healing. Clinical approaches of wound management include techniques like hyperbaric oxygen therapy, debridement, negative wound pressure therapy, and the use of wound dressings [97].
Among these methods, the role of nanotechnology role is prominent in wound dressings. Nanocellulose, with its unique properties including enhanced absorbency and easier removal compared to traditional materials like gauze, is widely adopted in biomedical applications for treating skin diseases. Its nanoscale morphology mimics the native extracellular matrix, rendering it a favorable substrate for skin cell adhesion and growth [98]. Combining nanocellulose with chitosan, poly (N-isopropylacrylamide) (PNIPAAm), polyvinyl alcohol (PVA), magnetic NPs, lactoferrin, collagen, and alginate in nanocomposites has demonstrated further improved effectiveness in repairing skin tissue [99].
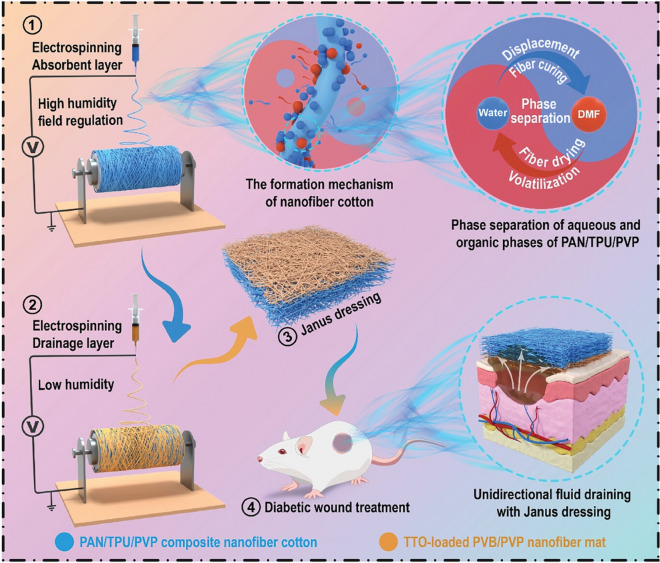
Autologous skin grafting is the “gold standard” of treatment for full-thickness injuries. However, due to the limitations of donor skin, the development of other tissue-engineered skin substitutes (such as skin regeneration scaffolds) is becoming increasingly important. Metal, ceramic and carbon-based nanomaterials have been introduced into scaffolds to reduce inflammation and enhance antimicrobial properties. Biomimicking scaffolds containing polymer nanofibres are able to recapitulate the native skin architecture and enhance cell proliferation [97]. Additionally, dual-faced polymeric nanofibers, or Janus nanofibers, can be engineered to integrate various materials and functionalities, yielding synergistic physicochemical effects and presenting a promising material for skin tissue engineering [100, 101]. Zhou et al. introduced a self-pumping Janus dressing with dual layers to enhance wound exudate management and accelerate healing [102]. This dressing consists of a hydrophobic drainage layer and a superabsorbent nanofiber layer, which work together to provide unidirectional fluid drainage and promote diabetic wound healing (Fig. 11).
Fig. 11.
Schematic preparation of a superabsorbent Janus dressing with polyvinylpyrrolidone-induced (PVP-induced) self-pumping for diabetic wound treatment by unidirectional draining excessive exudate. Adapted with permission from Zhou et al. [102]
In summary, nanotechnology, through the development of advanced wound dressings and biomimetic scaffolds, plays a crucial role in enhancing skin tissue engineering by improving wound healing, minimising scarring, and supporting cell growth and proliferation.
Drug delivery
Nanotechnology has transformed the field of drug delivery by providing precise and efficient drug-targeted delivery, smart-responsive drug release, and extraordinary in vivo stability, resulting in improved therapeutic efficacy, higher bioavailability, and reduced side effects [5]. A wide array of organic and inorganic nanomaterials has been developed to address various physicochemical challenges associated with drugs, including low solubility, stability, off-target deposition, and limited penetration across biological barriers [103].
The efficacy of NPs as drug carriers varies depending on factors like their shape, size, and other inherent biophysical or chemical characteristics [104]. Additionally, the method of drug loading, whether through conjugation or adsorption, plays a significant role in determining their performance. Conjugation involves the formation of covalent bonds between drug molecules and the surface of NPs, which can enhance drug stability, provide controlled release, and improve targeted delivery to specific tissues or cells [105]. In contrast, adsorption relies on non-covalent interactions, such as hydrophobic interactions or electrostatic forces, to physically load drugs onto NPs. While this approach is more straightforward and cost-effective, it may offer less stability and control over drug release compared to conjugation [106].
Enhancing active and passive transport
In nanomaterial drug delivery systems, the effectiveness of drug-targeted delivery can be further enhanced by employing both passive and active transport mechanisms, which complement the inherent characteristics of NPs as drug carriers [107]. Passive drug transport leverages the physicochemical properties of the nanocarriers and the specific physiological environment of the target tissue [108]. For instance, through the EPR effect, nanomedicines can passively accumulate at tumour sites, taking advantage of the leaky vasculature commonly found in tumours [107]. In contrast, active drug transport involves more precise interactions between nanomedicines and specific cellular targets, such as ligand-receptor binding, pH-responsive release, or the use of cell-penetrating peptides, which enable the drugs to efficiently target and enter specific cells [109]. These active mechanisms often work synergistically with passive transport to enhance the overall efficiency, specificity, and safety of drug delivery, particularly when combined with the strategic attachment methods of drug loading methods discussed earlier.
Doxil®, approved by the United States Food and Drug Administration (FDA) in 1995, marked the formal introduction of nanoparticle-based drug delivery formulations into clinical use. Traditional nanomaterial drug delivery systems, represented by Doxil®, have successfully achieved drug targeting and improved bioavailability. However, challenges such as drug toxicity and long-term biosafety remain [110]. Doxil® is a PEGylated liposomal formulation of doxorubicin that employs the EPR effect as a passive targeting mechanism, along with surface PEG modification. The hydrophilic PEG shell protects the drug from degradation by serum components and prevents opsonisation by the complement system, thereby avoiding rapid clearance by the mononuclear phagocytic system (MPS) and extending circulation time [111]. While these modifications enhance bioavailability and drug delivery, the reliance on the passive EPR effect alone results in low targeting efficiency, which can still lead to significant cardiotoxicity, similar to that of the original DOX [112].
To address the shortcomings, novel nanomaterial drug delivery platforms are being developed to achieve precise drug delivery by integrating both active and passive targeting mechanisms. This approach aims to reduce drug toxicity while enhancing therapeutic efficacy. Abraxane®, a nanoparticle-based formulation of paclitaxel bound to albumin, exemplifies this strategy and was approved by the FDA in 2005 [113, 114]. Albumin, a natural blood protein, contains hydrophobic pockets that can bind paclitaxel through hydrophobic interactions. Serving as a natural carrier, albumin increases paclitaxel's solubility in the bloodstream and offers advantages such as biodegradability, non-toxicity, and non-immunogenicity. Tumour tissues overexpress secreted protein acidic and rich in cysteine (SPARC), which functions similarly to albumin receptors by selectively binding albumin and accumulating it in tumour cells. Additionally, the high demand for albumin in tumour tissues further facilitates the concentration of drug-loaded albumin NPs at the tumour site. These characteristics enable albumin-based nanoparticle formulations to significantly increase local drug concentrations in tumour tissues, thereby reducing systemic toxicity and improving patient tolerance [115].
Additionally, numerous nanoparticle-based formulations that combine active and passive targeting mechanisms are currently undergoing clinical trials. For example, MBP-426 is a novel nano drug delivery system in which oxaliplatin is encapsulated within N-glutaryl phosphatidylethanolamine (NGPE) liposomes and bound to transferrin [116]. Transferrin acts as a targeting carrier by binding to transferrin receptors, which are highly expressed in tumour tissues, thereby efficiently delivering the drug to cancer cells [117]. Phase I clinical trials of MBP-426 have been completed, and the formulation has since advanced to a Phase Ib/II clinical trial in second-line patients with gastric, gastroesophageal, or esophageal adenocarcinoma [118].
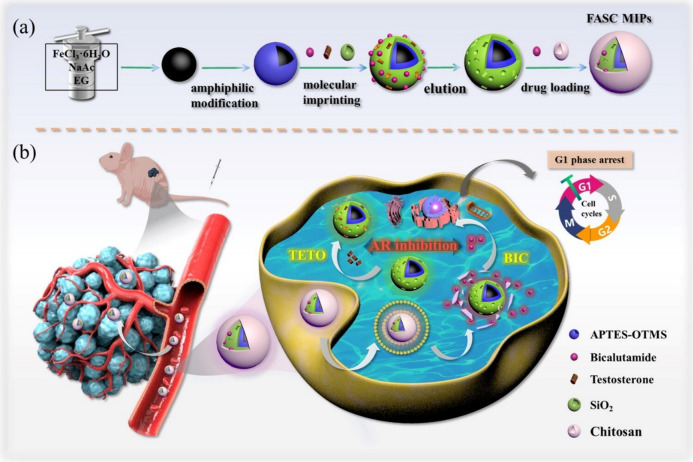
Recent studies have shown that molecularly imprinted polymeric nanoparticles (MIP NPs) are promising candidates for drug delivery systems that integrate both active and passive targeting mechanisms. MIP NPs have been designed with specific and complementary binding sites for drug molecules (referred to as “templates”) within their polymer matrix. These sites enable MIP NPs to recognise and load drugs or other templates with antibody-like affinity and selectivity, through either covalent or non-covalent binding [119–121]. This ability allows MIP NPs to improve the precision of drug delivery and release. Furthermore, the development of dual-imprinted polymers enables both drug loading and in vivo targeting, facilitating active targeted delivery and responsive release of drugs [122, 123]. For example, Liu et al. developed a novel pH-responsive core–shell MIP NPs (FASC MIPs) for prostate cancer treatment [124]. This formulation uses superparamagnetic tetraoxide NPs as the core material. The surface of these NPs was modified to create two types of imprinted cavities: one designed to sequester free testosterone (TETO) from solid tumours and another loaded with the anti-androgen drug bicalutamide (BIC). The NPs were further coated with pH-responsive chitosan, which facilitates targeted drug release in the acidic tumour microenvironment (Fig. 12). The FASC MIPs demonstrated synergistic antitumour effects by specifically targeting TETO through the imprinted cavities and releasing BIC in response to the acidic conditions, effectively inhibiting prostate cancer cells growth both in vitro and in vivo.
Fig. 12.
a Schematic illustration of the preparation route for FASC MIPs. b Strategy for achieving synergistic treatment of prostate tumours in vivo. Adapted with permission from Liu et al. [124]
Moreover, the high stability and robustness of MIP NPs protect the loaded drug molecules from complex biological environments, such as those in the gastrointestinal tract, making them suitable for various applications, including sustained transdermal formulations, therapeutic contact lenses, and oral formulations for protein delivery [122].
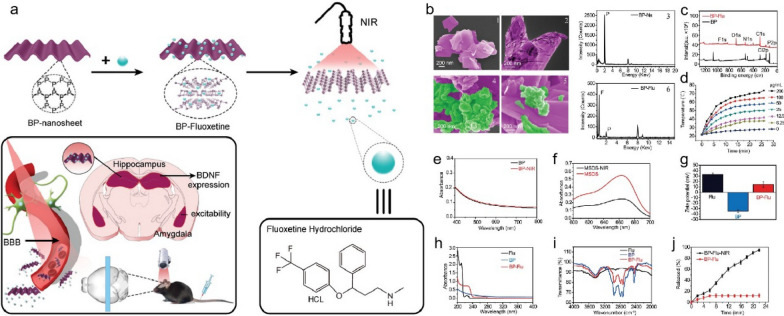
Enhancing the drug solubility and increasing stimulus-responsive release have been shown to effectively improve passive and active transport, respectively [125]. For example, Gou et al. developed carboxyl-functionalised mesoporous silica NPs (MSN-COOH) using a silicon coupling agent (hyd-TESPSA), which enhanced drug loading and solubility for poorly water-soluble non-steroidal antiinflammatory drugs (NSAIDs) like nimesulide and indomethacin [126]. The MSN-COOH improved drug bioavailability and efficacy by converting the drugs to an amorphous state and enabling pH-responsive release, thereby enhancing passive diffusion and active targeting to specific tissues, which reduces side effects. In addition to pH-responsive formulations, temperature, light, magnetic, ultrasound, and electrical-responsive drug delivery systems are also being increasingly developed [127]. An example of a light-responsive system is the use of black phosphorus (BP) nanosheets for targeted drug delivery. Jin et al. demonstrated that BP nanosheets, which possess excellent biodegradability and biocompatibility, can effectively load the antidepressant fluoxetine (Flu) through electrostatic interactions [128]. Upon exposure to near-infrared (NIR) light, these BP-Flu composites facilitated a rapid and controlled release of Flu, significantly reducing treatment duration and minimising side effects associated with the free drug (Fig. 13).
Fig. 13.
a Schematic procedure for fabricating BP-based drug delivery system for synergistic photothermal/chemotherapy of depression. b SEM, TEM, and EDS of BP nanosheets (b1, b2, b3) and BP-Fluoxetine (b4, b5, b6). Green colour represents Fluoxetine. c XPS survey spectra of BP nanosheets before and after Fluoxetine capturing. d The photothermal effect of BP nanosheets. e, f The UV–vis spectra of methylene blue (MSDS) and BP nanosheets before and after NIR irradiation. g Surface zeta analysis. h UV–vis absorption spectra of fluoxetine, BP, and BP-Fluoxetine (20 µg mL − 1). i FTIR spectra. j near-infrared defined fluoxetine releasing behaviour. Adapted with permission from Jin et al. [128]
In summary, nanomaterial drug delivery systems can provide targeted drug delivery, enhance therapeutic efficacy, improve the solubility and bioavailability of challenging drugs, and reduce toxicity. By integrating both active and passive targeting mechanisms, these systems achieve greater precision and improved biological safety. Additionally, they allow for precise drug release in diseased areas through internal responses to physiological changes and external responses to stimuli like pH and light.
Penetrating biological barriers
In addition to strategies for precise drug release to targeted tissues, effectively penetrating various biological barriers is crucial for maximising the efficacy of nanoformulations. These barriers include distribution barriers, microenvironmental barriers, and cellular and intracellular barriers [129].
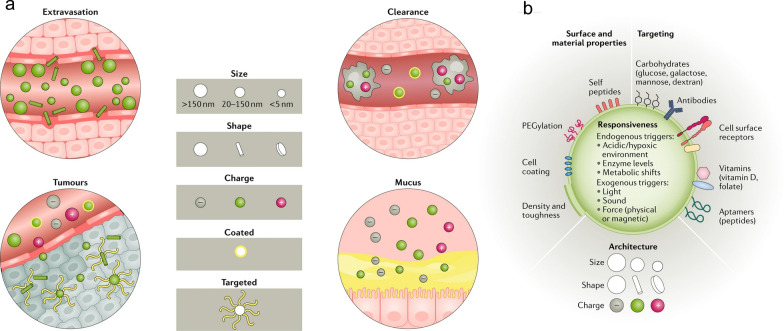
First, optimising the route of administration can significantly influence nanomaterials distribution. For example, intravenously injected polymeric NPs, such as PLGA NPs, tend to accumulate in the liver and spleen, while subcutaneous or lymphatic administration directs them more effectively to local lymph nodes [130]. Inhalation can deliver drugs directly to the lungs, bypassing systemic circulation and avoiding first-pass metabolism in the liver [131]. For systemic delivery, modifying the NP surface, such as with PEGylation, can prolong circulation time and enhance drug exposure. Adjusting NP size is also critical (Fig. 14a: NPs smaller than 10 nm are quickly cleared by the kidneys, whereas those larger than 200 nm can trigger immune responses unless specifically engineered [132].
Fig. 14.
a Factors such as size, shape, charge, and surface coating influence the behaviour of NPs in circulation and their interaction with local barriers. Spherical and larger NPs marginate more easily, while rod-shaped NPs extravasate better. Uncoated or positively charged NPs are cleared faster by macrophages. Rod-shaped, neutral, and targeted NPs penetrate tumours more effectively, whereas positively charged, smaller, and coated NPs traverse mucosal barriers more readily. b The surface properties, material composition, architecture, targeting moieties, and responsiveness of NPs can be modified to suit specific applications, allowing for a wide range of tailored nanoparticle designs. Adapted with permission from Langer et al. [129]
After reaching the target site, nanomaterial drug delivery systems encounter diverse microenvironments distinct from the circulatory system, such as acidic and enzyme-rich conditions in the gastrointestinal tract, low pH and high permeability in tumour tissues, and pH fluctuations in wound healing [133–135]. To overcome these challenges, NPs can be modified to respond to specific microenvironmental conditions. For example, pH-sensitive polymer coatings can enhance drug release in acidic environments [136], enzyme-resistant shells can protect NPs from degradation in the digestive tract [137], and penetration enhancers can improve NP delivery in tumour tissues [138]. Dynamic response modifications allow NPs to adapt to changing conditions, further enhancing therapeutic outcomes.
Modifying nano drug carriers can help them overcome cellular and intracellular barriers, ensuring effective drug delivery (Fig. 14b. Once inside the cell, NPs are often trapped in vesicles or endosomes, where the environment becomes increasingly acidic over time [139]. To promote drug escape from the endosome, some NPs are designed to respond to acidic or reductive environments, such as through the proton sponge effect or cleavable linkers, helping them disrupt the endosomal membrane and release the drug [140–142]. Additionally, complex-shaped NPs, such as nanostars, have shown promise in improving endosomal escape [143]. Once in the cytoplasm, drugs may need to traverse further intracellular membranes to reach specific organelles like the nucleus or mitochondria. pH-responsive nanoparticle systems are particularly useful in targeting these regions, especially for applications in gene editing or cancer immunotherapy [144, 145], as discussed further in the following sections.
Gene delivery
Expanding on drug delivery applications, these innovative nanoplatforms are now making significant strides in delivering gene therapeutics. Gene therapy is an experimental technique that exerts its therapeutic effects by introducing nucleic acids (DNA or RNA) into patient cells, enabling the expression of new genes or regulating the expression of target genes by correcting, disrupting or replacing them to prevent or treat a wide range of diseases [146, 147]. However, the effective in vivo delivery of nucleic acids into cells remains a significant challenge due to their low in vivo stability and susceptibility to rapid clearance from the bloodstream affecting their cellular uptake. Furthermore, nucleic acids have limited permeability through cell membranes owing to their electronegativity and large molecular size, posing a challenge to their effective cellular entry [148, 149].
Gene therapy can be stratified into DNA and mRNA-based therapy, with each encountering distinct challenges. In DNA-based therapies effective gene delivery involves overcoming extracellular barriers including enzymatic degradation and immune clearance, as well as intracellular barriers endosomal entrapment and nuclear transport across the nuclear envelope, a necessary step in DNA delivery and successful transgene expression [150]. Similarly, mRNA-based therapies must maintain the stability of the mRNA amid nucleases within the extracellular environment and avoid clearance [151, 152]. Unlike DNA therapies, in mRNA-based therapies, translation occurs in the cytosol rather than the nucleus, making endosomal escape a key intracellular barrier to conquer [154]. These challenges necessitate the development of vectors that can overcome extracellular and intracellular barriers to deliver genetic materials into target cells. Traditional vectors such as viral vectors are limited by obstacles such as immunogenicity, rapid clearance, limited genome packaging capacity, and potential carcinogenicity [149].
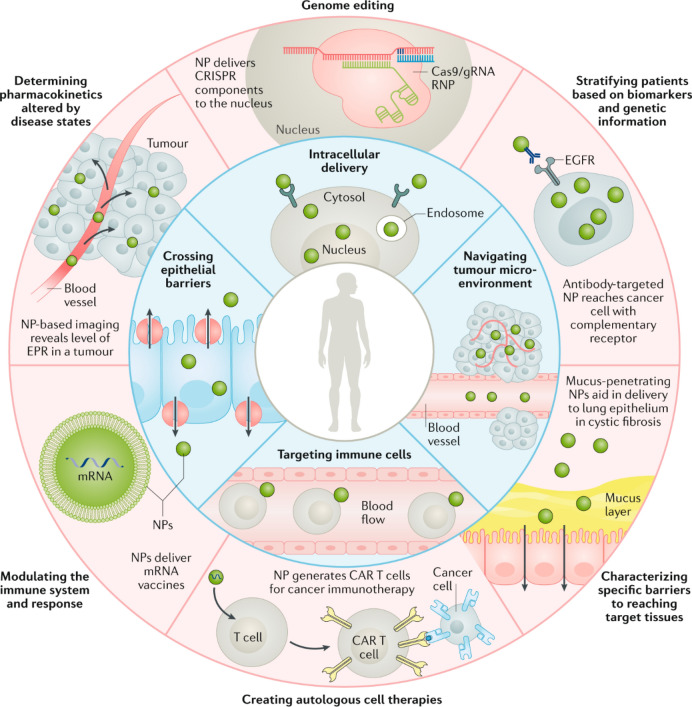
Therefore, the ideal vector should protect genetic material, avoid clearance, enable cellular uptake, promote early endosomal escape, and be biocompatible, biodegradable, non-immunogenic and non-toxic to host cells [149]. Thus, efforts have shifted towards non-viral vectors, such as NPs, which have emerged as powerful tools to overcome the challenges associated with viral vectors for gene delivery by enabling safe and efficient targeting. These NPs boast a high drug-loading capacity, low mutagenicity and the ability to overcome biological barriers without provoking immune responses (Fig. 15) [149, 153].
Fig. 15.
Schematic overview highlighting biological barriers that NPs can overcome (inner ring) and potential precision medicine applications that could benefit from NPs (outer ring). Intelligent NPs designs can improve the delivery of precision medicines, such as gene therapies, and accelerate their clinical translation. Abbreviations: CAR, chimeric antigen receptor; EGFR, epidermal growth factor receptor; EPR, enhanced permeation and retention; gRNA, guide RNA; RNP, ribonucleoprotein. Adapted with permission from Langer et al. [129]
Furthermore, NPs are hailed for their small size, high surface-to-volume ratio, and tunable properties that allow for selective targeting. Moreover, their ability to encapsulate genetic materials protects them from enzymatic digestion and facilitates efficient cellular uptake making them ideal vectors for gene delivery [155]. This protective capacity is exemplified with lipid NPs which have gained particular attention, especially with the recent success of the mRNA COVID-19 vaccines like BNT162b2 (Comirnaty® by BioNTech and Pfizer) which demonstrated 95.0% effectiveness in preventing COVID-19 [156]. These lipid NPs protect the mRNA encoding the SARS-CoV-2 spike protein by encapsulating it into the core, ensuring its safe delivery into target cells where it can be translated and trigger an immune response [157].
In addition to safeguarding genetic cargo, NPs must also facilitate cellular uptake and gene transfection, a process that can be hindered by the formation of a protein corona by serum proteins, leading to the physical destabilisation and agglomeration of NPs. Xiong et al. developed zwitterion CBAA-modified gold dendrimer-entrapped NPs (Au DENPs) for serum-enhanced gene delivery to address this. Remarkably, the zwitterion coating conferred the NPs with antifouling properties, resisting serum protein adsorption and increasing gene delivery efficiency between 1.4 to 1.7-fold in serum-containing media compared with serum-free medium (Fig. 16). Consequently, NPs endowed with anti-fouling properties effectively enhanced gene delivery by preventing serum adsorption, suggesting their potential to circumvent immune clearance [158, 159].
Fig. 16.
Overview of the synthesis procedure of Au DENPs (a) for gene delivery applications. b Notes for abbreviation: G5, generation 5; CBAA, carboxybetaine acrylamide; PEG-Mor, polyethylene glycol-morpholine; DENPs, dendrimer-entrapped NPs; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; NHS, N-hydroxysuccinimide; and COOH-PEG-Mor, PEG-Mor with the other end of the carboxyl group. Adapted with permission from Xiong et al. [158]
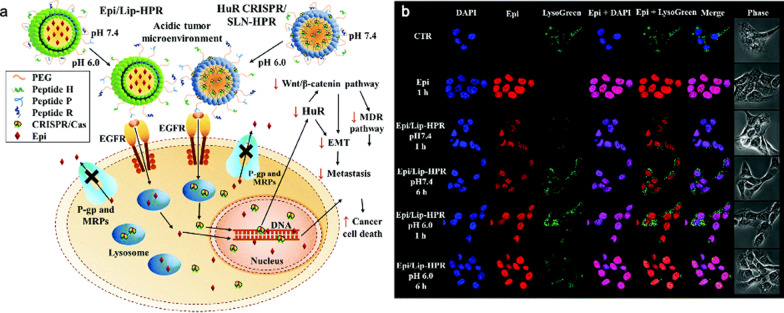
As previously discussed, DNA-based therapies require the DNA cargo to enter the nucleus for efficient transgene expression [150]. However, once inside the cell, NPs delivering DNA encounter several significant obstacles involving escaping from endosomal compartments before lysosomal trafficking and nucleus translocation which involves crossing the nuclear envelope, a formidable barrier, for transcription. To overcome this, NPs have been designed with nuclear targeting capabilities and nuclear localisation signals to ensure gene delivery across the nuclear envelope [160]. Wang et al. formulated pH-sensitive NPs delivering the CRISPR/Cas9 system and epirubicin to the nucleus in cancer cells (Fig. 17). These stimuli-responsive NPs exploit EGFR-targeting peptides and nucleus-localising sequences that enable endosomal escape and direct nuclear localisation under acidic tumour conditions [161]. By exposing targeting peptides at a lower pH, cellular uptake and transfection efficiency were enhanced. This demonstrates the potential of NPs to successfully target and traverse the nuclear envelope resulting in efficient gene delivery.
Fig. 17.
a Schematic illustration of how the HuR CRISPR/SLN-HPR and Epi/Lip-HPR NPs adapt in an acidic tumour environment due to the conformational changes of the H-peptide. The entry of NPs into SAS cells occurs via exposed P and R peptides. The CRISPR/Cas9 system knocked out the HuR gene, modulating several cancer pathways, while epirubicin accumulated in the nucleus, leading to cancer cell death. b pH-responsive localisation of Epi/Lip-HPR in SAS cells at pH 7.4 and 6.0, observed by CLSM. DAPI stains the nucleus; LysoTracker Green stains lysosomes. Adapted with permission from Wang et al. [161]
In conclusion, NPs are proving to be sophisticated vectors for overcoming key challenges in gene delivery. The development of these advanced nanomaterials has significantly enhanced the potential of gene delivery systems, offering solutions to the limitations of traditional viral vectors. The continued innovation in nanomaterial design and functionalisation is anticipated to result in more effective and safer gene delivery systems, ultimately enhancing therapeutic outcomes and broadening the scope of their applications.
Cancer therapy
Cancer remains a prominent cause of mortality globally, with conventional approaches identified for tumour-specific treatment: surgical resection, chemotherapy, and radiation therapy [162]. However, despite these treatment options, cancer patients often face short survival expectation and poor life quality.
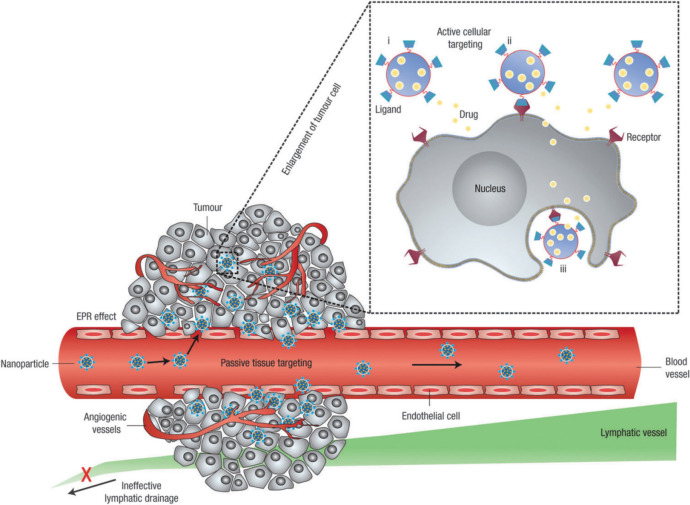
The rapid development of nanotechnology has introduced complementary and alternative strategies for cancer treatment by using both passive targeting, due to the small size of NPs, and active targeting, achieved through specific modifications to the NPs, thereby offering greater precision in therapy [163]. As mentioned in Sect. “Drug delivery”, passive targeting of NPs takes advantage of the EPR effect, which occurs due to the abnormal structure of tumour blood vessels. These vessels have looser walls and inadequate lymphatic drainage, allowing macromolecules or NPs to penetrate and accumulate more easily within tumour tissues [164]. Active targeting, in contrast, involves the surface modification of NPs with specific ligands such as antibodies, peptides, or carbohydrates that bind selectively to receptors on tumour cells (Fig. 18). This enhances the precision of drug delivery, exemplified by the use of trastuzumab, an antibody targeting the HER2 receptor, or folic acid ligands targeting the folate receptor [165, 166].
Fig. 18.
Passive and active tumour targeting. NPs achieve passive targeting by extravasating through the tumour’s permeable vasculature and poor lymphatic drainage (EPR effect). Active targeting is accomplished by functionalising NPs with ligands that bind specifically to target cells. Once targeted, NPs can (i) release their payload near target cells, (ii) adhere to the cell membrane as an extracellular sustained-release depot, or (iii) be internalised into the cell for direct drug delivery. Adapted with permission from Peer et al. [167]
Modified NPs frequently combine both passive and active targeting mechanisms to effectively direct them to tumour tissues, addressing the limitations of traditional cancer therapies, such as limited efficacy, toxicity, severe side effects, cancer recurrence, and drug resistance [168]. The following section explores the use of nanomaterials in surgical resection, radiation therapy, chemotherapy, and other novel therapies.
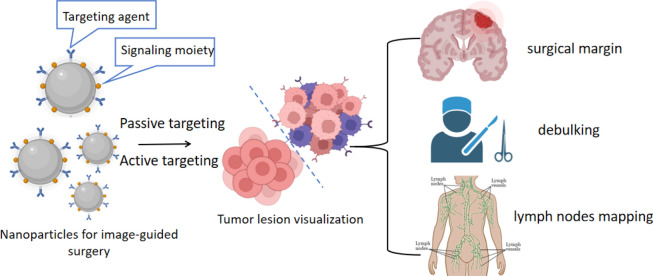
Accurate detection of malignant and healthy tissues is crucial for the therapeutic efficacy of surgical resection. As described in Sect. “Medical diagnostics”, nanomaterials such as QDs, surface-enhanced Raman spectroscopy (SERS) NPs, luminescent NPs and dye nanoformulations, are able to act as contrast agents for image-guided surgery [169]. These modified NPs differentiate between tumours and nearby normal tissues through active targeting and passive targeting effects, thus helping surgeons to identify surgical margins and local metastases with high resolution and sensitivity, leading to improved therapeutic outcomes (Fig. 19).
Fig. 19.
NPs development promotes improved precision in surgical tumour removal
In addition, many nanomaterials have been used to construct fast and efficient hemostatic measures that significantly improve safety during surgical treatments [170]. After surgery, wound healing involves three primary stages to stop bleeding. First, the damaged blood vessels constrict to reduce blood flow. Next, platelets aggregate to form an initial platelet plug that seals the wound. Finally, the coagulation cascade activates, producing fibrin that combines with the platelet plug to form a stable clot, effectively preventing further blood loss [171]. NPs have unique advantages in promoting hemostasis by enhancing platelet activation and aggregation, similar to platelet-activating factors. For example, Liang et al. synthesised zeolitic imidazolate framework (ZIF-8) nanoparticle-enhanced cryogels that provide rapid hemostasis during tumour resection surgery by promoting coagulation and stopping bleeding [172]. Additionally, these cryogels help prevent cancer recurrence after liver cancer surgery by releasing drugs in response to pH changes and generating reactive oxygen species under ultrasound to kill remaining cancer cells.
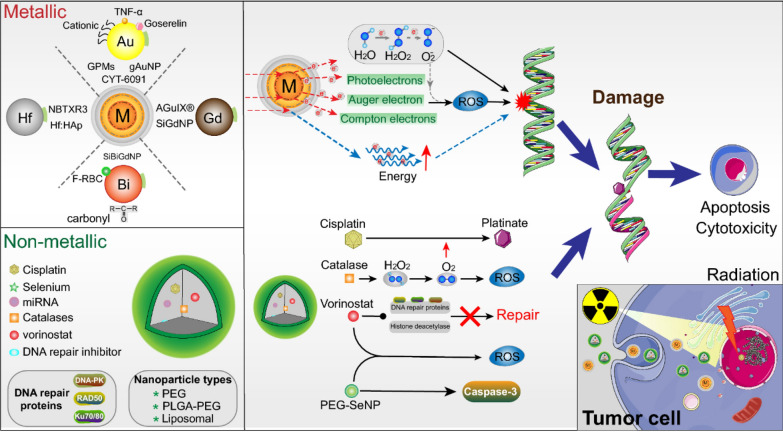
In radiation therapy, therapeutic effects often necessitate higher doses of irradiation, as tumour cells exhibit low radiation absorption. However, this can result in significant damage to surrounding normal tissues [173]. Therefore, the development of radiosensitisers and methods to protect normal tissues is a major strategy to increase the sensitivity of tumours to radiation therapy and to minimise side effects. Nanomaterials that incorporate high atomic number elements, including Au-, gadolinium (Gd)-, bismuth (Bi)-, hafnium (Hf)- and tungsten (W)-based nanomaterials can increase intracellular radiation deposition, making them potentially ideal sensitisers for radiotherapy (Fig. 20) [174].
Fig. 20.
Schematic of NP functional mechanisms in radiotherapy. Combing ionising radiation (IR) with NPs can boost radiosensitisation, cell apoptosis and cytotoxicity. Upper: Metallic NPs (Au, Hf, Gd and Bi) deposit the IR dose through interactions, such as electron secretion (Compton, Auger and photoelectric), ROS generation and energy amplification. Lower: Non-metallic NPs-encapsulated combined with radiotherapy further induced DNA damage and prevented rapid DNA repair, which will cause more cell apoptosis. Adapted with permission from Jin et al. [175]
Targeted modifications can further increase intratumoural enrichment to absorb radiation energy and promote the efficacy of radiation therapy [176]. Jyothi U et al. developed a novel nanoparticle system, R11-NU7441 NPs, which consists of PLGA NPs conjugated with the prostate cancer cell-penetrating peptide R11 and encapsulated with the potent radiosensitizer 8-dibenzothiophen-4-yl-2-morpholin-4-yl-chromen-4-one (NU7441). This system is designed for active targeting specifically to prostate cancer cells and provides a sustained release of NU7441, significantly enhancing the effectiveness of radiotherapy [177].
In addition to radiosensitisation, inorganic-based nanomaterials, exemplified by wide-ranging oxides of cerium (Ce), manganese (Mn), tantalum (Ta), and vanadium (V), play an important role in radioprotection due to their excellent enzyme-mimicking properties and their ability to scavenge ROS generated by radiotherapy in normal tissues, thus potentially reducing radioinflammation [178].
Conventional chemotherapeutic drugs typically lack specificity, causing potential damage to normal tissue cells alongside cancer cells during chemotherapy, resulting in severe adverse effects. Additionally, drug resistance and poor water solubility also hinder the effectiveness of chemotherapy drugs in clinical use [179]. As detailed in Sect. “Drug delivery”, NPs transport chemotherapeutic drugs to the tumour site by direct targeting through chemical modification or passive targeting of tumour cells through the EPR effect, thereby reducing systemic toxicity [180–182]. Moreover, nanocarriers can effectively address issues such as poor solubility, rapid metabolism, unstable absorption, insufficient permeability, and drug resistance associated with small molecule drugs [183].
Expanding on traditional treatments, advancements in tumour understanding have spurred rapid development in new therapeutic approaches such as biotherapy (including cellular immunotherapy, gene therapy, etc.), photothermal therapy (PTT), and photodynamic therapy (PDT). Some examples are shown in Fig. 21.
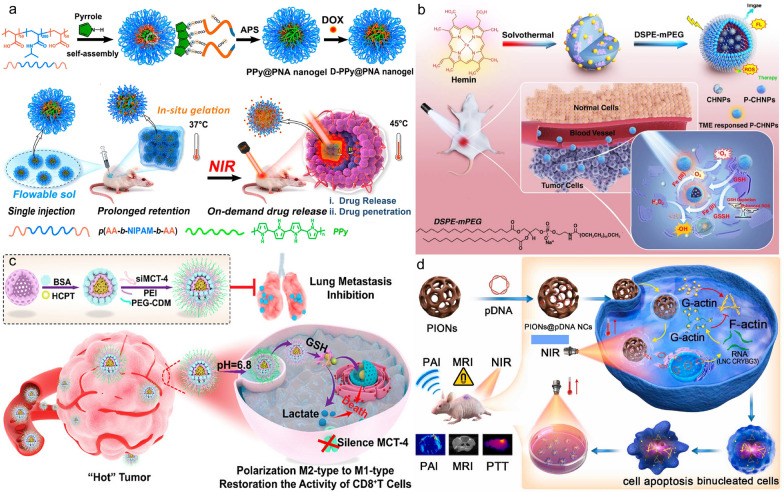
Fig. 21.
a The preparation of temperature-sensitive doxorubicin (DOX)-loaded complex polymeric nanogels of poly(acrylic acid-b-N-isopropylamide-b-acrylic acid/polypyrrole) (D-PPy@PNA nanogels) and in situ formation of D-PPy@PNA hydrogels for synergistic photothermo-chemotherapy. Adapted with permission from Geng et al. [184]. b Scheme of the synthesis approach of polymer encapsulated carbonised hemin NPs (P-CHNPs) and therapeutic mechanism of light amplified oxidative stress in tumour microenvironment by P-CHNPs for boosting photodynamic anticancer therapy. Adapted with permission from Lin et al. [185]. c Synthesis of HMONs@HCPT-BSA-PEI-CDM-PEG@siMCT-4, a surface-modified and redox (GSH)-responsive hollow mesoporous organosilica nanoplatform loaded with hydroxycamptothecin (HCPT) and monocarboxylate transporter 4-inhibiting siRNA (siMCT-4), administered for synergistic tumour chemo-immunotherapy. Adapted with permission from Li et al. [186]. d Schematic illustration of using porous iron oxide nanoagents (PIONs)-loaded with plasmid pcDNA3.1-LNC CRYBG3 nano-complexes (PIONs@pDNA NCs) as a photoporation nanoplatform for PTT and gene therapy. Adapted with permission from Huang et al. [187]
These emerging methods significantly enhance comprehensive tumour treatment, improving patient prognosis and quality of life. PTT and PDT offer safety, non-invasiveness, and high efficiency [188]. Yet, traditional photosensitisers lack specificity and suffer from low water solubility, limiting their bioavailability. Nanocarriers boost photosensitiser accumulation in tumours, enhancing phototherapy effectiveness while minimising damage to healthy tissues [189].
While immunotherapy is esteemed, it encounters challenges like low patient responsiveness, limited tumour specificity, and immunosuppressive tumour microenvironments. Nanomaterial-based approaches, such as inducing immunogenic cell death (ICD), combining with immune checkpoint blockade therapy, cancer vaccines, adoptive immunotherapy, and immune microenvironment modulation, show promise in enhancing tumour immunotherapy efficacy [190].
Gene therapy introduces genes into cells to correct abnormalities and produce therapeutic proteins. It sidesteps the systemic toxicity and tolerance issues typical of chemotherapy [191]. The development of gene therapy relies heavily on nanotechnologies. As mentioned in Sect. “Gene delivery”, gold, polymer, and lipid NPs are amongst the main non-viral carriers for gene delivery, which can effectively deliver small molecules of nucleic acids, prevent their extracellular degradation by nuclease and improve drug distribution [192]. In conclusion, nanotechnology plays a vital role in numerous cancer treatments.
Nanotechnology is essential in many cancer treatments; however, NPs face challenges in tumour therapy due to individual heterogeneity among patients. The primary advantage of NPs in cancer therapy is their ability to specifically accumulate in tumour tissues through passive and active targeting mechanisms. Passive targeting largely relies on the EPR effect, but this effect is significantly influenced by various factors. Different tumour types, such as solid tumours like breast cancer and pancreatic cancer, may show a more pronounced EPR effect, while blood tumours or tumours with poor vascularsation may demonstrate a weaker EPR effect. The uneven angiogenesis of tumours, depending on their anatomical location and vascular characteristics, can also impact the EPR effect, particularly in tumours that are less vascularised or have poor vascular permeability. Additionally, the diverse microenvironments of tumours, characterised by different pressure states and compositions, affect the efficacy of the EPR effect and consequently the accumulation of NPs in tumour tissues. Furthermore, individual patient differences, such as variations in physiological state, hemodynamic characteristics, inflammation levels, and vascular permeability, contribute to the variability in the EPR effect [193–195].
To address the challenges posed by this individual heterogeneity and reduced targeting effectiveness, several strategies have been proposed. These include personalised treatment plans that assess each patient's tumour vascularisation and microenvironment characteristics to develop tailored NP delivery strategies, combination therapies that integrate both EPR and active targeting strategies to enhance the accumulation of NPs in tumours, and auxiliary methods such as using ultrasound or vascular permeability enhancers to boost the EPR effect in tumour tissues. These strategies aim to overcome the limitations of individual differences and optimise the therapeutic potential of NPs in cancer treatment.
Antimicrobial and antiviral applications
Overuse or misuse of conventional antibiotics in various sectors such as medical care, livestock farming, and agriculture has resulted in heightened antibiotic resistance, diminished efficacy of antibiotic therapies, and the rise of superbugs [196–198]. This resistance develops when bacteria adapt to survive antibiotics, often through mechanisms such as modifying target sites, producing enzymes that deactivate antibiotics, using efflux pumps to expel the drugs, reducing membrane permeability to prevent antibiotic entry, or forming protective biofilms that blocks antimicrobials penetration [199]. These adaptations not only complicate infection management but also contribute to higher mortality rates, prolonged hospital stays, and the need for more expensive and potentially toxic alternative therapies. Given the limitations of traditional antibiotics in overcoming these resistance mechanisms, there is an urgent need for innovative antimicrobial strategies.
Advances in nanotechnology present hopeful avenues for addressing these challenges. Nanomaterials enable deliberate engineering designs, including size control, surface modification, crystalloid change, and stimuli-responsive functionalisation, which offer unique interactions with bacterial cells compared to conventional antibiotics [200]. Such interactions frequently lead to distinctive killing mechanisms and exceptional antimicrobial attributes, including broad-spectrum activity, long-lasting effects, inhibition of biofilm formation and resistance-independent antimicrobial effects [201, 202]. By circumventing traditional pathways of resistance, nanotechnology-based solutions have the potential to revolutionise the fight against resistance, offering new hope for effective and sustainable antimicrobial therapies.
Five classes of antimicrobial nanomaterials have been extensively researched and reported, encompassing metallic NPs (such as Ag, Cu, Au, ZnO, La2O3, CeO2, V2O5, etc.), carbon-based nanomaterials (including graphene, GO, CNTs), borides (like boron nitride), nanosized polymers (such as polycarbonate), and nanocomposites (for instance, La2O3/Ag-GO) [202]. Based on the initial interactions between the materials and bacteria, Xie et al. proposed a summary of several antimicrobial mechanisms, including membrane destruction, disruption of the electron transport chain, catalytic killing, cell division arrest, cell trapping, and ionic killing (Fig. 22a–f) [202]. In addition, they proposed four design principles for fabricating antimicrobial nanomaterials to prevent the resistance evolution include: engineering nanocomposites to diversify nano-microbe interactions, enabling stimuli-responsive functionalisation for controllable bactericidal effects, grafting targeting ligands for precise bacterial killing, and incorporating doping/shielding to prevent triggering defense mechanism in bacterial cells due to the release of metal ions (Fig. 22g).
Fig. 22.
The antimicrobial mechanisms of nanomaterials operate through their Molecular Initiating Events (MIEs), which include the following: a Membrane destruction via physical or chemical interactions; b Disruption of the electron transport chain by metallic or semiconductor nanomaterials; c Catalytic killing by enzyme-mimicking actions that directly destroy critical biomolecules or induce ROS-mediated damage; d Arrest of cell division by blocking or degrading the Z ring, a cytoskeletal structure composed of the protein FtsZ that is essential for bacterial cell division; e Prolonged ionic killing by inactivating cellular enzymes, disrupting respiratory processes, and increasing intracellular ROS levels. f Cell trapping through nanoparticle aggregation via noncovalent interactions. g Four proposed principles for designing antimicrobial nanomaterials: (i) diversifying nano-microbe interactions (e.g., structural destruction, catalysis, noncovalent binding); (ii) enabling stimuli-responsive functionalisation (e.g., pH, magnet, light, enzyme); (iii) grafting targeting ligands (e.g., charged ligands, peptides); and (iv) doping/shielding modifications to prevent metal ion release. Adapted with permission from Xie et al. [202]
In addition to directly targeting bacteria, nanomaterials can combat antimicrobial resistance (AMR) by targeting bacterial biofilms through various mechanisms. Biofilms, protective layers formed by bacterial communities, are significant contributors to chronic infections and increased antibiotic resistance, particularly in the case of multi-drug resistant (MDR) infections, where bacteria are resistant to multiple antibiotics [203]. These structured communities of bacteria are encased in a self-produced extracellular polymeric substance (EPS) that protects them from external threats, including antibiotics, thereby complicating treatment efforts. Therefore, strategies that enhance the penetration of antimicrobials into biofilms, disrupt mature biofilms, and prevent biofilm formation are crucial for effectively combating the development of AMR and MDR infections [204].
By modulating the size, shape, and surface properties of NPs, it is possible to enhance their penetration into biofilms, thereby weakening the protective barrier provided by the biofilm [205]. For instance, while EPS can inhibit the penetration of silver ions, Ag NPs with diameters less than 20 nm can effectively penetrate biofilms of Escherichia coli and Pseudomonas fluorescens [206].
Nanomaterials can also disrupt mature biofilms. NPs with unique shapes, such as nanosheets, nanoneedles, and sea urchin-shaped NPs, can mechanically disrupt the EPS of biofilms [207]. Magnetic nanomaterials have been shown to cause mechanical disruption of biofilms through the application of direct and alternating magnetic fields. For example, a magnetic urchin-like capsule robots (MUCRs) developed by Sun et al. loaded with magnetic liquid metal droplets (MLMDs) can change shape into spheroids and rods with sharp edges when triggered by an external magnetic field. These sharp edges, along with natural microspikes, can physically disrupt the protective function and mechanical stability of EPS (Fig. 23). Additionally, the applied mechanical force can break bacterial cells embedded within the biofilm, contributing to the overall destruction of the biofilm [208].
Fig. 23.
a Schematic of biofilm eradication on biliary stents using the MUCR@MLMD swarm. b Fabrication process of MUCR@MLMD. c TEM image of MLMD after sonication. d Size distribution histogram of MLMD. e EDX spectrum showing Ga, In, and Fe content in MLMD. f SEM image of MUCR post-chemical bath deposition and magnified TEM image of individual spines. g TEM image of MUCR@MLMD after vacuum loading and ultrasonic treatment. h Magnetic hysteresis loop of MUCR@MLMD. i Visualisation of biofilm eradication mechanism using microswarm; white arrows indicate deformed MLMDs. Reproduced with permission. Adapted with permission from Sun et al. [208]
Beyond mechanical damage, electron transfer can also be used to disrupt biofilms. 2D transition metal disulfide (MoS2) nanosheets with nanoholes containing atomic vacancies can enhance electron transport interactions with biofilms, disrupting key components of mature biofilms such as proteins, polysaccharide intercellular adhesin (PIA), and extracellular DNA, while downregulating the expression of related genes [209]. Moreover, oxidative stress can not only damage bacterial cell membranes but also lead to the degradation of EPS, achieving a synergistic antibacterial and anti-biofilm effect. Various advanced nanomaterials have been reported to generate ROS and reactive nitrogen species (RNS) through different mechanisms, including photocatalysis, nanozyme catalysis, photodynamic therapy, sonodynamic therapy, and nitric oxide (NO) therapy [210]. For example, in a recent study supramolecular photosensitiser NPs were developed, capable of producing ROS under light irradiation to disrupt biofilms and release antibiotics, effectively combating MDR bacteria by breaking down the biofilm structure and enhancing drug penetration [211]. Another example is a polymeric NPs internally loaded with the photosensitiser chlorin e6 designed for root canal irrigation, which combines photodynamic therapy with NO release. This system enhances biofilm penetration and eradication by generating ROS and NO simultaneously, providing an effective strategy against endodontic bacterial infections, especially in the treatment of apical periodontitis [212].
Certain nanomaterials do not combat bacteria and biofilm through direct interaction but instead inhibit biofilm formation and virulence expression by disrupting bacterial quorum sensing (QS) systems, the process by which bacteria communicate with each other [213–215]. This approach is expected to reduce the risk of developing resistance by avoiding selection pressure on bacteria. In addition, since QS systems are absent in mammals, therapies that block microbial QS reduce the risks of host toxicity. Chen et al. developed pH-sensitive curcumin-loaded nanoparticles (Cur-DA NPs and anti-CD54@Cur-DA NPs) to inhibit QS for treating biofilm-associated bacterial infections. These NPs enhance penetration and curcumin release in acidic biofilm environments, effectively inhibiting QS and improving antibiotic efficacy. The targeting modification with anti-CD54 boosts NPs accumulation at infection sites, significantly reducing bacterial load and inflammation in vivo, suggesting a promising antimicrobial strategy (Fig. 24).
Fig. 24.
a Illustration of Anti-CD54@Cur-DA NPs delivery for inhibiting QS and enhancing antibiotic therapy against biofilm-associated infections. b HPLC analysis of QS signal molecules in P. aeruginosa treated with Cur-DA NPs or free curcumin (n = 3). c Volcano plot showing significantly upregulated (red) and downregulated (blue) genes after treatment with Cur-DA NPs. d Heat map of multidrug efflux-related genes in P. aeruginosa biofilms treated with Cur-DA NPs or free curcumin. e Timeline for chronic P. aeruginosa lung infection model and treatments. f Ex vivo fluorescent imaging of heart, liver, spleen, lungs, kidneys after 24 h treatment with anti-CD54@Cur-DA NPs or Cur-DA NPs. g Quantitative bacterial count in lungs after treatment with tobramycin (TOB, aminoglycoside antibiotics), Cur-DA NPs + TOB, or anti-CD54@Cur-DA NPs + TOB (n = 8). h The H&E, Masson's trichrome, and Sirius Red staining of lung slices post-treatment with TOB, Cur-DA NPs + TOB, or anti-CD54@Cur-DA NPs + TOB. Statistical analysis (t-test) showing significance: *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar: 200 μm. Adapted with permission from Chen. et al. [216]
In addition, nanomaterials have been used as delivery of antimicrobial agents, such as antibiotics and antimicrobial peptides [217]. Immobilisation of nanomaterials on implantable devices, wound dressings, bone cement, or dental materials can serve as antibacterial coating [218].
Viral infections represent a significant health challenge, posing risks to individuals worldwide. Nanomaterials have shown great advantages in the study of antiviral therapy. In general, antiviral nanomaterials can prevent viral infections by directly targeting virus particles, interfering with virus-host cell interaction, and suppressing viral proliferation [219]. For example, the sharp edged structure of GO can cause physical damage to viral structures [220]; Carbon dots synthesised from glycyrrhizic acid (Gly-CDs) are reported to directly inactivate porcine reproductive and respiratory syndrome virus (PRRSV), inhibit viral invasion and replication, and activate innate immunity [221]; Ag NPs were shown to inhibit the interaction between spikes over the viral membrane and target cell membrane receptors, thereby inhibiting human immunodeficiency virus (HIV-1) attachment [222]; Au NPs were reported to inhibit replication of foot-and-mouth disease virus (FMDV) [223]; MIP NPs that use whole viral particles as templates are able to specifically capture and rapidly remove viral particles from the environment, thereby preventing viral infections [224].
In addition, nanomaterials can be used as drug carriers to deliver antiviral drugs. For example, PLGA NPs, cationic nanogels, and chitosan nanospheres can deliver bictegravir, azidothymidine, and acyclovir, respectively, resulting in benefits such as sustained release, reduced cytotoxicity and dosing, increased site-specific delivery, and improved stability and solubility [225–227].
Nanomaterials offer promising solutions for both antibacterial and antiviral therapies through unique mechanisms and targeted delivery systems. Their ability to prevent resistance evolution, enhance drug efficacy, and reduce toxicity highlights their potential to revolutionise infectious disease treatment in healthcare applications.
Future trends in nanotechnology for healthcare applications
Building on the previous discussion of nanotechnology's major applications in healthcare-medical diagnostics, tissue engineering, drug delivery, gene delivery, cancer therapy, and antimicrobial/antiviral applications-this section explores the future trends of nanotechnology in the field. As nanotechnology continues to revolutionise healthcare, it offers innovative solutions that promise to enhance the efficacy and personalisation of medical treatments. Key emerging trends such as personalised medicine, theranostics, and smart drug delivery systems are particularly noteworthy. These advancements not only represent cutting-edge technological progress but also have the potential to significantly impact the future of healthcare by enabling more precise, effective, and tailored medical interventions.
Personalised medicine aims to tailor treatments to the individual characteristics of each patient, making therapies more effective and reducing side effects. Nanotechnology plays a crucial role in this domain by enabling the development of nanoformulations that are specifically designed to interact with individual genetic profiles, thereby enhancing therapeutic outcomes. Jhawat et al. highlighted the integration of pharmacogenomics with nanotechnology, proposing that nanoformulations could lead to error-free and targeted therapeutic agent delivery, which is essential for personalised healthcare [228]. In addition, nanoinformatics, which combines nanotechnology with artificial intelligence, is becoming a powerful tool in personalised medicine. By analysing patient-specific data, nanoinformatics enables the design of better nanomaterials for personalised drug delivery, thus improving treatment precision [229].
Theranostics is an innovative approach in medicine that integrates therapeutic and diagnostic capabilities into a single platform, allowing for the simultaneous diagnosis, treatment, and monitoring of diseases. This dual functionality enhances the precision of medical interventions by utilising advanced nanomaterials and imaging techniques to deliver therapeutic agents directly to targeted sites while continuously monitoring treatment efficacy and disease progression [230]. The real-time feedback loop provided by theranostics enables clinicians to adjust treatment strategies based on individual patient responses, optimizing therapeutic outcomes. A notable advancement in this field is optical imaging-guided nanotheranostics, which utilises advanced imaging modalities such as MRI and CT to enhance the precision of cancer treatments. This approach not only allows for accurate visualisation of tumours but also improves the monitoring of therapeutic responses and enables real-time adjustments to treatment, thereby supporting personalised medicine (Fig. 25) [231].
Fig. 25.
Schematic illustration of nanotheranostics and personalised medicine. Nanotheranostics integrates diagnostic and therapeutic capabilities into a single platform for precise drug delivery and real-time treatment monitoring. Personalised nanomedicine allows for treatment adjustments based on patient response, optimizing effectiveness and minimising toxicity. Adapted with permission from Mura. et al. [232]
Theranostics is particularly promising in cancer care, where real-time monitoring ensures precise drug delivery to tumour sites, minimising damage to healthy tissues and reducing systemic toxicity [233]. Furthermore, it facilitates tracking therapeutic efficacy at the molecular level, offering insights into the biological responses of tumours, which aids in early detection of resistance and supports adaptive, personalised treatment plans tailored to the cancer's evolving nature [234]. These personalised strategies have been shown to significantly enhance patient outcomes by enabling more targeted and effective therapies [233]. Beyond oncology, the principles of targeted delivery and real-time monitoring that define theranostics have the potential to transform the management of other conditions, including cardiovascular diseases, neurological disorders, and infectious diseases, leading to more precise, personalised, and efficient healthcare across various medical fields [235].
Smart drug delivery systems are designed to release therapeutic agents in a controlled manner, specifically targeting diseased cells while minimising side effects on healthy tissues. This precision is achieved through the use of stimuli-responsive nanomaterials that can react to environmental changes such as pH, temperature, or magnetic fields. For example, polymeric nanodroplets have emerged as versatile carriers for gas and drug delivery, showing great potential in treating conditions like hypoxia and cancer [236]. Some cutting-edge examples have been presented in the Sect. “Drug delivery” and “Gene delivery”. The integration of 3D printing with nanotechnology is also advancing smart drug delivery systems, allowing for the creation of complex, multifunctional medical products tailored to individual patient needs. This combination is expected to lead to significant advancements in personalised medicine, particularly in tissue engineering and regenerative medicine [237].
Nanorobots are at the forefront of nanotechnology's impact on healthcare. These tiny robots can navigate through the body, delivering drugs precisely where they are needed, and are expected to play a significant role in personalised and precision medicine (Fig. 26). However, there are still challenges to overcome, particularly regarding safety and the scalability of production for clinical use [238]. Another exciting development is the use of molecularly imprinted polymers in drug delivery. As discussed in Sect. “Drug delivery”, MIPs are synthetic polymers with the ability to recognise and bind specific molecules, making them ideal for targeted drug delivery systems that require high specificity and efficiency [122].
Fig. 26.
Schematic of a nanorobot for drug or cell delivery, highlighting its four key components: propulsion, payload cargo hold, sensory and anchorage system, and delivery mechanism, designed for precise and controlled operation within body fluids and tissues. Adapted with permission from Agrahari. et al. [238]
Nanotechnology is set to transform healthcare by enabling more personalised, precise, and effective treatments. The continued development of personalised medicine, theranostics, and smart drug delivery systems promises to significantly enhance patient outcomes, particularly in the treatment of complex diseases like cancer. As these technologies evolve, their integration with other emerging fields, such as artificial intelligence and 3D printing, will likely lead to even more groundbreaking innovations in healthcare.
To fully harness the advancements described in the above sections, however, it is essential to translate these technologies from the laboratory to clinical practice. This process involves conducting rigorous clinical trials, developing scalable manufacturing processes, and obtaining regulatory approval. The following section will examine the progress in transitioning nanotechnology-based innovations to clinical settings, with a particular focus on global regulatory approvals for healthcare products.
Clinical translation and commercialisation of nanotechnology
Healthcare products are categorised into medicines (drugs) and medical devices, aligning with the regulatory frameworks of various countries. Therefore, nanotechnology and nanomaterials used in healthcare are necessarily regulated either as nanomedicines or as nanotechnology-based medical devices (NMDs) during clinical translation and commercialisation. Nanomedicines refer to medical applications that use nanomaterials to diagnose, treat, and prevent diseases. This category includes nanopharmaceuticals (i.e., drug formulations that enhance drug delivery and efficacy), nanoimaging agents (which improve the visualisation of biological processes for better diagnostics), and theranostics (which combine therapeutic and diagnostic functions into a single platform, thus allowing for simultaneous treatment and monitoring) [239]. NMDs, on the other hand, encompass a variety of medical tools and equipment that incorporate nanoscale properties to improve their functionality and compatibility with biological systems. These devices include: (i) diagnostic devices, which provide highly sensitive and specific disease detection; (ii) implantable devices, which integrate seamlessly with biological tissues; (iii) skin dressings, which offer superior wound healing properties; and (iv) surgical tools, which enhance precision and reduce invasiveness.
Before nanomedicines and NMDs can be applied clinically, they must undergo a rigorous approval process. This process involves preclinical studies and clinical trials to ensure their safety, efficacy, and quality. Regulatory bodies such as FDA, the European Medicines Agency (EMA), and China's National Medical Products Administration (NMPA) govern this process, requiring extensive testing and evaluation to meet stringent standards [240].
The current landscape of clinical research on nanomedicines and NMDs can be evaluated using data from the ClinicalTrials.gov database. Managed by the National Library of Medicine (NLM), a division of the United States National Institutes of Health (NIH), ClinicalTrials.gov is one of the largest global registries, offering extensive information on clinical trials conducted worldwide [241]. As of August 2024, a search of this database using "nano" as a keyword in the intervention/treatment field identified 336 relevant clinical trials. Of these, 154 trials have been completed, while 20 have been suspended, withdrawn, or terminated. The conditions targeted in these 336 nano-related trials span a broad spectrum of health issues, diseases, and disorders, as illustrated in Fig. 27. This diversity of research areas highlights the extensive exploration of nanotechnology applications across various medical fields, with a notable concentration of studies focusing on dental, orthopedic, and cancer treatments.
Fig. 27.
Distribution of clinical trials for nanotechnology-based healthcare products across different medical applications and specialties. Extracted from ClinicalTrials.gov database https://clinicaltrials.gov/ (keyword: nano)
Despite the challenges associated with transitioning from research and development (R&D) to market—such as high costs, complex manufacturing processes, and regulatory hurdles—several nanomedicines and NMDs have successfully reached the market [242]. These products are revolutionising healthcare by leveraging the unique properties of nanomaterials, such as improved drug bioavailability, targeting capabilities, and enhanced imaging capabilities. The emerging markets for nanomedicines and NMDs are experiencing rapid growth, driven by the increasing demand for advanced therapeutic and diagnostic solutions [243].
Commercialisation of nanomedicines
According to a report by Precedence Research, the global nanomedicine market was valued at approximately $170 billion in 2022 and is expected to reach around $494 billion by 2032 (Fig. 28), growing at a compound annual growth rate (CAGR) of 11.3% from 2023 to 2032 [243]. The rapidly expanding domain now includes over 200 enterprises across the globe, such as Sanofi SA, Pfizer Inc., Taiwan Liposome Company Ltd, Johnson & Johnson, and Bristol-Myers Squibb Company, all of which provide a wide variety of healthcare solutions based on nanotechnology [244]. Considerable financial resources are being allocated annually towards the advancement of nanotechnology R&D, with the total investment in the United States alone reaching approximately $40 billion over the past 20 years [245].
Fig. 28.
Nanomedicine market size forecast from 2022 to 2032. Adapted from Precedence Research [243]
The drug delivery segment held the largest portion of the healthcare nanotechnology (nanomedicine) market in 2022, accounting for 34% of its total, driven by the increasing prevalence of chronic and infectious diseases such as cancer and COVID-19, along with a heightened public understanding of the potential benefits of nanomedicine [246]. Liposomes (spherical vesicles made of phospholipids with the capability to deliver both hydrophilic and hydrophobic drugs) pioneered the successful transition of nanomedicine from concept to commercial reality [247, 248]. Since receiving approval from the FDA in 1995, lipid-based nanomedicines have been on the market for nearly three decades. Out of all the nanomedicines currently available in the market or undergoing different stages of clinical translation, liposomes or lipid-based NPs represent the most common category, adding up to 33% of the total [249]. Beside them, polymer-based nanomedicines, nanocrystals, inorganic-based NPs, carbon-based NPs and protein-based NPs have also gained approval and entered commercialisation [242], as shown in Table 1.
Table 1.
List of globally marketed nanomedicines approved by FDA, EMA, and NMPA. Adapted with permission from Halwani et al. [250]
| Type | Trade name | Company | Date of approval | Active ingredients | Indication |
|---|---|---|---|---|---|
| Lipid-based NPs | Doxil® | Johnson & Johnson | FDA (1995), EMA (1996) | Doxorubicin (adriamycin) | Metastatic ovarian cancer, HIV-associated Kaposi’s sarcoma (KS) |
| Lipodox® | Sun Pharma Global FZE | FDA (2013) | Doxorubicin hydrochloride | Metastatic ovarian cancer, HIV-associated KS | |
| DaunoXome® | Galen Ltd | FDA, EMA (1996) | Daunorubicin | Cancers and HIV-associated KS | |
| Onivyde® | Merrimack Pharmaceuticals | FDA (2015) | Irinotecan | Metastatic pancreatic cancer | |
| DepoCyt® | Pacira Pharmaceuticals | EMA (2002), FDA (2007) | Cytarabine | Lymphomatous meningitis | |
| Myocet® | Teva Pharmaceutical Industries Ltd | EMA (2000) | Doxorubicin hydrochloride | Breast cancer | |
| Caelyx® | Janssen Pharmaceuticals | EMA (1996) | Doxorubicin | Breast cancer, ovarian cancer, HIV-associated KS | |
| Mepact® | Takeda France SAS | EMA (2009) | Mifamurtide | Osteogenic sarcoma | |
| Marqibo® | Talon Therapeutics | FDA (2012) | Vincristine | Philadelphia chromosome-negative chronic myelogenous leukemia in adult patients | |
| Onpattro® | Alnylam | FDA & EMA (2018) | Patisiran | Hereditary transthyretin (TTR) mediated amyloidosis | |
| Lipusu® | Luye Pharma Group Ltd | FDA (2016) | Paclitaxel | Breast cancer, non-small-cell lung cancer (NSCLC) | |
| AmBisome® | NeXstar Pharmaceuticals | EMA (1990), FDA (1997) | Amphotericin B | Antifungal drug | |
| Vyxeos® | Jazz Pharmaceutics | FDA (2017), EMA (2018) | Daunorubicin and cytarabine | Acute myeloid leukemia | |
| Abelcet® | Defiante Farmaceutica | FDA (1995) | Amphotericin B | Antifungal drug | |
| DepoDur® | SkyePharma | FDA (2004), EMA (2006) | Liposomal morphine sulphate | Postoperative analgesia | |
| Curosurf® | Chiesi | FDA (1999) | Poractant alfa | Respiratory distress syndrome (RDS) | |
| Zevalin® | Bayer Pharma | FDA (2002) Disc. *, EMA (2004) | 90Y-ibritumomab tiuxetan | Lymphoma | |
| Inflexal® | Crucell Berna Biotech | EMA (1997) | Inactivated influenza virus vaccine | Prevents influenza infection | |
| Pfizer-BioNTech vaccine | Pfizer Pharmaceuticals | FDA (2020) | mRNA vaccine | prevents COVID-19 infection | |
| Moderna COVID-19 Vaccine | ModernaTX Inc | FDA (2020) | mRNA vaccine | Prevents COVID-19 infection | |
| Visudyne® | QLT Phototherapeutics | FDA & EMA (2000) | Photosensitiser (PS), benzoporphyrin | Choroidal neovascularisation caused by wet age-related macular degeneration | |
| Polymer-based NPs | Cimzia® | UCB | FDA (2008), EMA (2009) | IgG Fab’ fragment that specifically recognises and binds to TNF-α | Rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, and ankylosing spondylitis |
| Apealea® | Oasmia Pharmaceutical AB | EMA (2018) | Paclitaxel | Ovarian cancer, peritoneal cancer, fallopian tube cancer | |
| Adagen® | Enzon Pharmaceuticals Inc | FDA (1990) | Adenosine deaminase (ADA) | Adenosine deaminase (ADA)-severe combined immunodeficiency disorder | |
| Neulasta® | Amgen, Inc | FDA (2002) | Filgrastim | Febrile neutropenia, consequent infections arising due to lack of neutrophils | |
| Oncaspar® | Enzon Pharmaceuticals Inc | FDA (1994), EMA (2016) | L-asparaginase | Acute lymphoblastic leukemia, chronic myelogenous leukemia | |
| Genexol-PM® | Lupin Ltd | FDA (2007) | Paclitaxel | Breast cancer | |
| Pegasys® | Genentech USA, Inc | FDA, EMA (2002) | Recombinant human alfa-2a interferon | Hepatitis C, hepatitis B | |
| Diprivan® | Fresenius Kabi | FDA (1989), EMA (2001) | Propofol | (Sedative-hypnotic agent) used in surgery to induce relaxation before and during general anesthesia | |
| Somavert® | Pfizer Pharmaceuticals | EMA (2002), FDA (2003) | Analog of human growth hormone (acts as an antagonist of GH receptors) | Acromegaly | |
| Macugen® | Pfizer Pharmaceuticals | FDA (2004) | Pegatinib sodium | Choroidal neovascularisation caused by wet age-related macular degeneration | |
| Mircera® | Vifor | EMA (2007), FDA (2018) | Epoetin β (EPO) (EPO is a genetically recombinant form of erythropoietin) | Anemia | |
| PegIntron® | Merk & Co. Inc | EMA (2000), FDA (2001) | Alpha interferon (INF) molecule | Hepatitis C | |
| Krystexxa® | Savient Pharmaceuticals | FDA (2010) | Pegloticase is a recombinant porcinelike uricase | Refractory chronic gout | |
| Plegridy® | Biogene | FDA (2014) | Recombinant IFN-β | Relapsing remitting multiple sclerosis (RRMS) in adult patients | |
| Adynovate® | Baxalta US Inc | FDA (2015) | Coagulation factor VIII | Hemophilia A | |
| Copaxone®/FOGA | Teva Pharmaceutical Industries Ltd | FDA (1996), EMA (2016) | Glatiramer acetate | Multiple sclerosis (MS) | |
| Eligard® | Tolmar Pharmaceuticals Inc | FDA (2002) | Leuprolide acetate | Prostate cancer | |
| Renagel® | Sanofi | FDA (2000) | Sevelamer carbonate | Hyperphosphatemia caused by chronic kidney disease (CKD) | |
| Renagel®/renvela® | Genzyme | EMA (2007) | Sevelamer HCL | Hyperphosphatemia caused by CKD | |
| Restasis® | Allergan | FDA (2003) | Cyclosporine | Chronic dry eye | |
| Rebinyn® | NovoNordisk | FDA (2017) | Recombinant DNA-derived coagulation FIX | Hemophilia B | |
| Estrasorb™ | Novavax, Inc | FDA (2003) | Estradiol (17β-estradiol) hemihydrate | Moderate vasomotor symptoms due to menopause | |
| Zilretta® | Flexion Therapeutics | FDA (2017) | Triamcinolone acetonide | Knee osteoarthritis | |
| Nanocrystals | Emend® | Merk & Co. Inc | FDA (2003) | Aprepitant | Antiemetic drug |
| Ivemend® | Merk & Co. Inc | FDA, EMA (2008) | Fosaprepitant dimeglumine (prodrug of aprepitant) | Antiemetic drug | |
| Ostim® | Osartis GmbH & Co | FDA (2004) | Calcium hydroxyapatite | Bone-grafting material | |
| Rapamune® | Wyeth Pharmaceuticals Inc. (a subsidiary of Pfizer Inc.) | EMA (2001), FDA (2010) | Sirolimus (rapamycin) | Prevents rejection of kidney transplants (immunosuppressant) | |
| Rapamune® | Wyeth Pharmaceuticals Inc. (a subsidiary of Pfizer Inc.) | FDA (2015) | Sirolimus (rapamycin) | A rare progressive lung disease (lymphangioleiomyomatosis) | |
| Vitoss® | Orthovita Inc | FDA (2003) | β-tricalcium phosphate | Bone-grafting material | |
| Ritalin LX® | Novartis | FDA (2002) | Methylphenidate | Attention deficit hyperactivity disorder (ADHD) in children | |
| Avinza® | Pfizer Pharmaceuticals | FDA (2002) | Morphine sulfate | Psychostimulant | |
| Focalin XR® | Novartis | FDA (2008) | Dexamethylphenidate HCl | ADHD in children | |
| Invega® | Janssen Pharmaceuticals | FDA (2009) | Paliperidone | Schizophrenia | |
| Invega sustenna® | Janssen Pharmaceuticals | FDA (2009) | Paliperidone Palmitate | Schizophrenia | |
| Megace ES® | Par Pharmaceuticals | FDA (2005) | Megestrol acetate | Antianorexic | |
| NanOss® | RTI Surgical | FDA (2005) | Hydroxyapatite | Bone substitute | |
| EquivaBone® | Zimmer Biomet | FDA (2009) | Hydroxyapatite | Bone substitute | |
| OsSatura® | Isotis Othobiologics Inc | FDA (2003) | Hydroxyapatite | Bone substitute | |
| Epaxal® | Crucell Berna Biotech | EMA (1993) | Inactivated hepatitis A virus vaccine | Prevents hepatitis A infection | |
| Zanaflex® | Acorda | FDA (2002) | Tizanidine HCl | Muscle relaxant | |
| Ryanodex® | Eagle pharm | FDA (2014) | Dantrolene sodium | Malignant hyperthermia | |
| TriCor® | Abbott Laboratories | FDA (2004) | Fenofibrate | Antihyperlipidemia | |
| Inorganic NPs | Feraheme™ | AMAG Pharmaceuticals | FDA (2009) | Ferumoxytol | Anemia |
| Venofer® | Luitpold Pharm | FDA (2000) | Iron sucrose | Iron deficiency in CKD | |
| Dexferrum® | American Regent | FDA (1996) | Iron dextran | Iron deficiency in CKD | |
| Ferinject® | Vifor | FDA, EMA (2013) | Iron carboxymaltose colloid | Iron deficient anemia | |
| Ferrlecit® | Sanofi-Aventis | FDA (1999), EMA (2013) | Sodium ferric gluconate | Iron deficiency in CKD | |
| Hensify® | Nanobiotix | EMA (2019) | Hafnium oxide NPs | Locally advanced squamous cell carcinoma | |
| Infed® | Actavis Pharma | FDA (1992) | Iron dextran | Iron deficiency in CKD | |
| Feridex®/endorem® | AMAG Pharma | FDA (1996) Disc.* 2008 | SPION-dex | Imaging agent | |
| GastroMARK™/umirem® | Mallinckrodt Inc | FDA (2009)Disc.* 2012 | SPION-silicone | Imaging agent | |
| Carbon-based NPs | Canalin® | Chongqing Lummy Pharmaceutical Co., Ltd | NMPA (2020) | Carbon NPs | Lymph node tracers [251] |
| Protein-based NPs | Abraxane® | Celgene Pharmaceutical Co. Ltd | FDA (2005, 2012, 2013), EMA (2008) | Paclitaxel | Approved by the FDA for treatment of metastatic breast cancer (2005), lung cancer (2012), and metastatic pancreatic adenocarcinoma (2013) |
| Ontak® | Eisai | FDA (1999) | Diphtheria toxin | Leukemia, T-cell lymphoma |
*Disc.: discontinued
Commercialisation of nanotechnology-based medical devices (NMDs)
In addition to nanomedicines, NMDs are being approved for marketing worldwide. The first FDA 510(k) clearance for a medical device containing nanomaterials was given in 1980 [252]. A 510(k) is a premarket submission made to the FDA to demonstrate that the device to be marketed is as safe and effective (i.e., substantially equivalent) as a legally marketed device [253]. As of June 2024, 151 FDA 510(k)-cleared products featured the term “nano” in either the manufacturer or product name. However, not all of these products are NMDs. Table 2 lists those with published evidence confirming the use of nanomaterials or nanotechnology, including bone fillers (nano-structured hydroxyapatite), and dental resins (dimer acid derived nanohybrid composite). It also covers devices featuring nano-coatings or nano-structured surfaces, like dental implants (calcium phosphate coating), intervertebral fusion devices (hierarchical nano/microtextured surface), knee prostheses (hydroxyapatite nanocoating), vessel sealing devices (non-stick coating), trauma screws (surface with antimicrobial nanotopography) [254].
Table 2.
List of NMDs approved by FDA for marketing through the 510(k) process. Extracted from 510(k) Premarket Notification database https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm (keyword: nano)
| Medical application/specialty | Device/trade name | Applicant | Date of approval | Nanomaterial | Indication |
|---|---|---|---|---|---|
| Anesthesiology | UniPlex NanoLine cannula, PlexoLong-NanoLine set, StimuLong-NanoLine Set | Pajunk GmbH | 01/12/2006 | Nanoparticle-enhanced surface layer | Anesthesia conduction kits for temporary anesthetic delivery to peripheral nerve bundles during regional anesthesia |
| SonoLong Sono, Curl Sono, NanoLine® | PAJUNK GmbH Medizintechnologie | 03/01/2012 | Polymer nanocoating | Anesthesia conduction kits for delivery of continuous conduction anesthesia and/or analgesia of peripheral nerves | |
| Clinical chemistry | Nanosep™ N10 | Intercept, Ltd | 04/26/1994 | Nanoporous structures membrane | Clinical sample concentrator, for the concentration and purification of biological samples such as peptides, proteins, oligonucleotides, DNA, and RNA |
| Nano-Check™ AMI 3 in 1 Cardiac Disease Test | Nano-Ditech Corporation | 03/02/2006 | Colloidal Au NPs | Immunoassay method, a one-step lateral flowimmuno chromatography assay for the qualitative determination of three cardiac markers simultaneously in serum and heparin plasma | |
| Nano-Check™ AMI 2 in 1 Cardiac Marker, cTnI and Myoglobin | Nano-Ditech Corporation | 10/20/2010 | Colloidal Au NPs | Immunoassay method, to provide rapid and reliable results in diagnosing acute myocardial infarction (heart attack) by measuring the levels of cTnI and Myoglobin in the blood | |
| Nano-Check™ AMI cTnI Cardiac Marker Test | Nano-Ditech Corporation | 10/20/2010 | Colloidal Au NPs | A rapid immunoassay for the qualitative determination of cardiac troponin I (cTnI) in human serum and plasma specimens at cut off, as an aid in the diagnosis of Acute Myocardial Infarction (AMI) | |
| Dental | NanoTite PREVAIL, Certain, Parallel Walled, Tapered, External Hex | BIomet 3i, Inc | 01/31/2008 | Osseotite® nanoscale surface and nanocrystalline deposition of calcium phosphate | A root-form endosseous implant, for surgical placement in the jaw to support prosthetic attachments for single-tooth restorations, partially or fully edentulous spans, bridgework, and overdentures |
| Renamel® NANO™ + plus | Cosmedent, Inc | 02/24/2017 | Multifunctional acrylic monomers and silicaceous nanofillers | Tooth shade resin material, designed for Class I-V dental restorations, direct veneering of anterior teeth, splinting, and the repair of composite or ceramic restorations | |
| Nano composite | Cosmedent, Inc | 04/16/2007 | Light-cure nanocomposite | Tooth shade resin material, possesses the physical and mechanical properties to function in the oral cavity, mimicking the esthetic qualities of natural teeth | |
| DMRC NanoFlow; DMRC Nanocomposite; ebond; sure etch gel; sure etch liquid | Danville Materials, Inc | 09/25/2012 | Light-cure NPs | DMRC NanoFlow, a flowable composite for small cavities, repairs, linings, sealants, and marginal repairs; DMRC NanoComposite, a universal composite for direct and indirect restorations; eBond, a bonding agent to enhance adhesion between composites and tooth structure; Sure Etch Gel and Sure Etch Liquid, both used to prepare tooth surfaces for improved bonding by creating micropores | |
| Prime & Bond® NT™ Dual Cure Nano-Technology Universal Dental Adhesive System | Dentsply International, Inc | 02/23/2005 | Nanofiller | Tooth bonding agent for bonding all indirect restorations, including metal, ceramic, and composite crowns, inlays, onlays, veneers, bridge retainers, and endodontic posts to enamel and dentin without light | |
| Nanocomposite Restorative Kit | DMG USA, Inc | 05/30/2008 | Light-cure nanocomposite | Tooth shade resin material, designed for restorative procedures to repair and restore the function and aesthetics of teeth affected by cavities or other forms of dental damage | |
| NanoCeram-Bright | DMP, Ltd | 11/19/2010 | Light-cure nano-hybrid composite | Resin material for anterior and posterior restorations, cervical caries, root erosion, wedge-shaped defects, veneering discoloured anterior teeth, splinting mobile teeth, and repairing composite and ceramic veneers | |
| Nano Varnish, Plaquit, Lightpaint on Surface | Dreve Dentamid GmbH | 04/05/2018 | Light-cure NPs | Dental sealing lacquer for coating resin temporaries | |
| NanotiteTM Dental Implants | Implant Innovations, Inc | 02/22/2007 | Calcium phosphate nanocrystal | Dental implants with nanoscale calcium phosphate crystals on the surface | |
| Series of Nanova products for dental use | Nanova Biomaterials Inc | 01/16/2018 etc.* | Hydroxyapatite nanofiber | Tooth bonding agents; sealant, pit and fissure, conditioner; tooth shade resin materials | |
| Nano-TiCrown™ | Nano-Write Corporation | 06/11/2003 | Nanostructured titanium/titanium nitride material | Base metal alloy for making single-unit porcelain-fused-to-metal (PFM) prosthetics and custom dental prosthetics, such as porcelain-to-metal veneers | |
| Dimer Acid Derived Nano-hybrid Composite Restorative Material | Novocol, Inc | 09/13/2007 | Dimer acid derived nanohybrid composite | Tooth shade resin material, for class I, II, III, IV and V cavity classes | |
| NanoGen® | Orthogen, Llc | 05/06/2011 | Nanocrystalline calcium sulfate | Bone grafting material for bone regeneration, used alone, mixed with other bone filling agents to prevent particle migration, or as a resorbable barrier over other graft materials | |
| Pac-Dent Ceramic Nanohybrid Resin | Pac-Dent, Inc | 07/26/2021 | Ceramic nanohybrid | Tooth shade resin material, for fabricating permanent restorations such as inlays, onlays, veneers and full crownrestorations | |
| Nano-Bond II Adhesive System | Pentron Clinical Technologies | 08/10/2007 | Light-cure nanocomposite | Resin tooth bonding agent | |
| Sculpture® Plus Nano-Hybrid Composite | Pentron Laboratory Technologies | 01/17/2003 | Nanohybrid composite | Dental restorative material used to restore carious lesions, structural defects, or lost tooth structure | |
| CalApex Calplus, Cerafill RCS, Endoseal, Nanoseal S, Zical Ultra Paste, Zical Ultra Powder/Liquid | Prevest Denpro, Ltd | 05/23/2022 | Ag NPs | Antibacterial root canal sealer fortified with nanosilver used in conjunction with gutta-percha for effective sealing root canals of permanent teeth | |
| Camouflage™ NanoHybrid Composite (Universal and Flowable) | Prismatik Dentalcraft, Inc | 06/04/2014 | Ceramic-filled nanohybrid resin composite material | Tooth shade resin material for Class I-VI cavities in anterior and posterior teeth, suitable for direct restorations, inlays, and onlays | |
| Capo Hybrid, Capo Slow Flow, Capo Flow, Capo Natural, Capo Universal, Nano Paq, Nano Paq Flow | Schütz Dental GmbH | 10/24/2016 | Light-cure nanocomposite | Tooth shade resin material for direct and indirect restorations, fissure sealing on premolars and molars, stump buildup, and shape and colour correction | |
| General & plastic surgery | Series of LigaSure™ products | Covidien | 03/23/2021 etc.* | Nonstick nanocoating | Electrosurgical cutting and coagulation device and accessories, for open surgical procedures where ligation and division of vessels, tissue bundles, and lymphatics |
| Medline ReNewal Reprocessed LigaSure Exact Dissector, Nano-coated (LF2019) | Surgical Instrument Service And Savings, Inc | 04/29/2024 | Nanocoating to reduce tissue sticking | Electrosurgical cutting and coagulation device and accessories | |
| Immunology | Nanopia Wide Range C-Reactive ProTein (CRP) Reagent Kit | Clinical Data, Inc | 02/09/2006 | Antibody-coated latex particles | Quantitative latex agglutination immunoassay for measuring C-Reactive Protein (CRP) in serum or plasma, used to evaluate infection, tissue injury, and inflammatory disorders alongside a complete clinical evaluation |
| Microbiology | Nano-Check™ COVID-19 Antigen Test | Nano-Ditech Corporation | 01/23/2024 | Au NPs | Simple point-of-care device, used as an immunochromatographic lateral flow assay for detection of SARS-CoV-2 nucleo protein antigens in human anterior nasal swab specimens |
| Neurology | Series of Titan NanoLOCK products for neurology | Medtronic Sofamor Danek USA, Inc | 09/29/2022 etc.* | Titanium nanoscale featured surface | Stereotaxic Instruments, to be used during preparation and placement of Medtronic implants during spinal surgery to assist in precisely locating anatomical structures in either open, or minimally invasive, procedures |
| Orthopedic | Ascend VBR System, Ascend NanoTec VBR System | Alphatec Spine, Inc | 10/06/2023 | Hydroxyapatite nanocoating | Spinal intervertebral body fixation orthosis to replace a collapsed, damaged, or unstable vertebral body or for reconstructive decompression of the spinal cord and neural tissues in degenerative disorders |
| Calibrate PSX Interbody System, Calibrate NanoTec PSX Interbody System | Alphatec Spine, Inc | 07/13/2023 | Hydroxyapatite nanocoating | Intervertebral body fusion device for spinal fusion from L1 to S1 in skeletally mature patients, treating symptomatic degenerative disc disease (DDD), degenerative spondylolisthesis, and/or spinal stenosis at one or two adjacent levels | |
| Series of IdentiTi Porous Ti Interbody Systems | Alphatec Spine, Inc | 02/22/2024 etc.* | Hydroxyapatite nanocoating | Intervertebral body fusion devices for treating DDD and spinal instability in skeletally mature patients | |
| nanOss® Bone Void Filler | Angstrom Medica, Inc | 02/03/2005 | Hydroxyapatite nanocrystal | Resorbable calcium salt bone void filler device for non-structural bony voids or gaps in the extremities, spine, and pelvis | |
| Series of NanoBone® products | Artoss GmbH | 04/23/2019 etc.* | Nanocrystalline osteoconductive hydroxyapatite | Resorbable calcium salt bone void fillers device for bony voids or gaps in the extremities, posterolateral spine, and pelvis | |
| EMPOWR Porous Femur with HAnano SurfaceTM | Encore Medical, L.P | 03/30/2021 | Hydroxyapatite nanocoating | Knee joint patellofemorotibial metal/polymer porous-coated uncemented prosthesis for total knee arthroplasty, treating arthritis, avascular necrosis, joint configuration loss, deformities, fractures, and for salvage of failed surgeries | |
| NanoHive Medical Lumbar Interbody System | Ian Helmar | 07/12/2023 | Soft Titanium® lattice nanotextured surface | Intervertebral body fusion device for use in skeletally mature patients with DDD at one or two contiguous levels from L2 to S1 | |
| Series of Titan NanoLOCK products for orthopedic | Medtronic Sofamor Danek USA, Inc | 10/06/2022 etc.* | Titanium nanoscale featured surface | Intervertebral body fusion device for spinal fusion in skeletally mature patients with symptomatic DDD, degenerative spondylolisthesis, and/or spinal stenosis at one or two levels from L2 to S1 | |
| Series of Nanova products for orthopedic use | Nanova Biomaterials Inc | 05/05/2017 etc.* | Hydroxyapatite nanofiber | Metallic bone fixation appliances and accessories for securing soft tissue or bone-tendon-bone grafts during cruciate ligament reconstruction surgeries of the knee | |
| Series of Nanovis systems | Nanovis, Llc | 06/02/2023 etc.* | Bio-ceramic nanotube surface | Intervertebral body fusion devices for immobilising and stabilising spinal segments as an adjunct to fusion in patients with DDD, spinal stenosis, spondylolisthesis, spinal deformities, trauma, tumours, pseudarthrosis, and failed fusions | |
| Series of nanOss™ products | Pioneer Surgical Technology, Inc | 10/17/2014 etc.* | Nano-structured hydroxyapatite | Bone void fillers | |
| Series of SeaSpine Systems | SeaSpine Orthopedics Corporation | 07/07/2021 etc.* | Titanium nanoscale featured surface | Intervertebral body fusion devices | |
| OsteoFlo® NanoPutty®-Quadphasic Synthetic Bone Graft | SurGenTec, Llc | 08/14/2020 | Hydroxyapatite NPs | Resorbable calcium salt bone void filler device | |
| Tyber Medical BioTy® Nanotopography Trauma Screw | Tyber Medical, Llc |
06/20/2017 11/16/2016 |
Nanoscale featured surface | Metallic bone fixation fastener for bone reconstruction, osteotomy, arthrodesis, and joint fusion | |
| Radiology | DRX-Revolution Nano Mobile X-ray System | Carestream Health, Inc | 02/05/2018 etc.* | Carbon nanotube | Diagnostic mobile x-ray system with digital radiography (DR) technology, featuring a self-contained x-ray generator, image receptor(s), imaging display, and software for acquiring diagnostic images outside a standard x-ray room |
| Nanox.ARC | Nanox Imaging, Ltd | 04/28/2023 | Nano-cones field on a silicon chip | Tomographic X-ray system for producing images of the human musculoskeletal system from a single sweep, as an adjunct to conventional radiography for adult patients | |
| Nanox Cart X-ray System | Nanox Imaging, Ltd | 04/01/2021 | Nano-cones field on a silicon chip | Mobile X-ray system for radiographic examinations of hands, wrists, and fingers in adult patients, designed for use when transporting the patient to fixed equipment is not feasible | |
| Nanor® and Efficast/Nanor Hybrid Thermoplastic Materials | Orfit Industries N.V | 09/26/2013 | Hybrid thermoplastic NPs | Medical charged-particle radiation therapy system for retaining and reproducing a patient's position during radiation therapy | |
| Toxicology | Nano-Check™ DAT 5 Multi Drug Screening Test | Nano-Ditech Corporation | 05/15/2005 | Colloidal Au NPs | Qualitative immunoassay: a one-step, type II competitive immunochromatographic assay for detecting cannabinoids, opiates, benzoylecgonine, methamphetamine, phencyclidine, and their metabolites in human urine |
*Disc.: Due to the complex regulatory process and need for continuous improvement, medical devices often undergo multiple revisions and approvals, resulting in several approval dates throughout their life cycle
Some devices gain FDA approval via a different regulatory pathway, the premarket approval (PMA) process. The PMA process requires clinical trials to demonstrate the safety and efficacy of a device and is the most stringent type of device marketing application required by the FDA [252]. As of June 2024, five manufacturers had their 75 products, each bearing “nano” in the name and including different sizes or models of the identical device, approved via the PMA process [255]. Among these products, only the COBRA PzF NanoCoated Coronary Stent demonstrated clear evidence of using nanotechnology (Table 3). This particular product features a cobalt-chromium strut enveloped in a poly [bis(trifluoroethoxy)phosphazene] (Polyzene-F® polymer) coating with a thickness of approximately 50 nm, which has thrombo-resistant, anti-inflammatory and rapid healing effects [256]. Exploring the 510(k) and PMA databases reveals that FDA-approved NMDs are predominantly used in orthopedic and dental applications.
Table 3.
List of the NMD approved by FDA for marketing through the PMA process. Extracted from Premarket Approval (PMA) database https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm (keyword: nano)
| Medical application/specialty | Device/trade name | Applicant | Decision date | Withdrawal date | Nanomaterial | Indication |
|---|---|---|---|---|---|---|
| Cardiovascular | Cobra PzF NanoCoated Coronary Stent System | Celonova Biosciences, Inc. | 02/21/2017 etc.* | 12/22/2022 | Polyphosphazene nanocoating | Coronary stent system for improving coronary luminal diameter in patients, including those with diabetes mellitus, with symptomatic ischemic heart disease from de novo lesions in native coronary arteries |
*Disc.: Due to the complex regulatory process and need for continuous improvement, medical devices often undergo multiple revisions and approvals, resulting in several decision dates throughout their life cycle
NMDs approved by NMPA show a distinct emphasis in their applications compared to those cleared by FDA, which might be attributed to the variances in healthcare market demands, industrial chains, intellectual property rights, and regulatory frameworks between China and the United States. By June 2024, NMPA-approved NMDs from Chinese manufacturers are predominantly aimed at diagnostic purposes (Table 4), featuring in vitro diagnostic kits with elements like CNTs, gold nanocages, nano-enzymes, rare-earth nanospheres, and magnetic nanobeads for identifying conditions such as myocardial infarction, renal disease, cancer, blood clots, and COVID-19 [257]. The approval list of domestic products also comprises medical dressings containing Ag NPs or nano-ceramic materials, and bone fillers made with nano-structured hydroxyapatite. For the smaller number of overseas products approved (Table 5), diagnostic devices also represent the largest category. Furthermore, several of these products, including Adaptix™ Interbody System with Titan nanoLOCK™ Surface Technology [258], Nano-coated LigaSure™ Maryland Jaw Thoracic Sealer/Divider [259], and Nano-technology Universal Dental Adhesive [260], had already been approved by FDA.
Table 4.
List of domestic NMDs approved by NMPA for marketing. Extracted from NMPA database https://www.nmpa.gov.cn/datasearch/home-index.html#category=ylqx (Keywords: ‘纳米’ and its equivalent term in English, ‘nano’)
| Device/trade name | Applicant | Date of approval | Management category | Nanomaterial | Description |
|---|---|---|---|---|---|
| Urinary microalbumin detection kit (carbon nanotube colloidal gold method) | Benxi Tystj Biotechnology, Ltd. | 05/06/2020 | Class II | CNTs | Kit for qualitative urine microalbumin, used to diagnose kidney diseases |
| Series of thromboelastography assay kits (nano-enzyme clotting method) | Chongqing Kangjuquan Hong Biotechnology, Ltd. | 28/04/2024 etc.* | Class II | Nanoenzyme | Kit for thromboelastography (TEG) instrument to test coagulation in sodium citrate anticoagulated whole blood |
| Soluble ST2 protein (ST2) assay kit (nano-enzyme fluorescent immunoassay) | Chongqing Kangjuquan Hong Biotechnology, Ltd. | 09/04/2024 | Class II | Nanoenzyme | Kit to measure soluble suppression of tumourigenicity 2 protein (ST2) levels in human serum |
| Brain natriuretic peptide (BNP) Assay kit (nano-enzyme fluorescent immunoassay) | Chongqing Kangjuquan Hong Biotechnology, Ltd. | 17/04/2023 | Class II | Nanoenzyme | Kit to measure B-type natriuretic peptide (BNP) levels in human plasma |
| Activated coagulation assay kit (nano-enzyme coagulation method) | Chongqing Kangjuquan Hong Biotechnology, Ltd. | 01/03/2023 | Class II | Nanoenzyme | Kit for TEG to measure R, K, Angle, and MA values in whole blood samples |
| Thromboelastography quality control (nano-enzyme coagulation method) | Chongqing Kangjuquan Hong Biotechnology, Ltd. | 14/02/2023 | Class II | Nanoenzyme | Kit for quality control of R, K, Angle, and MA values in TEG testing |
| Nano-enzyme immunoassay analyser | Chongqing Kangjuquan Hong Biotechnology, Ltd. | 07/07/2022 | Class II | Nanoenzyme | Analyser using nanoenzyme immunochromatography for quantitative detection of serum amyloid A in serum and plasma |
| Cardiac troponin I/Creatine Kinase-MB/Myoglobin/D-Dimer/N-terminal pro-B-type Natriuretic Peptide (cTnI/CKMB/Myo/D-Dimer/NT-proBNP) Rapid Test Kit (rare earth nanofluorescence Immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 25/11/2022 | Class II | Nanofluorescent microsphere | Kit for in vitro quantification of cardiac troponin I/creatine kinase-MB/myoglobin/D-dimer/N-terminal pro-brain natriuretic peptide (cTnI/CKMB/Myo/D-Dimer/NT-proBNP) in plasma and whole blood |
| Procalcitonin/Interleukin-6 (PCT/IL-6) Rapid Test Kit (rare earth nanofluorescence immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 01/11/2022 | Class II | Nanofluorescent microsphere | Kit for in vitro quantification of procalcitonin/interleukin 6 (PCT/IL-6) in serum, plasma, and whole blood, aiding in bacterial infection diagnosis |
| Carcinoembryonic Antigen Test Kit (nanoenzyme immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 18/06/2021 | Class II | Nanoenzyme | Kit for qualitative detection of carcinoembryonic antigen (CEA) in nipple discharge, used for monitoring breast cancer treatment, prognosis, and recurrence |
| Interleukin-6 (IL-6) Test Kit (rare earth nanofluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying interleukin-6 (IL-6) in serum, plasma, and whole blood to monitor immune status and inflammation |
| S100-β Protein (S100-β) Test Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying S100-β protein in serum, aiding in brain injury diagnosis |
| Heart-type Fatty Acid Binding Protein (H-FABP) Test Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying heart-type fatty acid-binding protein (H-FABP) in serum, plasma, and whole blood, aiding in acute myocardial infarction diagnosis |
| Pepsinogen I (PGI)/Pepsinogen II (PGII) Test Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 25/05/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying pepsinogen I (PGI) and pepsinogen II (PGII) in serum and plasma, used to assess gastric function and fundus gland lesions |
| Serum Amyloid A (SAA) Test Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying serum amyloid A (SAA) in serum, plasma, and whole blood, used as non-specific inflammation marker |
| Anti-Müllerian Hormone (AMH) Test Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying anti-Müllerian hormone (AMH) in serum, plasma, and whole blood, indicating ovarian reserve function |
| β2-Microglobulin (β2-MG) Test Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying β2 microglobulin (β2-MG) in serum, plasma, and venous whole blood, used to monitor proximal renal tubule function |
| Cardiac Troponin I/Creatine Kinase-MB/Myoglobin (cTnI/CK-MB/Myo) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying cardiac troponin I/creatine kinase-MB/myoglobin (cTnI/CKMB/Myo) in serum, plasma, and venous whole blood, used to aid in diagnosing myocardial infarction and myopathy |
| 25-Hydroxy Vitamin D (25-OH VD3) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying 25-hydroxyvitamin D3 (25-OH VD3) in serum, plasma, and venous whole blood, used to diagnose vitamin D deficiency |
| Neutrophil Gelatinase-Associated Lipocalin (NGAL) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying neutrophil gelatinase-associated lipocalin (NGAL) in urine, used to assess kidney function damage |
| Myoglobin (Myo) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying myoglobin in serum, plasma, and whole blood, aiding in myocardial infarction diagnosis |
| N-terminal Pro-brain Natriuretic Peptide (NT-proBNP) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying N-terminal pro-brain natriuretic peptide (NT-proBNP) in serum, plasma, and whole blood, aiding in heart failure diagnosis |
| Creatine Kinase-MB (CK-MB) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying creatine kinase-MB (CKMB) in serum, plasma, and whole blood, aiding in diagnosing myocardial infarction and myopathy |
| Urinary Albumin (U-ALB) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying urinary albumin (U-ALB) in urine, aiding in kidney disease diagnosis |
| Cystatin C (CysC) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying cystatin C (CysC) in serum, plasma, and whole blood, indicating glomerular filtration rate |
| High-sensitivity C-reactive Protein (hsCRP + Conventional CRP) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying C-reactive protein (CRP) in serum, plasma, and whole blood |
| Procalcitonin (PCT) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying procalcitonin (PCT) in serum, plasma, and whole blood, aiding in diagnosing bacterial infectious diseases |
| D-Dimer Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying D-dimer in plasma and whole blood, aiding in diagnosing venous thrombosis, DIC, and monitoring thrombolytic therapy |
| Cardiac Troponin I (cTnI) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying cardiac troponin I (cTnI) in serum, plasma, and whole blood, aiding in myocardial infarction diagnosis |
| Glycated Hemoglobin (HbA1c) Assay Kit (rare earth nano fluorescent immunochromatography) | Hunan Naquan Aode Biotechnology, Ltd. | 26/04/2022 | Class II | Nanofluorescent microsphere | Kit for quantifying glycated hemoglobin (HbA1c) in whole blood, aiding in diabetes diagnosis and glucose monitoring |
| Series of rare earth nano fluorescent immunoassay analysers | Hunan Naquan Aode Biotechnology, Ltd. | 10/06/2022 etc.* | Class II | Nanofluorescent microsphere | Analyser for qualitative and quantitative analysis of analytes in human samples with compatible reagents |
| Cardiac Troponin I (cTnI) Detection Kit (nanogold cage colloidal gold method) | Nanjing Botian Kezhi Biotechnology, Ltd. | 05/02/2021 | Class II | Gold nanocages | Kit for qualitative detection of cardiac myosin-binding protein C in plasma |
| Troponin C (cTnC) Detection Kit (nanogold cage colloidal gold method) | Nanjing Botian Kezhi Biotechnology, Ltd. | 27/08/2021 | Class II | Gold nanocages | Kit for qualitative detection of cardiac myosin-binding protein C in plasma |
| Nano acupoint dressing | Shanghai Manjici Biology Limited Company | 29/01/2021 | Class II | Nano far-infrared ceramic | Dressing relieves pain from periarthritis, sprains, dysmenorrhea, cervical pain, joint pain, breast hyperplasia, and mastitis |
| Nano-silver medical antimicrobial dressing | Shenzhen Ajet Pharmaceutical Technology, Ltd. | 23/04/2021 | Class III | Ag NPs | Dressing protects burns and scalds, with nano-silver reducing infections |
| Nano-silver antimicrobial adhesive dressing | Shenzhen Ajet Pharmaceutical Technology, Ltd. | 27/05/2021 | Class III | Ag NPs | Dressing protects surgical incisions, with nano-silver as an antibacterial |
| Medical Nano Hydroxyapatite/Polycaprolactone 66 Composite Bone Filling Material | Sichuan Guona Keji, Ltd. | 14/11/2022 | Class III | Hydroxyapatite NPs | Bone filling material for repairing defects from cysts, tumours, fractures, spinal fusion, and joint grafting |
| Nano-silver burn dressing | Wuhan Canvest Biotechnology, Ltd. | 29/10/2021 | Class III | Ag NPs | Dressing for burns and scalds, with nano-silver reducing infections |
| Nano-silver wound dressing | Wuhan Canvest Biotechnology, Ltd. | 02/08/2021 | Class III | Ag NPs | Dressing with antibacterial nano-silver layer, adhesive, absorbent layer, release paper, and barrier paper; reduces wound infections |
| Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Test Kit (rare earth nanofluorescence immunoassay) | Xiamen AmonMed Biotechnology, Ltd. | 22/04/2022 | Class III | Nanofluorescent microsphere | Kit for qualitative detection of 2019-nCoV IgM/IgG antibodies in serum from unvaccinated and uninfected individuals |
| Nano-silver burn dressing | Anxin Nano Biotechnology (Zhuhai), Ltd. | 02/08/2021 | Class III | Ag NPs | Burn dressing for second-degree burns, scalds, ulcers, and wounds to reduce infections |
| Nano silver dressing | Anxin Nano Biotechnology (Zhuhai), Ltd. | 02/08/2021 | Class III | Ag NPs | Wound dressing to reduce infections |
*Disc.: Due to the complex regulatory process and need for continuous improvement, medical devices often undergo multiple revisions and approvals, resulting in several decision dates throughout their life cycle
Table 5.
List of imported NMDs approved by NMPA for marketing. Extracted from NMPA database https://www.nmpa.gov.cn/datasearch/home-index.html#category=ylqx (Keywords: '纳米' and its equivalent term in English, ‘nano’)
| Device/trade name | Applicant | Date of approval | Management category | Nanomaterial | Description |
|---|---|---|---|---|---|
| Nanofilled self adhesive light cured protective coating | GC Corporation | 28/08/2019 | Class II | Nanoscale light-cure filler | Dental coating material enhances gloss and hardness, protects glass ionomer cement and composite resin, and seals the bonding interface |
| Nano-checker 710 | Nano-Ditech Corporation | 21/10/2019 | Class II | Colloidal Au NPs | Analytical equipment quantitatively detects analytes in serum, plasma, or blood, including troponin I, myoglobin, CK-MB, procalcitonin, D-dimer, and NT-proBNP |
| In Vitro Nano-CheckTM AMI 2 in 1 Cardiac Marker, cTnI and Myoglobin | Nano-Ditech Corporation | 23/11/2020 | Class II | Colloidal Au NPs | Immunoassay reagent for qualitative detection of cTnI and myoglobin in serum or plasma |
| In vitro Nano-CheckTM AMI cTnI Cardiac Marker Test | Nano-Ditech Corporation | 27/11/2020 | Class II | Colloidal Au NPs | Immunoassay reagent for qualitative detection of cTnI in serum or plasma |
| In vitro Nano-CheckTM AMI 3 in 1 Cardiac Marker Test cTnI, CK-MB and Myoglobin | Nano-Ditech Corporation | 08/12/2020 | Class II | Colloidal Au NPs | Immunoassay reagent for qualitative detection of cTnI, CK-MB, and myoglobin in serum or plasma |
| Nanopia KL-6 | Sekisui Medical, Ltd | 21/08/2023 | Class II | KL-6-coated latex NPs | Reagent for quantitative in vitro detection of sialylated carbohydrate antigen (KL-6) in serum or plasma |
| Nanopia TDM carbamazepine | Sekisui Medical, Ltd | 03/04/2020 | Class II | Carbamazepine-coated latex NPs | Reagent for quantitative in vitro detection of carbamazepine in serum or plasma |
| Nanopia TDM digoxin | Sekisui Medical, Ltd | 18/05/2020 | Class II | Digoxin-coated latex NPs | Reagent for quantitative in vitro detection of digoxin in serum or plasma |
| Nanopia TDM valproic acid | Sekisui Medical, Ltd | 18/05/2020 | Class II | Valproic Acid-coated latex NPs | Reagent for quantitative in vitro detection of valproic acid in serum or plasma |
| Nanopia CRP | Sekisui Medical, Ltd | 17/04/2020 | Class II | CRP-coated latex NPs | Reagent for quantitative in vitro detection of C-reactive protein in serum or plasma |
| Nanopia P-FDP | Sekisui Medical., Ltd | 17/02/2020 | Class II | P-FDP-coated latex NPs | Reagent for quantitative detection of fibrin/fibrinogen degradation products (FDP) in plasma or serum |
| Nanopia MMP-3 | Sekisui Medical., Ltd | 10/02/2020 | Class II | MMP-3-coated latex NPs | Kit for quantitative detection of matrix metalloproteinase-3 (MMP-3) in serum or plasma |
| Nanopia D dimer | Sekisui Medical, Ltd | 26/11/2021 | Class II | D dimer-coated latex NPs | Kit for quantitative in vitro detection of D-dimer in serum or plasma |
| Series of LigaSure Sealer/divider, nano-coated systems | Covidien, Llc | 27/06/2022, etc.* | Class II and III | Nano-coated surface | Surgical equipment for cutting and coagulating tissue, blood vessels, and lymphatic vessels in open surgeries |
| Grandio light-curing nano-hybrid filling material | Voco GmbH | 03/08/2021 | Class III | Nano-hybrid composite | Dental filling material for Class I-V cavities, primary teeth, core buildups, and resin inlays |
| Filtek Z250 XT Nano Hybrid Universal Restorative | 3 M ESPE Dental Products | 22/01/2020 | Class III | Visible light-activated nanohybrid composite | Dental restorative material for direct restoration of teeth, core buildup, splinting, and indirect restorations like inlays, onlays, and veneers |
| Nano-technology dental adhesive | Dentsply Detrey GmbH | 18/08/2020 | Class III | Nanoscale light-cure filler | Dental adhesive for composite restorations, cavity lining, and protecting sensitive cervical areas |
| Adaptix™ Interbody System with Titan nanoLOCK™ Surface Technology | Medtronic Sofamor Danek USA, Inc | 10/07/2023 | Class III | Titanium nanoscale featured surface | Spinal fusion system for thoracolumbar fixation in DDD patients (L2-S1) |
*Disc.: Due to the complex regulatory process and need for continuous improvement, medical devices often undergo multiple revisions and approvals, resulting in several decision dates throughout their life cycle
In the European Union (EU), over 70 EU-certified private notified bodies regulate medical device approvals, issuing Conformité Européenne (CE) marks for EU-wide marketing. Differently from the United States and China, national authorities oversee post-market safety [261]. As of June 2024, the European Database on Medical Devices (EUDAMED) lists 130 approved medical devices that either contain nanomaterials or have "nano" in their name [262]. The products among them that can be identified as NMDs through publicly available information are listed in Table 6. They are mainly intended for use in the following areas: dental restorative materials, cosmetic injections, laboratory instruments, magnetic NPs for anti-cancer purposes, protein assays, COVID-19 rapid diagnostic kits, and others. Within this assortment, products related to dentistry represent the largest category, constituting over 30% of the total. This category specifically includes light-cure nanocomposites and zirconia-silica nanocomposite fillers for dental restoration. In addition, one particular example is aimed at cancer therapy: NanoTherm® AS1 is a water-based solution filled with superparamagnetic NPs, primarily made of Fe3O4 with a diameter of roughly 12 nm. These NPs function as energy transducers for magnetothermal therapy aimed at cancer treatment. To ensure the stability of these NPs within tumour tissues, their surface is coated with an aminosilane. In Europe, this formulation is applied in a dual treatment approach, combining magnetic hyperthermia therapy with radiation therapy, for patients suffering from recurrent glioblastoma multiforme (GBM) [263]. Another example, Proteonano™, represents instead a proteomics analysis technology that use magnetic NPs modified with peptides and small molecules for the identification of various protein biomarkers.
Table 6.
List of NMDs approved for EU-wide marketing. Extracted from EUDAMED database https://ec.europa.eu/tools/eudamed/#/screen/home (Keyword: nano)
| Device/trade name | Manufacturer/producer | Nomenclature code(s) | Risk class | Nanomaterial | Indication |
|---|---|---|---|---|---|
| Alergo Alnanogel | Alegro Medical GmbH | Medicated bandages, with zinc oxide and other components—other | Class I | Nanogel | The bandage is designed to provide superior protection against bacterial infection, while also offering relief from itching, burning, and other common symptoms associated with neuro dermatitis |
| JBP NanoLink Fille HA S | BioPlus, Ltd | Resorbable filling and reconstruction devices | Class III | Hyaluronic acid NPs | The gel is a transparent, viscous, and sterile substance in a pre-filled syringe for facial tissue augmentation by injection into the skin layer |
| Medental nanohybrid light cure composite | Conamco SA de CV dba Medental | Dental restoration compomers | Class IIa | light-cure nanocomposites | The dental composite for restorations of all cavity classes, Indirect inlays, onlays and veneers |
| Nano Synt. | Dentscare Ltda | Dental graft devices | Class III | Nano-hydroxyapatite | The synthetic bone replacement material is based on biphasic calcium phosphate with excellent osteoconductive action, being resorbed and replaced by living bone during bone remodelling |
| NanoTherm® AS1 | MagForce NT GmbH | Active-implantable devices—other | Class III | Superparamagnetic NPs | NPs for magnetothermal therapy in tumour treatment |
| Proteonano™ Whole Proteome Profiling Kit | Nanomics Biotechnology, Ltd | Immunochemistry reagents | Class A | Magnetic NPs | The kit uses modified magnetic NPs to compress the dynamic range of protein concentrations in complex biological samples, enhancing the detection of medium- and low-abundance proteins by reducing the dominance of high-abundance proteins |
| NanoSigma Oral Gel | NanoSigma Biotech,Ltd | Dressings for wounds, sores and ulcerations | Class I | Gel’s nanotechnology | The oral gel is a liquid bandage for oral ulcer and injured wound. It is suitable for oral mucosa wound |
| Saliva SARS-CoV-2 (2019-nCoV) Antigen Test Kit (Nanocarbon Assay) | Ningbo Beautiful Life Medical Biotechnology Development,Ltd | Rapid tests & point of care—immunochemisty—other | IVD General | Nanocarbon | The kit is a lateral flow, one-step immunoassay for the qualitative detection of N antigen in saliva sample |
| QNome-3841hexDx Nanopore Sequencer; QNome-3841 Nanopore Sequencer | Qitan Taike Biotechnology, Ltd | DNA synthesisers | Class A | Nano-sized protein pores | The instrument is used for determining the order, type and quantification of bases in DNA fragments |
| Znano | Zest Anchors, Llc | Dental conservation composites | Class IIa | zirconia-silica nanocomposite | The dental composite is a highly-filled, light cured, resin-based dental composite material |
From Tables 1–6, it is evident that while the classification of NMDs varies across regions, all systems fundamentally rely on the risk level associated with the device to determine regulatory requirements. The United States, Europe, and China all use a tiered approach, classifying devices into different categories to align regulatory requirements with the device’s potential risk, as shown in Table 7. FDA and NMPA both categorise devices into three classes: Class I for low-risk devices that require minimal oversight, Class II for moderate-risk devices needing more regulatory controls (such as 510(k) premarket notification for FDA and enhanced supervision for NMPA), and Class III for high-risk devices that support or sustain life, requiring stringent evaluation and approval processes [264]. The EU employs a more detailed four-class system, with Class I for low-risk, Class IIa and IIb for moderate to higher-risk depending on usage duration and invasiveness, and Class III for high-risk devices [265]. This system emphasises device invasiveness and duration of use more than the FDA or NMPA. Despite these differences, all systems aim to ensure device safety and effectiveness by increasing regulatory requirements as the risk level rises, particularly for devices that are life-supporting or implanted. These frameworks reflect a global commitment to maintaining high standards in medical device safety, adapted to each region's regulatory environment.
Table 7.
Comparison of medical device classification systems
| Classification Level | United States | Europe | China |
|---|---|---|---|
| Class I | Low-risk devices (general controls) | Low-risk devices (non-invasive or short-term use) | Low-risk devices (Routine management) |
| Class IIa | – | Medium-risk devices (short-term or moderately long-term use) | – |
| Class II | Moderate-risk devices [special controls, require 510(k)] | – | Moderate-risk devices (Enhanced supervision required) |
| Class IIb | – | Higher-risk devices (medium to long-term implantable or invasive devices) | – |
| Class III | High-risk devices (require PMA premarket approval) | High-risk devices (used to sustain life or implantable) | High-risk devices (Vital for life, strict requirements) |
The commercialisation decision for NMDs is heavily influenced by these regulatory classifications, as they dictate the level of evidence required for approval and market access. For example, devices intended for high-risk applications, such as implantable devices or those involving critical nanomaterial interactions with the human body, typically face stricter regulatory scrutiny and require more extensive clinical data. Understanding these regulatory frameworks is crucial for developers to strategise their product development and market entry effectively.
In addition, the divergent application focuses of nanotechnology in medical devices across the United States, Europe, and China reflect differences in the clinical translation and commercialisation of nanotechnology globally, which can be attributed to several factors, including differing healthcare priorities, regulatory environments, R&D capacities, and market demands in these regions. Different regions may prioritise healthcare needs based on prevalent diseases, demographic profiles, and public health challenges. For instance, the higher emphasis on orthopedics and spinal surgery in the United States could reflect a larger aging population suffering from musculoskeletal disorders [266]. The focus of Europe on dentistry might be due to a combination of high dental care standards and public health policies prioritising oral health [267]. In contrast, the high interest of China for diagnostics could stem from a strategic push to improve early disease detection and management in a populous country, aiming to reduce long-term healthcare costs [268]. In addition, the regulatory frameworks governing medical devices, including those using nanotechnology, vary significantly across regions, which can influence the type of products that reach the market [269]. Stricter or more lenient regulations in one area of healthcare over another can steer the R&D focus. Manufacturers with superior R&D capabilities can secure a larger market share by leveraging intellectual property protection, which limits similar technological advancements of their competitors, thus fostering differentiated competition. Market demands, driven by patient needs, insurance coverage, and societal values, also influence the focus of nanotechnology applications in medical devices. The economic incentives to address specific healthcare challenges can drive companies to innovate in areas with the highest return on investment.
The development and market introduction of nanomedicines and NMDs are ongoing, but the products available are still few compared to the extensive research indicated by 50,424 articles on 'nanomedicine' and 15,220 on 'nano medical device' found on PubMed (as of June 2024). This disparity underscores the significant hurdles in their clinical translation and commercialisation.
Market entry challenges and cost-effectiveness
The commercialisation of nanomedicines and NMDs faces several significant challenges, particularly in terms of market entry barriers and cost-effectiveness. This section explores these obstacles in detail, focusing on the financial, manufacturing, and quality control issues that impact the successful introduction of nanotechnology in healthcare.
Regulatory and approval challenges
One of the main challenges for bringing nanomedicines and NMDs to market is meeting the stringent regulatory requirements, which are crucial to ensuring the safety, efficacy, and quality of these products. Regulatory approval typically involves comprehensive preclinical and clinical evaluations, with toxicity testing and safety assessments being essential components [270]. Due to the unique size and surface characteristics of nanomaterials, they may exhibit toxicity profiles that differ from those of traditional materials, necessitating specific tests for acute, subacute, and chronic toxicity, as well as genotoxicity, immunotoxicity, and environmental toxicity to assess their potential impact on biological systems and the environment [271]. Sect. “Nanotoxicity and safety risks” will provide a more in-depth discussion on the health risks of nanomaterials in vivo and in the environment, as well as the mechanisms of toxicity. Additionally, safety evaluations generally include a range of in vitro and in vivo studies to understand the biocompatibility of nanomedicines and NMDs, as well as their absorption, distribution, metabolism, and excretion (ADME) properties in the body [272]. These studies must strictly adhere to Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) to ensure the reliability of the tests and the consistency of the products [273]. Figure 29 illustrates the steps necessary to bring nanomaterials to healthcare market. While these rigorous requirements help reduce uncertainties in the approval process, they also significantly increase the cost and time required for research and development, posing a major challenge to market entry [270]. By meeting these complex regulatory requirements, nanomedicines and NMDs can successfully enter the market and provide safe and effective treatment options for patients.
Fig. 29.
Summary of key steps for clinical application of NPs. Adapted from Webster [274]
Moreover, the requirements for these evaluations can vary significantly across different regions, creating additional obstacles for companies seeking to bring their products to the global market. Regulatory agencies in various countries have different standards and procedures for the approval of nanomedicines and NMDs. For example, as described in Table 7, different regions have distinct requirements for the classification of medical devices. These discrepancies mean that companies may need to conduct additional tests and modifications to meet the specific requirements of each market, thereby increasing both time and cost. This complexity necessitates that companies carefully consider the regulatory requirements and market characteristics of each region when developing global commercialisation strategies. Sect. “Current regulatory policies around the globe” will provide a detailed discussion on the current state of global regulations and offer recommendations for improvement, helping companies better navigate these challenges and achieve global market entry for nanomedicines and NMDs.
Financial barriers and R&D costs
Developing nanomedicines is an expensive and resource-intensive process that poses significant financial challenges for companies, particularly for smaller firms and start-ups [275]. The costs associated with R&D encompass a wide range of activities, including the design, synthesis, characterisation, and testing of nanomaterials. Each of these stages requires specialised equipment and expertise, which can drive up expenses. Additionally, scaling up production from laboratory-scale synthesis to manufacturing quantities suitable for clinical trials and eventual market supply involves substantial investment in infrastructure and technology.
Beyond the initial development costs, conducting extensive clinical trials to meet regulatory standards is another major financial hurdle [276]. Clinical trials, which are necessary to establish the safety, efficacy, and quality of nanomedicines, often involve multiple phases and require significant resources to manage and execute. These trials are not only costly due to the need for specialised staff, facilities, and materials but also time-consuming, which further delays the potential return on investment. Smaller companies and start-ups may find these expenses particularly prohibitive, as they typically lack the financial reserves and access to capital that larger pharmaceutical firms possess.
The financial burden of developing nanomedicines is further compounded by the uncertainty of return on investment. The innovative nature of nanomedicines means that there is often limited prior data to support their efficacy and safety, leading to a higher risk of delays or failures in the approval process [277]. This uncertainty can deter investors and make it difficult for companies to secure the necessary funding to carry their products through to market.
Given these substantial financial barriers, it is crucial for companies to carefully strategise their R&D investments and seek collaborative partnerships or alternative funding sources to mitigate risks and enhance their chances of successful market entry.
Manufacturing and quality control challenges
Scaling up the production of nanomedicines from laboratory research to commercial-scale manufacturing presents unique challenges due to the distinct properties of nanomaterials, such as their small size, high surface area, and reactivity [278]. These characteristics necessitate specialised manufacturing processes and equipment to maintain consistent quality. Even slight variations in particle size or surface properties can significantly impact the efficacy and safety of the final product.
Ensuring reproducibility across large-scale production is difficult, as processes optimised at a small scale may not perform identically when scaled up, potentially leading to variability. This requires strict control over production conditions and process parameters to ensure uniformity. Adherence to GMP guidelines is essential to meet regulatory standards, involving rigorous quality control measures, real-time monitoring, and validation of processes to detect and correct deviations [279].
Additionally, manufacturing nanomedicines requires advanced analytical techniques to accurately characterise nanomaterials, such as dynamic light scattering and electron microscopy, to ensure consistent particle size, morphology, and surface chemistry [280]. These requirements increase the complexity and cost of production but are crucial for ensuring product quality and safety.
Ethical implications
The integration of nanotechnology into healthcare brings forth several ethical considerations that must be addressed to ensure responsible and equitable use. One of the primary concerns is the issue of informed consent. Nanomedicines and NMDs often involve complex mechanisms and potential risks that may not be fully understood by patients. The novelty and intricacies of these technologies can make it challenging for patients to comprehend the full scope of benefits, risks, and long-term implications associated with their use. This can lead to difficulties in achieving truly informed consent, as patients may not have all the necessary information to make well-informed decisions about their treatments [281]. Therefore, healthcare providers have a duty to develop comprehensive educational materials and employ effective communication strategies that clearly explain the nature and implications of nanotechnology-based interventions in a way that is understandable to patients, thus safeguarding patient autonomy.
In addition to informed consent, ethical considerations also extend to issues of privacy and equitable access. Nano-enabled diagnostics and treatments could collect extensive biological and genetic data, raising concerns about data security, privacy breaches, and the potential misuse of sensitive information [282]. This data, if inadequately protected, could be exploited by third parties such as insurance companies or employers, leading to discrimination or stigmatisation based on genetic predispositions [283]. Furthermore, the high cost of developing and manufacturing nanotechnology-based healthcare products can create barriers to access, particularly for disadvantaged populations or those in low-income countries [284]. The unequal distribution of these advanced technologies risks widening existing healthcare disparities. To address these ethical challenges, it is crucial to implement robust data protection regulations and develop policies that promote equitable access to nanotechnology-based innovations, ensuring that all patients, regardless of their socioeconomic status, can benefit from these advancements in healthcare.
Cost-effectiveness consideration
The cost-effectiveness of nanomedicines and NMDs is a critical consideration in their development and market adoption. Although these technologies offer significant clinical advantages, such as improved therapeutic outcomes, targeted delivery, and enhanced diagnostic capabilities, they also involve substantial costs. The high initial investments required for the specialised synthesis of nanomaterials and the advanced technologies needed for their production contribute to increased development expenses compared to traditional pharmaceuticals and medical devices. Additionally, both nanomedicines and NMDs require extensive preclinical and clinical testing to meet stringent regulatory standards, further adding to the financial burden. However, these high costs can be offset by the potential long-term benefits, such as enhanced drug delivery, reduced side effects, fewer invasive procedures, and shorter recovery times, which can ultimately lead to lower overall healthcare costs. Moreover, implementing policies that promote equitable access and affordability, as mentioned previously, can enhance the cost-effectiveness of these technologies by ensuring that a wider patient population benefits from their advantages. Comprehensive economic evaluations that account for both direct and indirect costs are essential to determine the value these technologies bring to healthcare systems. By balancing these costs against the potential benefits, nanomedicines and NMDs can be seen as viable options that not only improve patient outcomes but also contribute to economic sustainability in healthcare.
Nanotoxicity and safety risks
The significant potential benefits of using nanotechnology in healthcare are undoubtedly appealing. However, the issue of biocompatibility must be addressed when any foreign substance is introduced into the human body for medical reasons, along with the environmental effects associated with commercialisation and market entry. Conducting a comprehensive and reliable evaluation of their toxicity throughout their lifecycle poses a challenge for professionals and organisations alike. This is because the reduction in size or dimensions, particularly to nanoscale in one or two dimensions, can lead to not only novel properties but also potentially unconventional toxicity behaviour.
Research has demonstrated that certain medical conditions may result from the improper exposure to nanomaterials. Mesothelioma, a type of cancer that forms in the protective lining of various internal organs, serves as a notable example, often linked to asbestos exposure. Asbestos, a collection of minerals composed of tiny, fibrous particles, was extensively used in the construction industry in the 1970s and shares similarities in size with nanomaterials. These tiny asbestos fibres can be inhaled and embedded in the lung tissue, causing damage over prolonged periods (Fig. 30).
Fig. 30.

Mechanism of Biopersistent Fiber-Induced Carcinogenesis. a Short asbestos fibers are cleared by macrophages, while long fibers (15–20 μm) cause persistent inflammation due to incomplete phagocytosis. TEM images show crocidolite phagocytosis by RAW264.7 cells. b Long fibers translocate to the pleural space, triggering frustrated phagocytosis at stoma openings and releasing proinflammatory cytokines and genotoxic activators, leading to mesothelial carcinogenesis. Small particles like coal dust, silica, and tangled multiwalled carbon nanotubes (MWCNTs) are cleared by phagocytes or lymphatic drainage. Retained long fibers cause chronic inflammation, leading to asbestosis or pulmonary carcinoma. Tangled MWCNTs and coal dust also cause lung carcinogenesis through persistent inflammation. Adapted with permission from Zhang et al. [285]
Although over 60 countries worldwide have enacted bans on asbestos [286], there remains a valid concern that other substances with similar sizes and shapes, such as nanofibers, could pose similar health risks if they are introduced into the human body in a comparable manner. NPs have been shown to be inhaled and deposited by diffusion in the small-diameter tubes of the lower part of the respiratory tract and in the alveoli [287]. Furthermore, epidemiological research indicates that exposure to NPs is linked to an increased risk of cardiovascular and respiratory diseases [288]. Regrettably, advancements in research on this subject have been limited; more specifically, there has not been a consensus on a standardised methodology for toxicological testing and analysis among medical professionals and toxicologists. This challenge arises partly due to the need for a multidisciplinary approach that encompasses materials science, chemistry, physics, toxicology, and environmental science.
While human health risk assessment focuses on determining the acceptability of health risks from medical products to humans under specific exposure scenarios [289], the life cycle assessment of medical products evaluates and compares the environmental impacts of the products or processes throughout their life cycle, encompassing their potential (negative) impacts on human health among other effects [290]. Currently, data on the impact of nanomaterials on aquatic life and plants are limited, with a shortage of suitable research methods [291]. Despite variations in their objectives, processes, and scope, both types of risk assessments depend on similar data for insights into nanomaterial toxicity [290]. There is a consensus on the importance of assessing the ecological and environmental safety of nanomaterials, which necessitates the development of suitable models and new methods. The following sections contain a discussion on the safety risks of nanomaterials from both health and environmental perspectives.
In vivo health risks
Typically, the body manages foreign chemicals through processes including exposure, absorption, distribution, metabolism, and excretion. These processes can be analysed through toxico-kinetics and a risk-based approach. Using logical and scientific rationale, along with ISO 10993, should elucidate the in vivo biosafety risk of a medical product. Yet, small particles are more easily taken up by cells, cross epithelial and endothelial membranes, undergo transcytosis, enter the blood and lymphatic circulatory systems, and ultimately accumulate in sensitive organs like the bone marrow, lymph nodes, spleen, and heart, therefore exhibiting significantly different dose sensitivities and kinetics [292]. Table 8 provides a comprehensive list of the primary and secondary biological barriers that NPs can penetrate, along with the organs they may accumulate in, offering an initial overview of the potential fate of NPs upon exposure to humans through different pathways.
Table 8.
Potential safety risk factors of nanotechnology in healthcare applications
| Potential safety risk factors in healthcare nanotechnology applications | |
|---|---|
|
Penetration through each physiological barrier and cause of complex dose-dependence toxicology; Primary biological barriers pulmonary barrier [295] skin barriers and etc. [296, 297] Secondary biological barriers vascular endothelial barrier [298, 299] |
Retention in each organ and cause of immunogenicity and/or toxicity; Liver [308] |
Specifically for the respiratory system, a predictive model has been developed to detail the behaviour of NPs within it [322]. The model suggests that smaller particles are more prone to settle in the respiratory tract, with their distribution across various regions being size-dependent. NPs measuring 5 nm are equally likely to settle in the nasopharyngeal (upper airways), tracheobronchial, and alveolar areas. However, for particles smaller than 5 nm, there is a higher tendency for them to settle in the nasopharyngeal area, while those larger than 5 nm predominantly settle in the alveolar region. This variance in settlement locations is likely to affect the biological interactions of the NPs. This model, however, exhibits limitations in two main areas: firstly, it was designed specifically for spherical NPs, leaving uncertainty about its applicability to NPs of different shapes; secondly, it uses default material behaviours primarily derived from studies on thorium, uranium, or technetium, despite the fact that the behaviour of a nanoparticle is highly influenced by its chemical composition and surface properties [287]. To add even more complexity to an already difficult challenge, the final destinations of NPs are diverse. To provide an example, after administering 20 nm colloidal Au NPs to mice through intratracheal instillation, these particles were found not only at the basal membrane between alveolar and endothelial cells but also on the surface of blood vessel endothelial cells, with a small fraction even detected in the bloodstream [323]. Extensive research has confirmed that NPs can cross biological barriers, impacting the parenchymal components of organs either toxically or therapeutically, as detailed in prior reviews [324–327].
These findings lead to two key agreements: firstly, the toxicity of metallic NPs cannot be inferred from the existing toxicology of their bulk metal counterparts [328], and secondly, once inside an organism, NPs can distribute across tissues in ways not governed by known physiological processes. Consequently, there is no clear guidance for nano-toxicology evaluation using traditional toxicological approaches, primarily due to the intricate interactions between nanomedicines and biological systems [329]. To advance in this area, it is crucial to understand the mechanisms behind the complex reactions to nanomaterial exposure. This section outlines common mechanisms of action, as listed in Table 9.
Table 9.
Potential safety risk factors of nanotechnology in healthcare applications
| Presently known mechanisms of nanotechnology potential risks | |
|---|---|
|
The complex dose-dependent toxicity linked to nanomaterials, which includes unintentional translocation or retention, can manifest through the following mechanisms: Transcellular/paracellular transport Physical damage to cell membrane |
Toxic phenomena related to nanomaterials, including lipid peroxidation, protein alterations, DNA damage, interference with signaling functions, and modulation of gene transcription, may occur through the following mechanism: Induction of oxidative stress |
Transcellular transport mechanisms
When nanomaterials enter a biological system, they inevitably engage with a variety of biological components, such as DNA, proteins, membranes, cells, and organelles, based on the specific context. The surfaces of these nanomaterials are quickly coated by biomolecules from the biological environment, with proteins being the most significant, forming what is known as a “protein corona.” This corona bestows a biological identity on the nanomaterials, influencing their subsequent interactions within the biological environment. Notably, the makeup of the “protein corona” is subject to change, determined by the concentrations and affinities of its different protein constituents to the nanomaterial surface [330]. Simultaneously, a key characteristic of nanomaterials is their relatively high surface-area-to-mass ratio, enabling them to adsorb significant quantities of materials per unit of mass. This capacity to bind a wide array of substances is thought to significantly influence their behaviour within cells under certain conditions. For instance, an in vitro investigation of polystyrene NPs demonstrated that NPs with a positive charge could penetrate cells 20 to 40 times more rapidly than those with a negative charge [331]. For NPs administered intravenously, their distribution across different organs is ultimately influenced by the plasma proteins that adhere to their surfaces. The distinct physical and chemical characteristics of these NPs—such as their size, surface area, modifications, charge, hydrophobic/hydrophilic properties, and redox activity—along with the in vivo microenvironment, dictate their varying patterns of interaction with proteins and lipids across various organ tissues and cells. This interaction determines the eventual location and destiny of the NPs within the body [332].
Research on inhaled nanomaterials has shown that while cells can engulf particles of most sizes, those smaller than 100 nm can move across alveolar epithelial cells via diffusion through the cell membrane lipid bilayer [333] or, in certain cases, cross the air-blood barrier through gaps between cells [334]. Experiments involving Au NPs and Ag NPs, coated with antibodies and of various sizes, demonstrated their role in the process of membrane receptor internalisation [335]. The binding and activation of these membrane receptors, along with the resulting protein expression, were notably influenced by the size of the NPs. NPs within the 2 to 100 nm range influenced signal pathways critical for cell function, with those measuring 7, 40, and 50 nm showing the most pronounced effects (Fig. 31). A summary of size-dependent cellular uptake of nanomaterials in regarding to the examined cell lines and major conclusions is presented in Table 10.
Fig. 31.
a Illustrations with corresponding fluorescence images of ErbB2 receptor localisation after treatment with different-sized Herceptin–colloidal gold nanoparticles complexes (Her–GNPs). Arrows indicate ErbB2 receptors, and the nucleus is counterstained with DAPI (blue) (scale bars = 10 µm). b Cross-sectional fluorescence intensity measurements of ErbB2 receptor localisation patterns with G2 (2 nm) and G40 (40 nm) Her–GNPs (scale bars = 10 µm). c Surface ErbB2 expression analysis using untreated cells normalised as 100% expression level (Ctrl). Cells were treated with unmodified 40-nm GNPs (Gold), Herceptin (Her) and Herceptin-modified GNPs of various sizes (* denotes statistical significance for G40/G50 compared to Her–GNPs of other sizes, p < 0.05, ANOVA). Error bars, ± s.d.; n = 4. Adapted with permission from Jiang et al. [335]
Table 10.
Size-dependent active and passive cellular uptake of nanomaterials with examined cell lines, and major conclusions. Adapted with permission from Kumar et al. [336]
| Nanomaterial | Size | Cell line | Major outcomes |
|---|---|---|---|
| Au NPs | 2–15 nm | MCF-7 | Higher uptake of smaller NPs; 2/6 nm located in cytoplasm and nucleus,15 nm only in cytoplasm |
| Aqueous QDs | 2–7 nm | A-427 | Size-dependent internalisation efficiency |
| Au NPs | 2.4–89 nm | Cos1 | 2.4 nm: in nucleus; 5.5 and 8.2 nm: partially in cytoplasm; 16 nm and above: no uptake |
| TiO2 | 5–80 nm | A549 | Uptake depends on overall size (with hard corona) |
| Au NPs | 13 nm, 45 nm | CF-31 |
45 nm: clathrin-mediated endocytosis 13 nm: mostly phagocytosis |
| Polystyrene NPs | 40 ~ 2000 nm | HeLa, A549, 1321 N1, HCMEC D3, RAW 264.7 | Uptake highly size-dependent for all cell lines; larger NPs enter more slowly |
| DPA-Quantum dot | 8 nm | RBCs | QDs penetrate cell membranes without pore formation |
| Mesoporous silica | 100 ~ 600 nm | RBCs | Strongly dependent on surface chemistry and nanoparticle size |
Paracellular transport mechanism
By interfering with specific transmembrane proteins, NPs can regulate internalisation and other fundamental cell functions, and also penetrate biological barriers. This is achieved by inducing the degradation, conformational change, and disintegration of proteins that link adjacent cells, facilitating paracellular transport. There are various types of intercellular junctions, including tight junctions (TJs), adherent junctions (AJs), desmosomes, and gap junctions (GJs) [337]. Although they serve different functions, desmosomes provide strong linkages that maintain stable adhesion between cells exposed to mechanical stress, such as those in the bladder and myocardium [338]; GJs are primarily used as channels for the exchange of ions and signal molecules between neighbouring cells, playing a crucial role in intercellular communication [339]. Regarding the function of paracellular permeability, which is an essential component in sealing the paracellular route in epithelial and certain endothelial barriers (e.g., blood–brain barrier), the structure of TJs is deemed responsible. They “glue” together portions of neighbouring cell membranes adjacent to the apical side, preventing lipids and specific proteins from mixing between the apical and basal sides of the cell, and limiting the space between cells to a very narrow range (from about 5 Å to 50 Å in different tissues) [340].
A detailed study investigated the impact of Au NPs size on their interactions with the paracellular AJs (Fig. 32) [341].
Fig. 32.
Au NPs-Induced Endothelial Leakiness in Swine Vessels. a Ex vivo construct scheme. b Size-dependent decrease in endothelial leakiness, measured by Evans blue dye (EBD) penetration. Smaller Au NPs (18 nm) at 8 × 10⁻3 m showed greater leakiness compared to 30 and 70 nm Au NPs. Results are means ± SD (n = 3), analysed by one-way ANOVA for 18, 30, and 70 nm Au NPs at 4 and 8 × 10⁻3 m concentrations. #P < 0.01, **, ##, &&P < 0.001. Adapted with permission from Lee et al. [341]
It was found that Au NPs with diameters of 18 nm and 30 nm were sufficiently small to migrate into the AJs and disrupt vascular endothelial (VE)-cadherin homophilic interactions, whereas Au NPs measuring 70 nm in diameter could not cause significant disruption. Another study investigated the impact of pristine graphene on brain microvascular endothelial cells (BMVECs), revealing a reduction in the expression of the TJ protein occludin, increased permeability, and a resulting increase in BMVEC cell death [342]. Additionally, a significant drop in mitochondrial membrane potential was observed in rats exposed to Ag NPs, leading to a decrease in adenosine triphosphate (ATP) levels and setting the stage for the onset of cell autophagy [343]. The study also revealed that autophagy is triggered in the brains of rats exposed to low doses of Ag NPs, unlike those exposed to silver citrate, highlighting how differences in surface chemistry can lead to distinct toxicity profiles.
The interaction between nanomaterials and proteins in the human body is particularly complex, especially since nanomaterials covered with proteins that trigger macrophage phagocytosis are identified by immune system cells with specific receptors. These interactions are not fixed and vary with the changing protein environment within the body, where abundant proteins may be gradually replaced by less abundant ones that have a higher affinity for the nanomaterials, indicating a dynamic process. The increasingly understood mechanism of interaction between nanomaterials and proteins at the molecular level has been illustrated in a recent publication [344].
Physical interaction
Surface chemistry may result in varied nanotoxicity behaviour via diverse biochemical interactions [345]. However, as the physical attributes of nanomaterials, such as shape and size, are scaled down, their significance becomes increasingly non-negligible in comparison to surface chemistry.
A study by Yuan et al. examined how diameter influences the uptake of NPs with a consistent surface ligand density through mathematical modeling [346]. The authors identified the most effective diameters for endocytosis as being approximately 30–50 nm. However, as each cell type has its distinct phenotype, the most effective size and distribution for NPs uptake should be determined individually, based on the specific cell type under examination and the surface chemistry involved. In another study published by Akcan et al., a series of toxicity behaviours of Au NPs were detailed [347]: 50 nm particles showed maximum uptake by HeLa cells; particles ranging from 45 to 50 nm activated membrane receptors in SK-BR-3 cells; those between 15 and 50 nm had the capacity to cross the blood–brain barrier in mice; and 15 nm particles displayed the most extensive distribution across various organs (in mice), including blood, liver, lungs, spleen, kidneys, brain, heart, and stomach. Inside the cell, size remains significant. Williams et al. reported that the entry of cadmium telluride quantum dots (CdTe QDs) into various subcellular organelles varies by size and cell type: QDs smaller than 2.1 nm penetrate the nucleus, while those measuring 4.4 nm localise within the cytoplasm [348].
Beyond the common size parameter for describing uniform nanomaterials (typically NPs), an important study published by Li et al. has showed that ultrathin 2D synthetic materials like graphene, ranging from 0.5 to 10 μm in lateral size, could be fully internalised by cells after initially attaching by their edges [349] (Fig. 33).
Fig. 33.
All-atom molecular dynamics simulations of corner piercing of a monolayer graphene across a lipid bilayer: a Spontaneous corner piercing observed. b Graphene–bilayer interaction energy vs. penetration distance, indicating a ~ 5kBT energy barrier; mean values from 11 simulations, error bars show SD. c Analytical model of corner piercing. Cellular uptake and internalisation of few-layer graphene microsheets: d–f Confocal images of human lung epithelial cells (d, e) and mouse macrophages (f) exposed to graphene microsheets (0.5–25 μm lateral dimension) after 24 h and 5 h, respectively. Nuclei (blue, DAPI), lung epithelial microtubules (green, FITC), and macrophage actin (red, rhodamine–phalloidin). Internalised graphene flakes (yellow arrows) disrupt cellular structures. g, h Transmission electron micrographs show graphene localisation in membrane-bound vacuoles in macrophages and lung epithelial cells after exposure to 10 ppm FLG sheets for 5 h and 24 h, respectively. Adapted with permission from Li et al. [349]
The study provided an energy barrier-based explanation, indicating that for small graphene pieces, thermal motion and entropic forces near the cell membrane initially align the piece with the membrane plane, leading to spontaneous piercing by corners with a minimal energy barrier (about KBT level). It was noted that in the absence of sharp edges, cell membranes present a significant energy barrier to the penetration by long graphene edges, even if they are only atomically thin. This highlights that local sharp features can facilitate penetration when the activation barriers are surpassed, regardless of whether the overall dimensions are in the nanometer or micrometer scale.
Oxidative stress and high reactivity
As the size of NPs decreases, the proportion of surface atoms to the total number of atoms in the NPs significantly increases, enhancing the surface capacity and surface tension of the particles. This leads to alterations in the physicochemical properties of the NPs, a phenomenon known as the surface effect or interface effect. When the surface atoms are exposed, they lack surrounding atoms, possess numerous unpaired bond forces, and are more likely to react with other atoms to reach a stable state, thus exhibiting high chemical reactivity. The substantial specific surface area of NPs increases the number of surface atoms, raises the level of structural disorder, and results in mismatched bonding states, creating many active sites.
Specifically, in the context of copper nanoparticles (Cu NPs) [350, 351], when these particles enter the digestive system, the acidic gastric fluid environment (with a pH of about 2) triggers the rapid conversion of ultra-reactive nano-metallic particles into their ionic form. Cu NPs transform hydrogen ions more swiftly than their micron-sized counterparts, leading to an excessive accumulation of Cu ions in the body and consequent toxicity. This toxicity escalates as the size of the Cu particles decreases, with significant toxicity observed at 17 μm for micron-sized copper particles and at 23.5 nm for Cu NPs. While inert micron-sized Cu only causes intestinal blockage at extremely high doses (5000 mg/kg), in gastric fluids, the highly reactive Cu NPs quickly convert to ionic form, rapidly relocating to the liver and kidneys for metabolic processing and elimination. The swift generation of numerous Cu ions by the NPs induces Cu ions overload in the body, resulting in liver and kidney damage and the disruption of tissue function.
The primary internal and biological toxic effects from nanomaterials are largely due to their ability to generate an overabundance of ROS such as hydroxyl radicals, singlet oxygen, hydrogen peroxide, and superoxide anion radicals [352–354] These free radical species including ROS and RNS, are highly reactive because of their unpaired electrons, leading them to interact with different cellular and subcellular structures and resulting in damage to cell membranes, lysosomes, DNA, and mitochondria, ultimately causing cell death through apoptosis and necrosis. The excessive generation of these radicals disrupts normal cellular signaling and mitogenic responses, impairing cell function [355, 356]
Oxidative stress is a critical factor in primary indirect genotoxicity, primarily due to the action of free radicals. These reactive species can interact with cellular biomolecules, including DNA, leading to significant cellular damage. ROS are particularly harmful as they can oxidize purines and pyrimidines in DNA, as well as cause strand breaks. Although cells have repair mechanisms to address this damage, these processes are not always successful, potentially resulting in gene mutations or extensive chromosomal abnormalities. Moreover, nanomaterials can induce DNA damage through additional mechanisms. They may directly interact with DNA or interfere with the processes of DNA replication and cell division. Nanomaterials can also affect protein kinases, which are crucial for regulating key cell cycle events, such as DNA replication and cell division, thereby further contributing to genomic instability [357].
Ag [356], silica [358, 359], amorphous silica [360, 361], Au [362], and ZnO [363] nanomaterials have been identified as causing human cell toxicity by both increasing ROS production and damaging DNA. Similarly, carbon-based nanomaterials like C60 fullerene [364] have been noted for their potential to generate free radicals. C60 and single-walled CNTs can become photoactivated, leading to intersystem crossing (ISC), which generates free electrons and subsequently results in ROS formation.
Moreover, free radicals can trigger an inflammatory response by accumulating in alveolar macrophages and inducing significant inflammation. If not addressed, this inflammation can lead to various pulmonary conditions, including lung cancer.
Toxicity of typical nanomaterials
From the standpoint of drugs and medical devices, potential safety concerns typically fall into four main categories: (i) systemic toxicity, which is especially relevant for long-term exposure in the case of permanent implants, (ii) local toxicity, (iii) carcinogenicity, and (iv) special considerations, which depend on the device's application, such as blood contact or interactions with the central nervous system. The introduction of nanomaterials complicates the evaluation of each of these safety issues. Therefore, alongside fundamental research into the interactions between these novel materials and biological systems, this review provides a concise examination of the safety concerns associated with extensively studied nanotechnology materials to enhance understanding.
Systemic toxicity
Systemic toxicity of dispersive nanomaterials, whether they are manufactured or naturally occurring, is heavily influenced by their biokinetics. This is typically evaluated using the ADME(T) framework, where ‘A’ represents administration, ‘D’ denotes distribution, ‘M’ stands for metabolism, ‘E’ signifies elimination, and ‘T’ refers to toxicity [365]. Understanding the biodistribution and clearance rates of nanomaterials is particularly complex and challenging, which has significantly impeded the broader clinical application of nanomedicines and nanotechnology-based devices.
Joël Bourquin’s review examined the long-term biological fate of various clinically relevant nanomaterials, including liposomes, micelles, Au NPs, SPIONs, silica, and polymers [366]. The review identified the kidneys, testes, liver, and spleen as primary target organs for dispersive nanomaterials. For Au NPs, the study found that their distribution and retention in these organs were influenced by factors beyond particle size, such as surface properties and coating materials. As shown in Table 11, Au NPs of different sizes (ranging from 2 to 110 nm) demonstrated similar patterns of organ accumulation and variable clearance times, indicating that size alone does not reliably predict their behaviour in biological systems. This data highlights that Au NPs exhibit consistent biodistribution across various sizes, with accumulation in several organs regardless of particle dimensions. Additionally, the clearance times for these NPs are not directly correlated with size, suggesting that other factors, including surface chemistry and shape, have a significant impact. The importance of these factors emphasises that a comprehensive understanding of NPs behaviour requires considering properties beyond size, such as surface characteristics and coatings, to accurately predict their biological interactions and clearance.
Table 11.
Long-term biodistribution data of gold-based nanomaterials based on collected evidence. Adapted with permission from Bourquin et al. [366]
| Material | Size (nm) | Animal | Primary organs with accumulation | Clearance observed (days) | Comments on biodistribution |
|---|---|---|---|---|---|
| Ethanediamine-coated Au NPs | 3.2 | Mouse | Kidney, Testis, Liver, Lung, Heart, Spleen | 90 | Detected in multiple organs, size not a clear predictor |
| Ethanedioic-acid-coated Au NPs | 3.7 | Mouse | Liver, Spleen, Lung, Heart, Brain | 90 | Similar to 3.2 nm, broad distribution |
| Au NPs | 2 ± 0.5 | Mouse | Liver, Spleen, Lung, Heart, Brain | 90 | Consistent with 3.2 and 3.7 nm, suggests size-independent distribution |
| Au NPs | 5 ± 1 | Mouse | Liver, Spleen, Lung, Heart, Brain | 90 | Distribution similar across size range |
| Au NPs | 10 ± 2 | Mouse | Liver, Spleen, Lung, Heart, Brain | 90 | Broad organ distribution regardless of size increase |
| CALNN-coated Au NPs | 16 | Rat | Thymus, Heart, Liver, Kidney, Brain, Spleen, Testis, Bladder | 28 | Rapid clearance, diverse organ distribution |
| Citrate-coated Au NPs | 20 | Rat | Liver, Spleen, Kidney, Brain, Bone Marrow | 60 | Increased size but similar clearance time |
| Au nanorods | 55 × 13 | Rat | Femur, Muscle, Liver, Heart, Kidney, Spleen, Lung | 28 | Larger dimensions, yet cleared similarly to smaller NPs |
| PEGylated Au NPs containing TNF-α | 27 | Mouse | Spleen, Liver, Kidney | 120 | Longer retention, not purely size-dependent |
| PEG stabilised silica coated Au NPs | 110 | Mouse | Bladder, Liver, Lung, Spleen, Brain | 28 | Large size, rapid clearance contradictory to expectations |
High surface activity can lead to aggregation, which makes nanomaterials more easily recognised and phagocytised by macrophages, particularly in blood-rich organs such as the liver and spleen. This recognition and capture process significantly impacts the biodistribution and retention of NPs in the body. Furthermore, surface chemistry plays a vital role in NPs behaviour; certain surface characteristics may cause NPs to cluster, resulting in increased accumulation in organs like the kidneys and complicating their excretion. Additionally, variations in surface charge can affect how NPs interact with and are internalised by tissue cells, further influencing their biodistribution. These findings underscore that understanding the biological fate of NPs requires a comprehensive analysis of surface properties, as these factors are critical determinants of their interactions within biological systems and their eventual clearance from the body.
In addition to size and surface properties, the shape of nanomaterials is a critical factor in evaluating their toxic properties. Carbon-based nanomaterials, such as graphene oxide (GO), exemplify how different shapes—such as sheets, flakes, and nanoribbons—can affect the rate and extent of internalisation and interactions with biological structures. For instance, irregularly shaped GO sheets are cleared more slowly, leading to prolonged exposure and increased toxicity, whereas spherical GO NPs are cleared more rapidly, reducing long-term toxicity [367].
Ultimately, the systemic toxicity of nanotechnology-based drugs and devices depends on a comprehensive understanding of biodistribution and clearance processes. This understanding requires not only a detailed study of the size, shape, and physicochemical properties of nanomaterials but also consideration of how these materials are applied in the human body. Manufacturers must also consider the potential generation of nanoscale debris during long-term use, which could impact safety and efficacy.
Local toxicity
Local toxicity of nanomaterials is often associated with their route of administration, such as topical application or the site of implantation. Research has shown that Ag NPs, for example, can induce local toxicity at the point of entry, triggering an inflammatory response from the immune system [368, 369]. When nanomaterials interact with local tissues, they can provoke inflammation and lead to tissue damage. For instance, prolonged exposure to long asbestos fibers can result in fibrosis, characterised by the accumulation of excess fibrous connective tissue, which can impair organ function and lead to disease [370]. This local toxicity can arise from the intrinsic properties of the materials themselves or from their interactions with biological molecules, leading to chronic inflammation. A notable example is breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), which has been identified in patients with various types of textured implants, used for both breast cancer reconstruction and cosmetic purposes [371]. Due to the lack of specific tracking of implant textures, the risk of this disease remains inconclusive at both individual and societal levels [372]. Furthermore, the effects of nanostructured surface topography on interactions with surrounding tissues have not been extensively studied from a nanotoxicology perspective, as highlighted in the regulatory discussions in Sect. “Current regulatory policies around the globe”.
Patient-based investigations have confirmed that the surface roughness of silicone mammary implants significantly influences both acute and early-stage chronic fibrotic responses [373]. The adherence of proteins to rough surfaces can mediate pro-inflammatory and profibrotic processes, suggesting that surface roughness plays a role in modulating fibrosis and enhancing the immune response [373]. Additionally, another study demonstrated that nanostructures with a higher aspect ratio on titanium substrates can alter the elastic modulus of the material surface, thereby inducing macrophage polarisation toward the M1 phenotype and triggering intense immune responses [374]. These findings underscore the importance of considering surface topography in the design of nanomaterials, as it plays a crucial role in interactions with proteins and subsequent local biological reactions, beyond the well-studied effects of surface chemistry.
Furthermore, the inflammatory response is amplified by the activation of the complement system, which leads to the production of chemoattractant molecules that recruit and activate additional innate immune cells [375]. Over the years, there has been growing recognition of the role that complement activation plays in the interaction of engineered nanomaterials, including nanomedicines, with the immune system. While it is well-established that surface properties such as chemistry, area, and energy influence protein adsorption, including that of complement proteins, the mechanisms by which the complement system is specifically activated at sites of local exposure to nanomaterials remain less understood. These gaps in understanding highlight the need for further research into how surface properties contribute to immune responses at the molecular level.
Carcinogenicity
The assessment of carcinogenicity risk for nanomaterials typically relies on theoretical analysis and existing data rather than new carcinogenicity testing, especially when the risks can be adequately evaluated or managed without additional studies [376]. Long-term carcinogenicity studies are costly and time-consuming, often requiring two to three years to complete, which poses significant economic concerns for manufacturers. Therefore, understanding the genotoxicity of nanomaterials is crucial, as genotoxicity is a key aspect of carcinogenesis. Recent research has extensively studied the genotoxic effects of nanomaterials and the mechanisms leading to transient or permanent genetic changes [377–379]. Genotoxicity is generally categorised into two main mechanisms: primary and secondary.
Primary genotoxicity can be further divided into direct and indirect mechanisms. Direct genotoxicity occurs when nanomaterials physically interact with DNA within the nucleus, leading to DNA damage such as breaks, lesions, or chromosomal alterations. Indirect genotoxicity, on the other hand, is often associated with the generation of ROS induced by nanomaterials or the release of toxic ions from their dissolution. These ROS can cause significant DNA damage through oxidative stress mechanisms, such as the Fenton-type reaction described in Sect. “Oxidative stress and high reactivity”. To determine whether primary genotoxicity is direct or indirect, it is essential to investigate the mechanisms of nanomaterial uptake and their ability to enter the nucleus, as discussed in Sects. “Transcellular transport mechanisms” and “Paracellular transport mechanism”. For instance, smaller nanomaterials measuring only a few nanometres can penetrate the nucleus via nuclear pores. However, larger nanomaterials have also been observed within the nucleus, suggesting alternative pathways for nuclear entry, such as endocytosis [380].
Secondary genotoxicity represents the predominant mechanism of nanomaterial-induced genotoxicity and is mediated by ROS produced by inflammatory cells [357]. When nanomaterials activate phagocytes, they can initiate an oxidative burst, triggering an inflammatory response aimed at clearing foreign materials. If this clearance is unsuccessful, as is often the case with inhaled nanomaterials, it can lead to a chronic immune response and sustained genotoxic effects. This form of genotoxicity is challenging to study using standard in vitro methods and has primarily been investigated in vivo, focusing on chronic inflammation caused by immune cells such as macrophages and neutrophils [377, 381]. These findings underscore the importance of carefully designing the surface chemistry and size of nanomaterials to control their ROS-inducing potential, which is critical during the manufacturing development phase.
Several studies have highlighted the variability in genotoxic effects depending on the type and coating of NPs. For example, Uygur et al. compared the toxicity of dialysed pristine Ag NPs with a size of 15 nm, PEG-coated Ag NPs (PEGylated Ag NPs), and silver nanorods across various cell lines [382]. Their findings demonstrated that all these forms of Ag NPs were genotoxic to peripheral blood cells. Similarly, Foldbjerg et al. investigated polyvinylpyrrolidone (PVP)-coated Ag NPs, with primary sizes exceeding 50 nm, using human adenocarcinomic alveolar epithelial cells (A549) [383]. They detected bulky DNA adducts in the nucleus through 32P post-labelling, which strongly correlated with oxidative stress. Conversely, Singh et al. utilised sophorolipid, a novel glycolipid, to conjugate 10 nm Ag NPs and found no genotoxicity in human liver carcinoma cells (Hep G2) at concentrations up to 100 μg/mL [384]. Additionally, Kim et al. reported that Ag NPs caused DNA damage in BEAS-2B cells and mouse lymphoma cells (L5178Y), although they did not exhibit mutagenicity [385]. Recent studies have also found that starch-coated Ag NPs, with primary sizes ranging from 6 to 20 nm, were cytotoxic, genotoxic, and antiproliferative in human fibroblasts and glioblastoma cells [386]. These findings suggest that the genotoxicity of Ag NPs is highly dependent on their size and surface coating, highlighting the importance of surface properties in determining their biological interactions and effects.
The role of surface properties is also evident in semiconductors like metal oxides, which generate electron–hole pairs upon energy exposure (e.g., from light) a and subsequently produce ROS. The risk of oxidative stress from these materials is significantly influenced by the chemical interactions of their surfaces with the environment. Rossi et al. demonstrated that the pulmonary effects observed in mice following acute and subacute inhalation of various coated or uncoated TiO2 NPs could not be attributed to particle size or surface area alone [387]. Instead, these effects were primarily influenced by the surface coating of the TiO2 particles. Among four types of TiO2 NPs with diameters ranging from 10 to 40 nm, only the uncoated TiO2 NPs did not induce pulmonary inflammation, likely due to differences in electron–hole recombination tendencies caused by the coatings. This electron–hole recombination effect is a well-known mechanism in the photocatalytic field [388, 389].
In summary, the genotoxicity and carcinogenicity of nanomaterials are complex phenomena influenced by multiple factors, including size, surface chemistry, and coating materials. A comprehensive understanding of these factors is essential for accurately assessing the risks associated with nanomaterials and for guiding their safe design and application in various fields.
Special considerations
In risk analysis, special considerations must be tailored to specific product scenarios. For example, for products that involve blood contact, beyond conventional toxicity risks, it is crucial to consider haemocompatibility. Given the broad scope of this comprehensive review, which encompasses a wide range of product types, it is challenging to systematically analyse each specific hazard in detail. Therefore, we have chosen to highlight some recent studies on the risks associated with nanomaterials. Keeping abreast of the latest research findings is vital for risk analysis and is particularly important for R&D engineers and scientists in developing safe and effective nanomaterials.
Jack et al. recently conducted a study on the toxicity potential of highly purified, metal-free, and endotoxin-free GO nanosheets [390]. The study focused on two precisely defined size ranges of GO nanosheets: small (ranging from 2 μm to 50 nm) and ultrasmall (ranging from 300 to 10 nm). The findings revealed that when introduced into the human airway, these GO nanosheets formed airborne aggregates with a median size of 80–90 nm. This size distribution is significant for blood-contact products, as the study also found that both small and ultrasmall GO increased blood thrombogenicity in in vitro coagulation assays compared to air exposure. These results suggest that the thrombotic potential of GO must be carefully considered in the design of products involving blood contact.
Similarly, a study on nano-silicon dioxide (nano-SiO2) investigated the cytotoxic effects of orally administered nano-SiO2 particles ranging in size from 20 to 30 nm at doses of 300, 600, and 900 mg/kg/day over 20 days [391]. The results indicated varying levels of cytotoxicity, with the highest doses causing significant toxicity in the liver and lungs, and the intermediate dose primarily affecting the kidneys and lungs. These findings provide essential insights into the risk assessment for oral nanomedicine applications, highlighting the need for careful dose and toxicity evaluations based on specific organ sensitivity.
These studies not only deepen our understanding of nanotoxicity but also underscore the complexity of assessing risks associated with nanomaterials. They highlight the importance of not assuming all risks are fully controlled and the need for thorough biological risk evaluations. For scientists and R&D engineers, regularly reviewing and analysing the latest research developments is crucial for guiding the development of safe and effective nanomaterials. By integrating the most current scientific insights into their design and evaluation processes, researchers and developers can better anticipate and mitigate potential risks associated with nanomaterial use.
Environmental risks
Nanotechnology has the potential to be a beneficial tool in environmental cleanup efforts by aiding in the treatment and prevention of pollution from hazardous substances [392–394], but it can also present risks to the ecosystem if not properly managed. One major concern is that the unique properties of nanomaterials, such as their small size and high reactivity, can lead to unexpected ecological consequences. Studies have shown that nanomaterials, such as Ag NPs and TiO2 NPs, can induce toxicity in aquatic and terrestrial organisms, which may disrupt local ecosystems and biodiversity [395, 396].
In the evaluation of risks from newly created engineered nanomaterials, it is widely agreed, and we share this view, that while the conventional approach to environmental risk assessment, which involves assessing both exposure and potential hazards, remains applicable to nanotechnology, it must be further adapted to consider the specific mechanisms unique to nanomaterials. Handy et al. have offered an extensive review based on regulatory guidelines, including a flowchart for assessing toxicity in water and soil environments and a summary of standard tests of the Organisation for Economic Co-operation and Development (OECD) for ecotoxicity [397].
Additionally, there have been calls for caution regarding environmental considerations, suggesting that hazard assessments for nanomaterials should also encompass studies on their behavior in environmentally aged states, including non-nanomaterial entities like dissolution products. Once nanomaterials are released into natural ecosystems, they may undergo transformations that significantly affect their toxicity and environmental fate. Thus, more comprehensive research is needed on how environmental conditions, such as pH, temperature, and the presence of natural organic matter, affect the surface chemistry, aggregation state, and solubility of nanomaterials. These factors, in turn, influence their interaction with biota and ecological systems.
Generally, risk assessment for nanomaterials should cover five stages across the lifecycle: resource acquisition, production process, utilisation stage, elimination process, and waste management. Furthermore, the risk assessment must ensure the integrity and standardisation of the evaluation across these stages, with special emphasis on the waste management stage. A critical challenge in this assessment is the development of reliable environmental monitoring techniques that can track the concentration, transformation, and bioavailability of nanomaterials in different environmental compartments (e.g., air, water, soil). A more integrated approach is suggested, combining multiple techniques such as single-particle inductively coupled plasma mass spectrometry (spICP-MS), transmission electron microscopy (TEM), and field-flow fractionation (FFF) coupled with light scattering detection to achieve real-time and precise detection of nanomaterials in complex matrices [398, 399].
Upon entering natural environments, nanomaterials may degrade into various forms under the influence of environmental processes, resulting in the final environmental aged state, which includes changes like surface transformation and dissolution [400]. For instance, the aging process of nanomaterials in aquatic environments can result in the formation of new nanomaterial species with altered reactivity and toxicity. Therefore, risk assessments should not only focus on pristine nanomaterials but should also extend to the aged forms, as these might pose different risks. Long-term monitoring and predictive modeling of these aged forms are crucial to assess their cumulative ecological impacts.
A research work about the lifecycle aging of Ag and TiO2 nanomaterials and its distribution of the different environmental aged states was done by Adam et al. [401]. It was modeled that release into surface water and soil primarily occur in altered forms (with 34–58% and 78–86% in the 25th and 75th percentiles, respectively, averaging 53% and 82%), whereas air releases were mainly in unaltered or matrix-encapsulated forms (ranging 38–46% and 36–44%, with averages of 42% and 40%). The variation in the release pathways across different environmental compartments highlights the need for a more systematic study of the nanomaterial release patterns in each medium and their potential environmental and ecological impact [401–403].
In response to the complex transformation pathways of nanomaterials, such as hetero-aggregation, dissolution, and sulphidation, it is recommended that risk assessments incorporate detailed monitoring and predictive models of these processes within natural ecosystems [404, 405]. Monitoring should focus on both short-term and long-term effects of nanomaterial exposure in critical environmental compartments. Furthermore, the integration of environmental data from different regions and ecosystems is essential to better understand the global distribution and impact of nanomaterials, which can support more informed regulatory frameworks.
Once nanomaterials enter ecosystems through industrial discharges or agricultural activities, they may affect the growth and development of rare medicinal plants, crop yield, and the metabolism of primary and secondary metabolites [406]. TiO2 NPs, commonly used in daily life, were studied for their effect on Luteolin, a sensitive anticancer flavonoid [407]. The findings showed that the impact was directly proportional to the level of nanoparticle contamination. Flavonoids can undergo various chemical modifications when exposed to metallic oxide NPs, depending on factors such as pH, temperature, and the presence of other chemical species.
In conclusion, the environmental risk analysis approach should be improved to fully account for the environmental aging of nanomaterials through specific transformation pathways such as hetero aggregation [408], dissolution [409], and sulphidation [404]. These transformations largely depend on the characteristics of the nanomaterial like size and surface coating, as well as the nanomaterial’s interact with various environmental components, such as water, soil, and living organisms which also determined largely on the surface chemistry of the nanomaterial. For a scientifically sound risk assessment, tailored methods should be developed by the manufacturers or other responsible entities based on specific application scenarios.
Safety risk analysis consideration
Based on the mechanisms outlined above, we recommend the following guidelines for medical product manufacturers conducting risk analysis of nanotechnology-derived medical products:
pay higher attention to NPs due to their higher mobility;
carefully consider the surface properties of all materials. For example, a nano-structured coating might not release NPs but could still pose cytotoxicity through physical interaction mechanisms when in direct contact;
when evaluating the properties of a nanotechnology-derived medical product, especially the nanoscale materials it might contain or produce throughout its lifecycle (such as size, size distribution, surface modification, redox potential, etc.), remember that no universal rule has been established. Therefore, a characterisation protocol based on scientific rationale is necessary for both documentation and result reliability;
for designing a reasonable characterisation protocol, refer to relevant technical manuals [410–412]. However, these manuals are just a starting point aimed at distinguishing “normal bulk materials” from “nanomaterials” to facilitate future evaluation. Medical product manufacturers should thoroughly scrutinise their product;.
regardless of the characterisation results, they only serve as input data for further toxicity or safety evaluation rather than as rigid indicators. For example, a material with nanoscale dimensions does not unconditionally show higher toxicity than its bulk form;
the nano-safety risks associated with nanoscale materials or other forms of products involving nanotechnology can be analysed or controlled using the mechanisms outlined above.
Current regulatory policies around the globe
Previous reviews have highlighted that key regional and national regulatory agencies have been proactively engaging in the oversight of nano-enabled products and collaborating to form a global consensus on standardisation and regulation [413]. Initiatives aimed at the evaluation and regulation of nanomaterials have been launched by Europe, the United States, and China.
In 2003, the United States officially proposed examining the possible impacts of synthetic nanomaterials on human health and the environment. By 2004, the Environmental Protection Agency (EPA) in the United States had established the direction for environmental and health safety research concerning synthetic nanomaterials, focusing on their environmental distribution, movement, and transformation, as well as their toxicological attributes. In 2007, the EPA in the United States released the Nanotechnology White Paper, which comprehensively addressed the risks to ecological toxicity and human health presented by synthetic nanomaterials. Following this, in 2009, the EPA issued the “Nanomaterials Research Strategy,” aimed at guiding scientists and regulatory bodies in investigating the possible environmental and health hazards associated with synthetic nanomaterials. In 2011, the President's Office along with the National Science and Technology Council released a White paper titled “Nanotechnology Environment, Health, and Safety Research Strategy,” incorporating nanotoxicology into the national research agenda. In the same year, the FDA published preliminary guidance to assist companies in determining the applicability of nanomaterials to their products.
The European Union has also conducted research on the environmental and biosafety assessments of synthetic nanomaterials. In 2005, the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) of the European Commission released “Artificial Products and by-products of Nanotechnology,” providing a systematic review of the toxicity, environmental impacts, and potential health dangers posed by nanomaterials.
In 2007, discussions commenced in Europe regarding the adaptation of the existing REACH regulation (Registration, Evaluation, Authorisation, and Restriction of Chemicals) for application to nanomaterials. Moreover, the updated European cosmetic regulations mandated that starting from 2013, any products containing synthetic nanomaterials must clearly label them as such.
Overall, for regulatory purposes, an initial clear definition was necessary. Factors to be considered in defining nano include: (i) size, (ii) size distribution, (iii) specific surface area, (iv) surface modification, (v) other physicochemical properties, (vi) whether the nanomaterials are organic or inorganic, (vii) nanocomposites, (viii) durability, (ix) whether the nanomaterials are manufactured or natural.
Size is considered the primary criterion when defining nanomaterials. The European Commission Recommendation from 10th June 2022, regarding nanomaterial definitions, reinforces this by adhering to the 2010 SCENIHR opinion, defining 'nanoscale' as ranging from 1 to 100 nm. Similarly, the FDA's 2014 Guidance for Industry on nanotechnology applications specifies that for a product to be considered under nanotechnology, it should be engineered with at least one dimension, or have internal or surface structures, within the nanoscale range of approximately 1 nm to 100 nm. Additionally, the NMPA's 2021 Guiding Principles for medical devices using nanomaterials describe nanomaterials as having any external dimension or internal/surface structure in the nanoscale, specifically between 1 and 100 nm, a range which often, but not always, presents unique properties not seen in larger scales. Despite the global consensus on the 100 nm threshold, it is emphasised that this measurement is a guideline rather than a strict indicator of toxicity or safety. Materials smaller than 100 nm are not inherently toxic, nor are those larger necessarily safe. As stated in the “Scientific Basis for the Definition of the Term ‘Nanomaterial’”, the term ‘nanomaterial’ simply refers to a material categorised by the size of its parts and does not inherently indicate a specific risk or hazard. The designation ‘nano’ does not automatically mean the material possesses novel properties compared to its larger or smaller counterparts. However, the size of a material does affect its biodistribution and distribution kinetics within organisms and ecosystems, a statement that is widely agreed upon in the scientific community.
This leads to a secondary criterion for defining nanomaterials, the “change of physicochemical properties”. Both the FDA and NMPA guidelines address whether a material or finished product is designed to demonstrate properties or effects, including physical, chemical, or biological, attributable to its dimensions, even if these dimensions are beyond the nanoscale range, extending up to one micrometer (1000 nm).
However, it seems the EU regulatory body has not set a similar standard, which we believe is due to an intentional emphasis on NPs rather than nanostructures, such as coatings, as indicated in clause 11: “The definition should not apply to large, solid products or components, even when they possess an internal or surface structure at the nanoscale, such as coatings, some ceramic materials, and complex nanocomponents, including nanoporous and nanocomposite materials.” This implies that while there is a consensus on the nano dimension being defined as 1 to 100 nm, regulations across different regions are not completely aligned regarding whether zero-dimensional (e.g., QDs), one-dimensional (e.g., nanotubes, nanofibers), or two-dimensional (e.g., surface coatings with nano thickness or dispersed nanosheets) materials should be uniformly regulated under the same criteria or framework.
Currently, among the various parameters considered for defining nanomaterials, the size requirement has been commonly addressed in many regions, whilst the EU has uniquely mentioned the specific surface area as a reference, and the FDA and NMPA have highlighted unique physicochemical properties. Another aspect that requires clarification is whether the materials are “manufactured or incidentally produced”. The EU’s Clause 6 expressly states that the nanomaterial definition should encompass natural, incidental, or manufactured materials, mirroring the stance in China where NMPA includes incidental nanomaterials under its regulatory framework. Conversely, the FDA’s guidelines specifically employ the term “engineered” to differentiate between deliberately modified nanotechnology products and those that might inadvertently contain nanoscale materials, pointing out that incidental NPs in conventionally manufactured products fall outside the scope of the guidance. It is also noteworthy that ISO 80004 series standards are dedicated to engineered nanotechnologies, including coatings among others.
As of now, there are noticeable differences and ambiguities in the regulatory boundaries of nanotechnology, which could lead to confusion among companies without a solid scientific background, relying solely on regulatory texts. Nevertheless, a definitive compilation of terms would still be beneficial. The Joint Research Centre of the European Commission provides a summary of the core elements found in nanomaterial definitions across global organisations, and an analysis illustrating the similarities and distinctions in the regulatory definitions of nanomaterials across Europe, the United States, and China, and other major nations is presented in Fig. 34.
Fig. 34.
Schematic representation of the overall assessment of environmental safety risks induced by nanomaterials, and the variety in the final state and distribution of nanomaterials cause by environmental processes, leading to the complexity of ecotoxicity caused by NPs
Although regulatory stipulations vary from one nation to another, this does not imply that potential risks, such as nano-structured coatings in the EU or accidentally produced NPs in the US, should be disregarded; instead, these should be addressed through a framework designed for risk analysis papers. In fact, a case-by-case methodology for the safety evaluation and risk assessment of nanomaterials has been advocated by various authorities (EFSA in 2009, FDA in 2007, SCENIHR in 2009), since deducing risks from one nanomaterial to another is not deemed practical, even if they share the same basic chemical composition. The provision of our interpretation of both the scientific basis and the regulations is aimed at assisting companies and professionals in acquiring a more defined direction for this type of risk assessment.
In the field of medicine and medical device registration, the adoption of a harmonised biological risk evaluation framework based on ISO 10993, which prioritises a risk-analysis approach over rigid testing protocols, indicates that the absence of precise definitions is unlikely to impede the innovative use of nanotechnology in the development of global healthcare products. For the safety assessment of specific nanomaterials, the European Parliament's REACH framework provides a versatile method for hazard evaluation and risk characterisation, making it particularly suitable for nanomaterials. REACH also promotes the use of computational methods, such as Quantitative Structure–Activity Relationships (QSARs), which has facilitated significant advancements in risk assessment despite the scarcity of experimental data. Moreover, the regulatory landscape is progressively aligning with the view that vertebrate animal testing should be employed only as a last resort, advocating for alternative testing approaches. However, to further enhance regulatory frameworks, there is a critical need to develop standardised nanomaterial characterisation data, employing harmonised testing standards and ensuring consistent reporting of endpoints. Currently, the availability of test data from the industrial sector is constrained by financial limitations and technological competition.
To improve the regulatory framework for nanomaterials globally, it is crucial to implement several key strategies that enhance clarity, consistency, and scientific rigor. First, establishing a unified global database for nanomaterials would facilitate the consolidation of definitions, classifications, uses, and risk assessments from different regions. Such a database would provide a centralised resource that fosters information sharing and regulatory harmonisation, enabling companies to better navigate and comply with various regional regulations. Second, developing standardised tools and methods for the risk assessment of nanomaterials is essential. Standardisation would not only streamline the evaluation processes across different regulatory bodies but also ensure that risk assessments are comparable and consistent globally. This would help mitigate discrepancies in regulatory practices and provide a more coherent approach to understanding and managing the potential risks associated with nanomaterials. Third, introducing a dynamic regulatory mechanism that evolves in response to technological advancements and emerging scientific research is vital. Regulations should be adaptable to accommodate new findings and innovations in nanotechnology, ensuring that they remain relevant and scientifically sound. This approach would prevent regulations from becoming outdated and inadequate, thus maintaining a high standard of safety and efficacy. Fourth, promoting interdisciplinary research collaboration is necessary for a comprehensive understanding of nanomaterials' behaviour and their potential environmental and health impacts. By integrating insights from fields such as chemistry, materials science, biology, and toxicology, regulatory strategies can be more effectively tailored to address the complex nature of nanomaterials. Finally, developing clear guidance documents and technical standards would address current ambiguities within existing regulations. This includes providing explicit definitions and regulatory requirements for incidentally produced nanomaterials, which would reduce confusion among companies and ensure a more straightforward interpretation of regulations.
Implementing these improvements would enhance the efficiency and effectiveness of nanomaterial regulation, fostering safer and more sustainable development of nanotechnology on a global scale.
Conclusions and outlook
Nanotechnology is based on a variety of materials including organic, inorganic, carbon-based, and composite. As dimensions are reduced to the nanometer scale, materials scientists provide a range of design options such as discrete particles, unique shapes, or nanostructured coatings, among others. These designs showcase distinctive benefits as innovative nanomedicines, functioning as drug carriers or therapeutic agents, or when incorporated to improve the performance of NMDs, such as enhancing detection capabilities, antibacterial properties, and so forth. Yet, when entering the market, a comprehensive examination of product lifecycle risks associated with nanotoxicity reveals numerous safety concerns, and the potential mechanisms were explored here.
The safety risks associated with nanotechnology in healthcare are multifaceted, often influenced by factors such as surface reactivity, size, and shape of nanomaterials. These same attributes, crucial for the efficacy of nanomedicine, also pose potential safety hazards. Understanding and addressing these risks require a focus on surface characteristics and their deviations from the original state.
Environmental considerations are paramount throughout the lifecycle of nanotechnology products. While traditional hazard evaluation methods can be adapted for nano-specific aspects, the aging process of nanomaterials significantly impacts their environmental fate, emphasising the need for comprehensive assessments.
Moreover, the complex nature of nanomaterials necessitates tailored risk assessments that account for individual material properties and toxicological profiles. A nuanced approach, aligned with regulatory standards, would enhance risk management strategies and ensure the responsible use of nanotechnology in healthcare.
The evolution of nanotechnology in healthcare underscores the critical need for close collaboration among researchers, industry professionals, regulatory bodies, and healthcare practitioners. Successfully translating research advancements into market-ready applications requires prioritizing comprehensive risk assessment strategies, particularly with regard to long-term toxicity, which remains both the costliest and most critical aspect. Given the complexity of this research area, advancing the safe and effective integration of nanotechnology into healthcare hinges on robust interdisciplinary collaboration. By working together, we can harness the transformative potential of nanotechnology while safeguarding human health and environmental sustainability. This will involve the development of a more comprehensive regulatory framework, informed by cutting-edge scientific insights and supported by transparent communication across all sectors. Through these efforts, we can ensure the safe, effective, and sustainable deployment of nanotechnology in healthcare.
Abbreviations
- 510(k)
510(k) of the food, drug and cosmetic act
- ADA
Adenosine deaminase
- Ag
Silver
- AJ
Adherent junction
- Al
Aluminum
- ALD
Atomic layer deposition
- ATP
Adenosine triphosphate
- Au
Gold
- BET
Brunauer–Emmett–Teller
- BIA-ALCL
Breast implant-associated anaplastic large cell lymphoma
- Bi
Bismuth
- BMVEC
Pristine graphene on brain microvascular endothelial cell
- BP
Black phosphorus
- C60
Fullerenes
- CAGR
Compound annual growth rate
- Cd
Cadmium
- CDs
Carbon dots
- CdTe
Cadmium telluride
- CeO2
Cerium oxide
- CNA35
Collagen-binding adhesion protein 35
- CFs
Carbon nanofibers
- CKD
Chronic kidney disease
- CNTs
Carbon nanotubes
- CoFe2O4
Cobalt ferrite
- CT
Computerised tomography
- Cu
Copper
- CuO
Copper oxide
- CVD
Chemical vapor deposition
- DOX
Doxorubicin
- EFSA
European Food Safety Authority
- EMA
European Medicines Agency
- EPA
Environmental Protection Agency
- EPO
Erythropoietin
- EPR
Permeability and retention
- EPS
Extracellular polymeric substance
- EU
European Union
- EUDAMED
European Database on Medical Devices
- FDA
The United States Food and Drug Administration
- Fe
Iron
- Fe3O4
Iron oxide
- FFF
Field-flow fractionation
- FMDV
Foot-and-mouth disease virus
- GBM
Glioblastoma multiforme
- Gd
Gadolinium
- Gel
Gelatin
- Gd
Gadolinium
- GH
Growth hormone
- GJ
Gap junction
- Gly
Glycyrrhizic acid
- GO
Graphene oxide
- GQDs
Graphene quantum dots
- Gr
Graphene
- HA
Hydroxyapatite
- HIV-1
Human immunodeficiency virus
- ICD
Immunogenic cell death
- INF
Alpha interferon
- ISC
Intersystem crossing
- KS
Kaposi’s sarcoma
- La2O3
Lanthanum oxide
- L-cysteine
Chiral amino acid
- LipSiNs
Lithiated porous silicon nanowires
- MgAl2O4
Magnesium aluminum oxide
- MgO
Magnesium oxide
- MIP NPs
Molecularly imprinted polymeric nanoparticles
- Mn
Manganese
- MOF
Metal–organic frameworks
- MPS
Mononuclear phagocytosis system
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- MSNs
Mesoporous silica nanoparticles
- NGPE
N-glutaryl phosphatidylethanolamine
- NIH
The United States National Institutes of Health
- NLM
National Library of Medicine
- NMDs
Nanotechnology-based medical devices
- NMPA
National Medical Products Administration
- NO
Nitric oxide
- NPs
Nanoparticles
- NSCLC
Non-small-cell lung cancer
- OECD
The Organisation for Economic Co-operation and Development
- Pb
Lead
- PDT
Photodynamic therapy
- PET
Positron emission tomography
- PETE
Polyethylene terephthalate
- PGA
Poly (glycolic acid)
- PIII
Plasma-immersion ion implantation
- PIA
Polysaccharide intercellular adhesin
- PLA
Poly (lactic acid)
- PLGA
Poly (lactic-co-glycolide acid)
- PMA
Premarket approval
- PMONPs
Periodic mesoporous silica nanoparticles
- PNIPAAm
Poly (N-isopropylacrylamide)
- PRRSV
Porcine reproductive and respiratory syndrome virus
- PS
Photosensitiser
- pSiNPs
Porous silicon nanoparticles
- PTT
Photothermal therapy
- PU
Polyurethane
- PVA
Polyvinyl alcohol
- PVP
Polyvinylpyrrolidone
- QS
Quorum sensing
- QSARs
Quantitative structure–activity relationships
- R&D
Research and development
- REACH
Registration evaluation, authorisation, and restriction of chemicals
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- RRMS
Relapsing remitting multiple sclerosis
- SBA2
Silica-based glass particles
- SCENIHR
Scientific committee on emerging and newly identified health risks
- SERS
Surface-enhanced Raman spectroscopy
- spICP-MS
Single-particle inductively coupled plasma mass spectrometry
- SF
Silk fibroin
- SPARC
Secreted protein acidic and rich in cysteine
- SPIONs
Superparamagnetic iron oxide nanoparticle
- Sr
Strontium
- TCP
Tricalcium phosphate
- TEM
Transmission electron microscopy
- TiO2
Titanium dioxide
- TJ
Tight junction
- TPE
Tetraphenylethene
- TTR
Transthyretin
- V2O5
Vanadium pentoxide
- VE
Vascular endothelial
- W
Tungsten
- Zn
Zinc
- ZnO
Zinc oxide
- ZrO2
Zirconium dioxide
Author contributions
X.M., Y.T., R.Y., H.W., L.A., J.C.: writing—original draft, methodology, data curation, formal analysis, visualisation. G.W., J.K., A.P.: writing—review & editing, conceptualisation, supervision, project administration, funding acquisition. All authors contributed to the article and approved the final manuscript.
Funding
This work was supported by grants from the Royal Society of Chemistry, the Wellcome Trust (ISSF3), the National Institute for Health and Care Research, the Kuwait University and the University College London.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaohan Ma and Yaxin Tian have contributed equally.
Contributor Information
Xiaohan Ma, Email: xiaohan.ma.21@ucl.ac.uk.
Alessandro Poma, Email: a.poma@ucl.ac.uk.
References
- 1.Armarego WLF. Nanomaterials. In: Armarego WLF, editor. Purification of laboratory chemicals. Oxford: Butterworth-Heinemann; 2022. p. 586–630. [Google Scholar]
- 2.Singh A, Amiji MM. Application of nanotechnology in medical diagnosis and imaging. Curr Opin Biotechnol. 2022;74:241–6. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa AI, Rebelo R, Reis RL, Bhattacharya M, Correlo VM. Current nanotechnology advances in diagnostic biosensors. Med Devices Sens. 2021;4(1): e10156. [Google Scholar]
- 4.Sardari S, Hheidari A, Ghodousi M, Rahi A, Pishbin E. Nanotechnology in tissue engineering: expanding possibilities with nanoparticles. Nanotechnology. 2024;35(39): 392002. [DOI] [PubMed] [Google Scholar]
- 5.Malik S, Muhammad K, Waheed Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules. 2023;28(18):6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Liu H, Ye Y, Lei Y, Islam R, Tan S, Tong R, Miao YB, Cai L. Smart nanoparticles for cancer therapy. Sign Transduct Target Ther. 2023;8(1):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vodyashkin A, Stoinova A, Kezimana P. Promising biomedical systems based on copper nanoparticles: synthesis, characterization, and applications. Coll Surf B Biointerface. 2024;237: 113861. [DOI] [PubMed] [Google Scholar]
- 8.Kumah EA, Fopa RD, Harati S, Boadu P, Zohoori FV, Pak T. Human and environmental impacts of nanoparticles: a scoping review of the current literature. BMC Public Health. 2023;23(1):1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali F, Neha K, Parveen S. Current regulatory landscape of nanomaterials and nanomedicines: a global perspective. J Drug Deliv Sci Technol. 2023;80: 104118. [Google Scholar]
- 10.Paras K, Yadav P, Kumar DR, Teja S, Chakraborty M, Chakraborty SS, Mohapatra A, Sahoo MMC, Chou CT, Liang DRH. A review on low-dimensional nanomaterials: nanofabrication. Charact Appl Nanomater. 2022;13(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barhoum A, Garcia-Betancourt ML, Jeevanandam J, Hussien EA, Mekkawy SA, Mostafa M, Omran MM, Abdalla MSM, Bechelany. Review on natural, incidental, bioinspired, and engineered nanomaterials: history, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials. 2022;12(2):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta D, Das KP, Deepak KS, Das B. Candidates of functionalized nanomaterial-based membranes. In: Dutta S, Hussain CM, editors. Membranes with functionalized nanomaterials. Amsterdam: Elsevier; 2022. p. 81–127. [Google Scholar]
- 13.Rafik ST, Vaidya JS, MacRobert AJ, Yaghini E. Organic nanodelivery systems as a new platform in the management of breast cancer: a comprehensive review from preclinical to clinical studies. J Clin Med. 2023;12(7):2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Yang J, Gao F, Zhang Q. Covalent organic frameworks: recent progress in biomedical applications. ACS Nano. 2023;17(3):1879–905. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi A, Bonilla-Cruz J. Review on healthcare biosensing nanomaterials. Acs Appl Nano Mater. 2023;6(7):5042–74. [Google Scholar]
- 16.Armentano I, Dottori M, Fortunati E, Mattioli S, Kenny JM. Biodegradable polymer matrix nanocomposites for tissue engineering: a review. Polym Degrad Stab. 2010;95(11):2126–46. [Google Scholar]
- 17.Zhang Y, Fang F, Li L, Zhang J. Self-assembled organic nanomaterials for drug delivery, bioimaging, and cancer therapy. ACS Biomater Sci Eng. 2020;6(9):4816–33. [DOI] [PubMed] [Google Scholar]
- 18.Xu M, Yim W, Zhou JJ, Zhou JC, Jin ZC, Moore C, Borum R, Jorns A, Jokerst JV. The application of organic nanomaterials for bioimaging, drug delivery, and therapy Spanning various domains. IEEE Nanatechnol Mag. 2021;15(4):8–28. [Google Scholar]
- 19.Mantri Y, Jokerst JV. Engineering plasmonic nanoparticles for enhanced photoacoustic imaging. ACS Nano. 2020;14(8):9408–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong G, Zou Y, Antaris AL, Diao S, Wu D, Cheng K, Zhang X, Chen C, Liu B, He Y, Wu JZ, Yuan J, Zhang B, Tao Z, Fukunaga C, Dai H. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat Commun. 2014;5(1):4206. [DOI] [PubMed] [Google Scholar]
- 21.He X, Jiang Z, Akakuru OU, Li J, Wu A. Nanoscale covalent organic frameworks: from controlled synthesis to cancer therapy. Chem Commun. 2021;57(93):12417–35. [DOI] [PubMed] [Google Scholar]
- 22.Yao S, Liu Z, Li L. Recent progress in nanoscale covalent organic frameworks for cancer diagnosis and therapy. Nanomicro Lett. 2021;13(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allahou LW, Madani SY, Seifalian A. Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. Int J Biomater. 2021; 20211–16. 10.1155/2021/3041969. [DOI] [PMC free article] [PubMed]
- 24.Rommasi F, Esfandiari N. Liposomal nanomedicine: applications for drug delivery in cancer therapy. Nanoscale Res Lett. 2021;16(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Kodidela S, Godse S, Thomas-Gooch S, Kumar A, Raji B, Zhi K, Kochat H, Kumar S. Targeted drug delivery to the central nervous system using extracellular vesicles. Pharmaceuticals. 2022;15(3):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng YT, Oz Y, Gu YM, Ahamad N, Shariati K, Chevalier J, Kapur D, Annabi N. Rational design of polymeric micelles for targeted therapeutic delivery. Nano Today. 2024;55: 102147. [Google Scholar]
- 27.Alshammari BH, Lashin MMA, Mahmood MA, Al-Mubaddel FS, Ilyas N, Rahman N, Sohail M, Khan A, Abdullaev SS, Khan R. Organic and inorganic nanomaterials: fabrication, properties and applications. RSC Adv. 2023;13(20):13735–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natesan V, Kim SJ. The trend of organic based nanoparticles in the treatment of diabetes and its perspectives. Biomol Ther. 2023;31(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhong X, Li J, Liu Z, Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem Soc Rev. 2021;50(15):8669–742. [DOI] [PubMed] [Google Scholar]
- 30.Zarschler K, Rocks L, Licciardello N, Boselli L, Polo E, Garcia KP, De Cola L, Stephan H, Dawson KA. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomedicine. 2016;12(6):1663–701. [DOI] [PubMed] [Google Scholar]
- 31.Lenders V, Koutsoumpou X, Sargsian A, Manshian BB. Biomedical nanomaterials for immunological applications: ongoing research and clinical trials. Nanoscale Adv. 2020;2(11):5046–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayda S, Hadla M, Palazzolo S, Riello P, Corona G, Toffoli G, Rizzolio F. Inorganic nanoparticles for cancer therapy: a transition from lab to clinic. Curr Med Chem. 2020;25(34):4269–303. [DOI] [PubMed] [Google Scholar]
- 33.Knezevic NZ, Kaluderovic GN. Silicon-based nanotheranostics. Nanoscale. 2017;9(35):12821–9. [DOI] [PubMed] [Google Scholar]
- 34.Murali A, Lokhande G, Deo KA, Brokesh A, Gaharwar AK. Emerging 2D nanomaterials for biomedical applications. Mater Today. 2021;50:276–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakubczak M, Szuplewska A, Rozmyslowska-Wojciechowska A, Rosenkranz A, Jastrzebska AM. Novel 2D MBenes-synthesis, structure, and biotechnological potential. Adv Funct Mater. 2021;31(38):2103048. [Google Scholar]
- 36.Riley PR, Narayan RJ. Recent advances in carbon nanomaterials for biomedical applications: a review. Curr Opin Biomed Eng. 2021;17: 100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Guo X, Wu Y, Chen X, Feng L, Xie N, Shen G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment. Signal Transduct Target Ther. 2024;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao N, Yan L, Zhao X, Chen X, Li A, Zheng D, Zhou X, Dai X, Xu FJ. Versatile types of organic/inorganic nanohybrids: from strategic design to biomedical applications. Chem Rev. 2019;119(3):1666–762. [DOI] [PubMed] [Google Scholar]
- 39.Poma A, Brahmbhatt H, Watts JK, Turner NW. Nucleoside-tailored molecularly imprinted polymeric nanoparticles (MIP NPs). Macromolecules. 2014;47(18):6322–30. [Google Scholar]
- 40.Poma A, Brahmbhatt H, Pendergraff HM, Watts JK, Turner NW. Generation of novel hybrid aptamer-molecularly imprinted polymeric nanoparticles. Adv Mater. 2015;27(4):750–8. [DOI] [PubMed] [Google Scholar]
- 41.Brahmbhatt H, Poma A, Pendergraff HM, Watts JK, Turner NW. Improvement of DNA recognition through molecular imprinting: hybrid oligomer imprinted polymeric nanoparticles (oligoMIP NPs). Biomater Sci. 2016;4(2):281–7. [DOI] [PubMed] [Google Scholar]
- 42.Park W, Shin H, Choi B, Rhim WK, Na K, Han DK. Advanced hybrid nanomaterials for biomedical applications. Prog Mater Sci. 2020;114: 100686. [Google Scholar]
- 43.Ling D, Park W, Park YI, Lee N, Li F, Song C, Yang SG, Choi SH, Na K, Hyeon T. Multiple-interaction ligands inspired by mussel adhesive protein: synthesis of highly stable and biocompatible nanoparticles. Angew Chem Int Ed Engl. 2011;50(48):11360–5. [DOI] [PubMed] [Google Scholar]
- 44.Chiozzi V, Rossi F. Inorganic-organic core/shell nanoparticles: progress and applications. Nanoscale Adv. 2020;2(11):5090–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hideshima S, Hinou H, Ebihara D, Sato R, Kuroiwa S, Nakanishi T, Nishimura S, Osaka T. Attomolar detection of influenza a virus hemagglutinin human H1 and avian H5 using glycan-blotted field effect transistor biosensor. Anal Chem. 2013;85(12):5641–4. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, He Z, Li B, Cheng T, Liu G. AND logic-like pH- and light-dual controlled drug delivery by surface modified mesoporous silica nanoparticles. Mater Sci Eng C Mater Biol Appl. 2017;73:1–7. [DOI] [PubMed] [Google Scholar]
- 47.Vodyashkin A, Sergorodceva A, Kezimana P, Morozova M, Nikolskaya E, Mollaeva M, Yabbarov N, Sokol M, Chirkina M, Butusov L. Synthesis and activation of pH-sensitive metal–organic framework Sr (BDC)∞ for oral drug delivery. Dalton Trans. 2024;53(3):1048–57. [DOI] [PubMed] [Google Scholar]
- 48.Sun XJ, Li H, Qi LJ, Wang F, Hou YC, Li JG, Guan SK. Construction and biocompatibility evaluation of MOF/ S-HA composite coating on the surface of magnesium alloy vascular stent. Prog Org Coat. 2024;189: 108177. [Google Scholar]
- 49.Wang H, Fang T, Wang J, Zhang M, Mu X, Gao T, Wei T, Dai Z. Adaptive size evolution of an MOFs-in-MOF nanovehicle for enhanced nucleus-targeted tumor chemotherapy. Nano Lett. 2024. 10.1021/acs.nanolett.4c02817. [DOI] [PubMed] [Google Scholar]
- 50.Huang R, Zhou X, Chen G, Su L, Liu Z, Zhou P, Weng J, Min Y. Advances of functional nanomaterials for magnetic resonance imaging and biomedical engineering applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14(4): e1800. [DOI] [PubMed] [Google Scholar]
- 51.Li HC, Yang SQ, Hui D, Hong RY. Progress in magnetic Fe O nanomaterials in magnetic resonance imaging. Nanotechnol Rev. 2020;9(1):1265–83. [Google Scholar]
- 52.Gao ZY, Ma TC, Zhao EY, Docter D, Yang WS, Stauber RH, Gao MY. Small is smarter: nano MRI contrast agents—advantages and recent achievements. Small. 2016;12(5):556–76. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Xiong H, Zhou Q, Zhao Z, Zhang Y, Li Y, Wang S, Shi S. A pH-activatable MnCO(3) nanoparticle for improved magnetic resonance imaging of tumor malignancy and metastasis. ACS Appl Mater Interfaces. 2021;13(16):18462–71. [DOI] [PubMed] [Google Scholar]
- 54.Mao Q, Gu M, Hong C, Wang H, Ruan X, Liu Z, Yuan B, Xu M, Dong C, Mou L, Gao X, Tang G, Chen T, Wu A, Pan Y. A contrast-enhanced Tri-modal MRI technique for high-performance hypoxia imaging of breast cancer. Small. 2024;20(28): e2308850. [DOI] [PubMed] [Google Scholar]
- 55.Lai J, Luo Z, Chen L, Wu Z. Advances in nanotechnology-based targeted-contrast agents for computed tomography and magnetic resonance. Sci Prog. 2024;107(1):368504241228076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kee PH, Danila D. CT imaging of myocardial scar burden with CNA35-conjugated gold nanoparticles. Nanomedicine. 2018;14(6):1941–7. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Zhu J, Wang S, Zhao L, Wei P, Yi T. Self-assembled nano-CT contrast agent leveraging size aggregation for improved in vivo tumor CT imaging. Adv Mater. 2024;36(2): e2309789. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Cai W, Chen X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc Chem Res. 2015;48(2):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen D, Yang D, Dougherty CA, Lu W, Wu H, He X, Cai T, Van Dort ME, Ross BD, Hong H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano. 2017;11(4):4315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li YP, Pan ZW, Jiang YJ, Peng YY, Cai T, Hong H, Wang XF. Zirconium-containing nanoscale coordination polymers for positron emission tomography and fluorescence-guided cargo delivery to triple-negative breast tumors. Acta Biomater. 2024;179:313–24. [DOI] [PubMed] [Google Scholar]
- 61.Jung KO, Kim TJ, Yu JH, Rhee S, Zhao W, Ha B, Red-Horse K, Gambhir SS, Pratx G. Whole-body tracking of single cells via positron emission tomography. Nat Biomed Eng. 2020;4(8):835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu JC, Tang Z, Eremina OE, Sofias AM, Lammers T, Lovell JF, Zavaleta C, Cai W, Cormode DP. Nanomaterial-based contrast agents. Nat Rev Method Prim. 2023;3(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malhotra BD, Ali MA. Nanomaterials in biosensors. In: Malhotra BD, Ali MA, editors. Nanomaterials for biosensors. Norwich: William Andrew Publishing; 2018. p. 1–74. [Google Scholar]
- 64.Malitesta C, Mazzotta E, Picca RA, Poma A, Chianella I, Piletsky SA. MIP sensors—the electrochemical approach. Anal Bioanal Chem. 2012;402(5):1827–46. [DOI] [PubMed] [Google Scholar]
- 65.Malik S, Singh J, Goyat R, Saharan Y, Chaudhry V, Umar A, Ibrahim AA, Akbar S, Ameen S, Baskoutas S. Nanomaterials-based biosensor and their applications: a review. Heliyon. 2023. 10.1016/j.heliyon.2023.e19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Wang T, Zhong Y, Li R, Deng W, Xiao X, Xu Y, Zhang J, Hu X, Wang Y. A review of nanomaterials for biosensing applications. J Mater Chem B. 2024;12(5):1168–93. [DOI] [PubMed] [Google Scholar]
- 67.Wozniak M, Ploska A, Siekierzycka A, Dobrucki LW, Kalinowski L, Dobrucki IT. Molecular imaging and nanotechnology-emerging tools in diagnostics and therapy. Int J Mol Sci. 2022;23(5):2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poma A, Turner APF, Piletsky SA. Advances in the manufacture of MIP nanoparticles. Trend Biotechnol. 2010;28(12):629–37. [DOI] [PubMed] [Google Scholar]
- 69.Poma A, Whitcombe M, Piletsky S. Plastic antibodies. In: Piletsky SA, Whitcombe MJ, editors. Designing receptors for the next generation of biosensors. Berlin: Springer; 2013. p. 105–29. [Google Scholar]
- 70.Muzyka K, Karim K, Guerreiro A, Poma A, Piletsky S. Optimisation of the synthesis of vancomycin-selective molecularly imprinted polymer nanoparticles using automatic photoreactor. Nanoscale Res Lett. 2014;9(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furth ME, Atala A. Tissue engineering. In: Lanza R, Langer R, Vacanti J, editors. Principles of tissue engineering. Boston: Academic Press; 2014. p. 83–123. [Google Scholar]
- 72.Zheng X, Zhang P, Fu Z, Meng S, Dai L, Yang H. Applications of nanomaterials in tissue engineering. RSC Adv. 2021;11(31):19041–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar R, Aadil KR, Ranjan S, Kumar VB. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J Drug Deliv Sci Technol. 2020;57: 101617. [Google Scholar]
- 74.Heo C, Yoo J, Lee S, Jo A, Jung S, Yoo H, Lee YH, Suh M. The control of neural cell-to-cell interactions through non-contact electrical field stimulation using graphene electrodes. Biomaterials. 2011;32(1):19–27. [DOI] [PubMed] [Google Scholar]
- 75.Lu X, Li G, Jiao W, Li K, Zhang T, Liu X, Fan H. Magnetic nanomaterials-mediated neuromodulation. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(4): e1890. [DOI] [PubMed] [Google Scholar]
- 76.Chaparro CIP, Simões BT, Borges JP, Castanho MARB, Soares PIP, Neves V. A promising approach: magnetic nanosystems for alzheimer’s disease theranostics. Pharmaceutics. 2023;15(9):2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu J, Cui X, Ke PC, Mortimer M, Wang X, Bao L, Chen C. Nanomaterials as novel agents for amelioration of Parkinson’s disease. Nano Today. 2021;41: 101328. [Google Scholar]
- 78.Gong W, Zhang T, Che M, Wang Y, He C, Liu L, Lv Z, Xiao C, Wang H, Zhang S. Recent advances in nanomaterials for the treatment of spinal cord injury. Mater Today Bio. 2023;18: 100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang XD, Cui X, Wang DC, Wang S, Liu ZR, Zhao GR, Zhang Y, Li Z, Wang ZL, Li LL. Piezoelectric nanotopography induced neuron-like differentiation of stem cells. Adv Funct Mater. 2019;29(22):1900372. [Google Scholar]
- 80.Mishra A, Kumar R, Mishra J, Dutta K, Ahlawat P, Kumar A, Dhanasekaran S, Gupta AK, Sinha S, Bishi DK, Gupta PK, Nayak S. Strategies facilitating the permeation of nanoparticles through blood-brain barrier: an insight towards the development of brain-targeted drug delivery system. J Drug Deliv Sci Technol. 2023;86: 104694. [Google Scholar]
- 81.Barot T, Rawtani D, Kulkarni P. Nanotechnology-based materials as emerging trends for dental applications. Rev Adv Mater Sci. 2021;60(1):173–89. [Google Scholar]
- 82.Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12(9):8882–92. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S, Zhou H, Kong N, Wang Z, Fu H, Zhang Y, Xiao Y, Yang W, Yan F. l-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration. Bioact Mater. 2021;6(10):3288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaasalainen M, Zhang R, Vashisth P, Birjandi AA, S’Ari M, Martella DA, Isaacs M, Makila E, Wang C, Moldenhauer E, Clarke P, Pinna A, Zhang X, Mustfa SA, Caprettini V, Morrell AP, Gentleman E, Brauer DS, Addison O, Zhang X, Bergholt M, Al-Jamal K, Volponi AA, Salonen J, Hondow N, Sharpe P, Chiappini C. Lithiated porous silicon nanowires stimulate periodontal regeneration. Nat Commun. 2024;15(1):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tukmachi MS, Safi IN, Ali MMM. Evaluation of mechanical properties and cytotoxicity of maxillofacial silicone material after incorporation of zirconia nanopowder. Mater Today Proceed. 2021;42:2209–17. [Google Scholar]
- 86.Abdalqadir M, Mohammed K, Azhdar B. The impact of zirconium dioxide nanoparticles on the color stability of artificially aged heat-polymerized maxillofacial silicone elastomer. Sci Prog. 2023;106(4):368504231205392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watcharajittanont N, Tabrizian M, Putson C, Pripatnanont P, Meesane J. Osseointegrated membranes based on electro-spun TiO(2)/hydroxyapatite/polyurethane for oral maxillofacial surgery. Mater Sci Eng C Mater Biol Appl. 2020;108: 110479. [DOI] [PubMed] [Google Scholar]
- 88.Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: a review. J Endod. 2016;42(10):1417–26. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Gulati K, Li Z, Di P, Liu Y. Dental implant nano-engineering: advances limitations and future directions. Nanomaterials (Basel). 2021;11(10):2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao Z, Bian Y, Hu T, Yang Y, Cui Z, Wang T, Yang S, Weng X, Liang R, Tan C. Recent advances in two-dimensional nanomaterials for bone tissue engineering. J Mater. 2023;9(5):930–58. [Google Scholar]
- 91.Bozorgi A, Khazaei M, Soleimani M, Jamalpoor Z. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater Sci. 2021;9(13):4541–67. [DOI] [PubMed] [Google Scholar]
- 92.Sun H, Xu J, Wang Y, Shen S, Xu X, Zhang L, Jiang Q. Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact Mater. 2023;24:477–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie C, Ye J, Liang R, Yao X, Wu X, Koh Y, Wei W, Zhang X, Ouyang H. Advanced strategies of biomimetic tissue-engineered grafts for bone regeneration. Adv Healthc Mater. 2021;10(14): e2100408. [DOI] [PubMed] [Google Scholar]
- 94.Hou C, An J, Zhao D, Ma X, Zhang W, Zhao W, Wu M, Zhang Z, Yuan F. Surface modification techniques to produce micro/nano-scale topographies on Ti-based implant surfaces for improved osseointegration. Front Bioeng Biotechnol. 2022;10: 835008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu H, Zhang H, Chen S, Guan S, Lu W, Zhu H, Ouyang L, Liu X, Mei Y. Fe-NC nanozymes-loaded TiO2 nanotube arrays endow titanium implants with excellent antioxidant capacity for inflammation inhibition and soft tissue integration. Compos B Eng. 2023;267: 111054. [Google Scholar]
- 96.Guan S, Hou Z, Tan J, Zhang X, Liu J, Du H, Zhu H, Qiao Y, Liu Z, Liu X. Straddle-type heterostructure films endow titanium implant with NIR photocatalysis property for rapid sterilization. Appl Catal B. 2023;334: 122826. [Google Scholar]
- 97.Goonoo N, A. Bhaw-Luximon, nanomaterials combination for wound healing and skin regeneration. In: Toit LCD, Kumar P, Choonara YE, Pillay V, editors. Advanced 3D-printed systems and nanosystems for drug delivery and tissue engineering. Amsterdam: Elsevier; 2020. p. 159–217. [Google Scholar]
- 98.Bacakova L, Pajorova J, Bacakova M, Skogberg A, Kallio P, Kolarova K, Svorcik V. Versatile application of nanocellulose: from industry to skin tissue engineering and wound healing. Nanomaterials (Basel). 2019. 10.3390/nano9020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leong MY, Kong YL, Harun MY, Looi CY, Wong WF. Current advances of nanocellulose application in biomedical field. Carbohydr Res. 2023;532: 108899. [DOI] [PubMed] [Google Scholar]
- 100.Lamberger Z, Zainuddin S, Scheibel T, Lang G. Polymeric Janus fibers. ChemPlusChem. 2023;88(2): e202200371. [DOI] [PubMed] [Google Scholar]
- 101.Qian S, Zhao B, Mao J, Liu Z, Zhao Q, Lu B, Mao X, Zhang L, Cheng L, Zhang Y, Cui W, Sun X. Biomedical applications of Janus membrane. Biomed Technol. 2023;2:58–69. [Google Scholar]
- 102.Zhou L, Liu F, You J, Zhou B, Guo W, Qu W, Ren X, Gao G. A novel self-pumping Janus dressing for promoting wound immunomodulation and diabetic wound healing. Adv Healthc Mater. 2024;13(10): e2303460. [DOI] [PubMed] [Google Scholar]
- 103.Liu R, Luo C, Pang Z, Zhang J, Ruan S, Wu M, Wang L, Sun T, Li N, Han L, Shi J, Huang Y, Guo W, Peng S, Zhou W, Gao H. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin Chem Lett. 2023;34(2): 107518. [Google Scholar]
- 104.Sahu T, Ratre YK, Chauhan S, Bhaskar LVKS, Nair MP, Verma HK. Nanotechnology based drug delivery system: current strategies and emerging therapeutic potential for medical science. J Drug Deliv Sci Technol. 2021;63: 102487. [Google Scholar]
- 105.Eras A, Castillo D, Suarez M, Vispo NS, Albericio F, Rodriguez H. Chemical conjugation in drug delivery systems. Front Chem. 2022;10: 889083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsu C-Y, Rheima AM, Kadhim MM, Ahmed NN, Mohammed SH, Abbas FH, Abed ZT, Mahdi ZM, Abbas ZS, Hachim SK, Ali FK, Mahmoud ZH, Kianfar E. An overview of nanoparticles in drug delivery: properties and applications. S Afr J Chem Eng. 2023;46:233–70. [Google Scholar]
- 107.Li S, Wang H, Shan Y. The mechanism of nano-drug delivery. Curr Pharmacol Rep. 2019;5(6):410–20. [Google Scholar]
- 108.Rabanel JM, Aoun V, Elkin I, Mokhtar M, Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19(19):3070–102. [DOI] [PubMed] [Google Scholar]
- 109.Salahpour Anarjan F. Active targeting drug delivery nanocarriers: ligands. NanoStruct NanoObject. 2019;19:100370. [Google Scholar]
- 110.Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. [DOI] [PubMed] [Google Scholar]
- 111.Zielinska A, Carreiro F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A, Souto EB. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16):3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gabizon AA, Lyass O, Berry GJ, Wildgust M. Cardiac safety of pegylated liposomal doxorubicin (Doxil/Caelyx) demonstrated by endomyocardial biopsy in patients with advanced malignancies. Cancer Invest. 2004;22(5):663–9. [DOI] [PubMed] [Google Scholar]
- 113.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (abraxane ABI-007) in the treatment of breast cancer. Int J Nanomed. 2009;4:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fraguas-Sanchez AI, Lozza I, Torres-Suarez AI. Actively targeted nanomedicines in breast cancer: from pre-clinal investigation to clinic. Cancers (Basel). 2022;14(5):1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Rev. 2018;130:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Senzer NN, Matsuno K, Yamagata N, Fujisawa T, Wasserman E, Sutherland W, Sharma S, Phan A. Abstract C36: MBP-426, a novel liposome-encapsulated oxaliplatin, in combination with 5-FU/leucovorin (LV): phase I results of a phase I/II study in gastro-esophageal adenocarcinoma, with pharmacokinetics. Mol Cancer Therapeutics. 2009;8(12_Supplement):C36–C36. [Google Scholar]
- 117.Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820(3):291–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kadhum WR, Majeed AA, Saleh RO, Ali E, Alhajlah S, Alwaily ER, Mustafa YF, Ghildiyal P, Alawadi A, Alsalamy A. Overcoming drug resistance with specific nano scales to targeted therapy: focused on metastatic cancers. Pathol Res Pract. 2024;255: 155137. [DOI] [PubMed] [Google Scholar]
- 119.Moczko E, Poma A, Guerreiro A, de Vargas Sansalvador IP, Caygill S, Canfarotta F, Whitcombe MJ, Piletsky S. Surface-modified multifunctional MIP nanoparticles. Nanoscale. 2013;5(9):3733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guerreiro A, Poma A, Karim K, Moczko E, Takarada J, de Vargas-Sansalvador IP, Turner N, Piletska E, de Magalhães CS, Glazova N, Serkova A, Omelianova A, Piletsky S. Influence of surface-imprinted nanoparticles on trypsin activity. Adv Healthcare Mater. 2014;3(9):1426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poma A, Guerreiro A, Caygill S, Moczko E, Piletsky S. Automatic reactor for solid-phase synthesis of molecularly imprinted polymeric nanoparticles (MIP NPs) in water. RSC Adv. 2014;4(8):4203–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu R, Poma A. Advances in molecularly imprinted polymers as drug delivery systems. Molecules. 2021;26(12):3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma X, Knowles JC, Poma A. Biodegradable and sustainable synthetic antibodies—a perspective. Pharmaceutics. 2023. 10.3390/pharmaceutics15051440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Zhang P, Song H, Tang X, Hao Y, Guan Y, Chong T, Hussain S, Gao R. Unveiling a pH-responsive dual-androgen-blocking magnetic molecularly imprinted polymer for enhanced synergistic therapy of prostate cancer. ACS Appl Mater Interfaces. 2024;16(4):4348–60. [DOI] [PubMed] [Google Scholar]
- 125.Soni V, Pandey V, Asati S, Jain P, Tekade RK. Design and fabrication of brain-targeted drug delivery. In: Tekade RK, editor. Basic fundamentals of drug delivery. Amsterdam: Academic Press; 2019. p. 539–93. [Google Scholar]
- 126.Gou K, Wang Y, Guo X, Wang Y, Bian Y, Zhao H, Guo Y, Pang Y, Xie L, Li S, Li H. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021;134:576–92. [DOI] [PubMed] [Google Scholar]
- 127.Pham SH, Choi Y, Choi J. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics. 2020;12(7):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jin L, Hu P, Wang Y, Wu L, Qin K, Cheng H, Wang S, Pan B, Xin H, Zhang W, Wang X. Fast-acting black-phosphorus-assisted depression therapy with low toxicity. Adv Mater. 2020;32(2): e1906050. [DOI] [PubMed] [Google Scholar]
- 129.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolen Y, Valente M, Tagit O, Jager E, Van Dinther EAW, van Riessen NK, Hruby M, Gileadi U, Cerundolo V, Figdor CG. Nanovaccine administration route is critical to obtain pertinent iNKt cell help for robust anti-tumor T and B cell responses. Oncoimmunology. 2020;9(1):1738813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhong Q, Merkel OM, Reineke JJ, da Rocha SR. Effect of the route of administration and PEGylation of poly (amidoamine) dendrimers on their systemic and lung cellular biodistribution. Mol Pharm. 2016;13(6):1866–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11(6):673–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J Nucl Med. 2010;51(8):1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kruse CR, Singh M, Targosinski S, Sinha I, Sorensen JA, Eriksson E, Nuutila K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: in vitro and in vivo study. Wound Repair Regen. 2017;25(2):260–9. [DOI] [PubMed] [Google Scholar]
- 136.Al-Shaeli M, Benkhaya S, Al-Juboori RA, Koyuncu I, Vatanpour V. pH-responsive membranes: mechanisms, fabrications, and applications. Sci Total Environ. 2024;946: 173865. [DOI] [PubMed] [Google Scholar]
- 137.Vitulo M, Gnodi E, Meneveri R, Barisani D. Interactions between nanoparticles and intestine. Int J Mol Sci. 2022;23(8):4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9(2):223–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beach MA, Nayanathara U, Gao Y, Zhang C, Xiong Y, Wang Y, Such GK. Polymeric nanoparticles for drug delivery. Chem Rev. 2024;124(9):5505–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen G, Abdeen AA, Wang Y, Shahi PK, Robertson S, Xie R, Suzuki M, Pattnaik BR, Saha K, Gong S. A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nat Nanotechnol. 2019;14(10):974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hocking KM, Evans BC, Komalavilas P, Cheung-Flynn J, Duvall CL, Brophy CM. Nanotechnology enabled modulation of signaling pathways affects physiologic responses in intact vascular tissue. Tissue Eng Part A. 2019;25(5–6):416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao Y, Jia L, Wang Q, Hu H, Zhao X, Chen D, Qiao M. pH/Redox dual-responsive polyplex with effective endosomal escape for codelivery of siRNA and doxorubicin against drug-resistant cancer cells. ACS Appl Mater Interfaces. 2019;11(18):16296–310. [DOI] [PubMed] [Google Scholar]
- 143.Yue J, Feliciano TJ, Li W, Lee A, Odom TW. Gold nanoparticle size and shape effects on cellular uptake and intracellular distribution of siRNA nanoconstructs. Bioconjug Chem. 2017;28(6):1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yan Y, Ding H. pH-responsive nanoparticles for cancer immunotherapy: a brief review. Nanomaterials. 2020;10(8):1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fang T, Cao X, Ibnat M, Chen G. Stimuli-responsive nanoformulations for CRISPR-Cas9 genome editing. J Nanobiotechnol. 2022;20(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ran T, Zhigang X. Gene therapy: a double-edged sword with great powers. Mol Cell Biochem. 2020;474(1-2):73-81. 10.1007/s11010-020-03834-3. [DOI] [PubMed]
- 147.Uddin F, Rudin CM, Sen T. CRISPR gene therapy: applications limitations and implications for the future. Front Oncol. 2020;10:1387. 10.3389/fonc.2020.01387. [DOI] [PMC free article] [PubMed]
- 148.Mendes BB, Conniot J, Avital A, Yao D, Jiang X, Zhou X, Sharf-Pauker N, Xiao Y, Adir O, Liang H, Shi J, Schroeder A, Conde J. Nanodelivery of nucleic acids. Nat Rev Method Prim. 2022;2(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang T, Gonzalez KM, Cordova LE, Lu J. Nanotechnology-enabled gene delivery for cancer and other genetic diseases. Expert Opin Drug Deliv. 2023;20(4):523–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jinturkar KA, Misra A. Challenges and opportunities in gene delivery. In: Misra A, editor. Challenges in delivery of therapeutic genomics and proteomics. Amsterdam: Elsevier; 2011. p. 45–82. [Google Scholar]
- 151.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed]
- 153.Jiao L, Sun Z, Sun Z, Liu J, Deng G, Wang X. Nanotechnology-based non-viral vectors for gene delivery in cardiovascular diseases. Front Bioeng Biotechnol. 2024;12:1349077. 10.3389/fbioe.2024.1349077. [DOI] [PMC free article] [PubMed]
- 154.Durymanov M, Reineke J. Non-viral delivery of nucleic acids: insight into mechanisms of overcoming intracellular barriers. Front Pharmacol. 2018;9:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anjum S, Ishaque S, Fatima H, Farooq W, Hano C, Abbasi BH, Anjum I. Emerging applications of nanotechnology in healthcare systems: grand challenges and perspectives. Pharmaceuticals21. 2021;14(8):707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81(4):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilson B, Geetha KM. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J Drug Deliv Sci Technol. 2022;74: 103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiong Z, Alves CS, Wang J, Li A, Liu J, Shen M, Rodrigues J, Tomas H, Shi X. Zwitterion-functionalized dendrimer-entrapped gold nanoparticles for serum-enhanced gene delivery to inhibit cancer cell metastasis. Acta Biomater. 2019;99:320–9. [DOI] [PubMed] [Google Scholar]
- 159.Panico S, Capolla S, Bozzer S, Toffoli G, Dal Bo M, Macor P. Biological features of nanoparticles: protein corona formation and interaction with the immune system. Pharmaceutics. 2022;14(12):2605. 10.3390/pharmaceutics14122605. [DOI] [PMC free article] [PubMed]
- 160.Yao J, Fan Y, Li Y, Huang L. Strategies on the nuclear-targeted delivery of genes. J Drug Target. 2013;21(10):926–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang CS, Chang CH, Tzeng TY, Lin AM, Lo YL. Gene-editing by CRISPR-Cas9 in combination with anthracycline therapy via tumor microenvironment-switchable, EGFR-targeted, and nucleus-directed nanoparticles for head and neck cancer suppression. Nanoscale Horiz. 2021;6(9):729–43. [DOI] [PubMed] [Google Scholar]
- 162.Długosz O, Matyjasik W, Hodacka G, Szostak K, Matysik J, Krawczyk P, Piasek A, Pulit-Prociak J, Banach M. Inorganic nanomaterials used in anti-cancer therapies: further developments. Nanomaterials. 2023;13(6):1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Subhan MA, Yalamarty SSK, Filipczak N, Parveen F, Torchilin VP. Recent advances in tumor targeting via EPR effect for cancer treatment. J Pers Med. 2021;11(6):571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bazak R, Houri M, Achy SE, Hussein W, Refaat T. Passive targeting of nanoparticles to cancer: a comprehensive review of the literature. Mol Clin Oncol. 2014;2(6):904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jahan S, Karim ME, Chowdhury EH. Nanoparticles targeting receptors on breast cancer for efficient delivery of chemotherapeutics. Biomedicines. 2021;9(2):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Samani RK, Tavakoli MB, Maghsoudinia F, Motaghi H, Hejazi SH, Mehrgardi MA. Trastuzumab and folic acid functionalized gold nanoclusters as a dual-targeted radiosensitizer for megavoltage radiation therapy of human breast cancer. Eur J Pharm Sci. 2020;153: 105487. [DOI] [PubMed] [Google Scholar]
- 167.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nanoenabled Med Appl. 2020. 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 168.Piktel E, Niemirowicz K, Watek M, Wollny T, Deptula P, Bucki R. Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J Nanobiotechnol. 2016;14(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wojtynek NE, Mohs AM. Image-guided tumor surgery: the emerging role of nanotechnology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(4): e1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang L, You X, Dai C, Tong T, Wu J. Hemostatic nanotechnologies for external and internal hemorrhage management. Biomater Sci. 2020;8(16):4396–412. [DOI] [PubMed] [Google Scholar]
- 171.Malik A, Rehman FU, Shah KU, Naz SS, Qaisar S. Hemostatic strategies for uncontrolled bleeding: a comprehensive update. J Biomed Mater Res B Appl Biomater. 2021;109(10):1465–77. [DOI] [PubMed] [Google Scholar]
- 172.Liang Y, Li M, Huang Y, Guo B. An integrated strategy for rapid hemostasis during tumor resection and prevention of postoperative tumor recurrence of hepatocellular carcinoma by antibacterial shape memory cryogel. Small. 2021;17(38): e2101356. [DOI] [PubMed] [Google Scholar]
- 173.Song G, Cheng L, Chao Y, Yang K, Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv Mater. 2017;29(32):1700996. [DOI] [PubMed] [Google Scholar]
- 174.Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv Mater. 2019;31(3): e1802244. [DOI] [PubMed] [Google Scholar]
- 175.Jin J, Zhao Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: recent advances and future challenges. J Nanobiotechnoly. 2020;18(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clement S, Campbell JM, Deng W, Guller A, Nisar S, Liu G, Wilson BC, Goldys EM. Mechanisms for tuning engineered nanomaterials to enhance radiation therapy of cancer. Adv Sci. 2020;7(24):2003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Menon JU, Tumati V, Hsieh JT, Nguyen KT, Saha D. Polymeric nanoparticles for targeted radiosensitization of prostate cancer cells. J Biomed Mater Res A. 2015;103(5):1632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu DX, Cao F, Xu ZF, Zhao CH, Liu ZK, Pang JD, Liu ZX, Moghiseh M, Butler A, Liang SX, Fan WJ, Yang J. Selective organ-targeting hafnium oxide nanoparticles with multienzyme-mimetic activities attenuate radiation-induced tissue damage. Adv Mater. 2024. 10.1002/adma.202308098. [DOI] [PubMed] [Google Scholar]
- 179.Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol. 2016;13(11):659–73. [DOI] [PubMed] [Google Scholar]
- 180.Luo C, Sun J, Sun B, He Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trend Pharmacol Sci. 2014;35(11):556–66. [DOI] [PubMed] [Google Scholar]
- 181.Oz UC, Bolat ZB, Poma A, Guan L, Telci D, Sahin F, Battaglia G, Bozkır A. Prostate cancer cell-specific BikDDA delivery by targeted polymersomes. Appl Nanosci. 2020;10(9):3389–401. [Google Scholar]
- 182.Ellis E, Zhang K, Lin Q, Ye E, Poma A, Battaglia G, Loh XJ, Lee TC. Biocompatible pH-responsive nanoparticles with a core-anchored multilayer shell of triblock copolymers for enhanced cancer therapy. J Mater Chem B. 2017;5(23):4421–5. [DOI] [PubMed] [Google Scholar]
- 183.Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J, Shao A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geng S, Zhao H, Zhan G, Zhao Y, Yang X. Injectable in situ forming hydrogels of thermosensitive polypyrrole nanoplatforms for precisely synergistic photothermo-chemotherapy. ACS Appl Mater Interfaces. 2020;12(7):7995–8005. [DOI] [PubMed] [Google Scholar]
- 185.Lin L, Pang W, Jiang X, Ding S, Wei X, Gu B. Light amplified oxidative stress in tumor microenvironment by carbonized hemin nanoparticles for boosting photodynamic anticancer therapy. Light Sci Appl. 2022;11(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li K, Lin C, He Y, Lu L, Xu K, Tao B, Xia Z, Zeng R, Mao Y, Luo Z, Cai K. Engineering of cascade-responsive nanoplatform to inhibit lactate efflux for enhanced tumor chemo-immunotherapy. ACS Nano. 2020;14(10):14164–80. [DOI] [PubMed] [Google Scholar]
- 187.Huang H, Yuan G, Xu Y, Gao Y, Mao Q, Zhang Y, Bai L, Li W, Wu A, Hu W, Pan Y, Zhou G. Photoacoustic and magnetic resonance imaging-based gene and photothermal therapy using mesoporous nanoagents. Bioact Mater. 2022;9:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–74. [DOI] [PubMed] [Google Scholar]
- 189.Overchuk M, Weersink RA, Wilson BC, Zheng G. Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano. 2023;17(9):7979–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zuo S, Song J, Zhang J, He Z, Sun B, Sun J. Nano-immunotherapy for each stage of cancer cellular immunity: which, why, and what? Theranostics. 2021;11(15):7471–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Scheller EL, Krebsbach PH. Gene therapy: design and prospects for craniofacial regeneration. J Dent Res. 2009;88(7):585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roma-Rodrigues C, Rivas-Garcia L, Baptista PV, Fernandes AR. Gene therapy in cancer treatment: why go nano? Pharmaceutics. 2020;12(3):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. [DOI] [PubMed] [Google Scholar]
- 194.Sun R, Xiang J, Zhou Q, Piao Y, Tang J, Shao S, Zhou Z, Bae YH, Shen Y. The tumor EPR effect for cancer drug delivery: current status, limitations, and alternatives. Adv Drug Deliv Rev. 2022;191: 114614. [DOI] [PubMed] [Google Scholar]
- 195.Islam R, Maeda H, Fang J. Factors affecting the dynamics and heterogeneity of the EPR effect: pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin Drug Deliv. 2022;19(2):199–212. [DOI] [PubMed] [Google Scholar]
- 196.Collignon P, Beggs JJ. Socioeconomic enablers for contagion: factors impelling the antimicrobial resistance epidemic. Antibiotics. 2019. 10.3390/antibiotics8030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shelar A, Singh AV, Chaure N, Jagtap P, Chaudhari P, Shinde M, Nile SH, Chaskar M, Patil R. Nanoprimers in sustainable seed treatment: molecular insights into abiotic-biotic stress tolerance mechanisms for enhancing germination and improved crop productivity. Sci Total Environ. 2024;951: 175118. [DOI] [PubMed] [Google Scholar]
- 198.Singh AV, Shelar A, Rai M, Laux P, Thakur M, Dosnkyi I, Santomauro G, Singh AK, Luch A, Patil R, Bill J. Harmonization risks and rewards: nano-QSAR for agricultural nanomaterials. J Agric Food Chem. 2024;72(6):2835–52. [DOI] [PubMed] [Google Scholar]
- 199.Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Burgmann H, Sorum H, Norstrom M, Pons MN, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez JL. Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol. 2015;13(5):310–7. [DOI] [PubMed] [Google Scholar]
- 200.Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ebrahimi M, Asadi M, Akhavan O. Graphene-based nanomaterials in fighting the most challenging viruses and immunogenic disorders. ACS Biomater Sci Eng. 2022;8(1):54–81. [DOI] [PubMed] [Google Scholar]
- 202.Xie M, Gao M, Yun Y, Malmsten M, Rotello VM, Zboril R, Akhavan O, Kraskouski A, Amalraj J, Cai X, Lu J, Zheng H, Li R. Antibacterial nanomaterials: mechanisms, impacts on antimicrobial resistance and design principles. Angew Chem Int Ed Engl. 2023;62(17): e202217345. [DOI] [PubMed] [Google Scholar]
- 203.Goyal B, et al. Structural effects of nanoparticles on their antibacterial activity against multi-drug resistance. Inorganic Nano Metal Chem. 2022; 1–13. 10.1080/24701556.2021.2025103.
- 204.Zhao X, Tang H, Jiang X. Deploying gold nanomaterials in combating multi-drug-resistant bacteria. ACS Nano. 2022;16(7):10066–87. [DOI] [PubMed] [Google Scholar]
- 205.Li B, Mao J, Wu J, Mao K, Jia Y, Chen F, Liu J. Nano-bio interactions: biofilm-targeted antibacterial nanomaterials. Small. 2024;20(7): e2306135. [DOI] [PubMed] [Google Scholar]
- 206.Le Ouay B, Stellacci F. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10(3):339–54. [Google Scholar]
- 207.Luan Y, Liu S, Pihl M, van der Mei HC, Liu J, Hizal F, Choi C-H, Chen H, Ren Y, Busscher HJ. Bacterial interactions with nanostructured surfaces. Curr Opin Coll Interface Sci. 2018;38:170–89. [Google Scholar]
- 208.Sun M, Chan KF, Zhang Z, Wang L, Wang Q, Yang S, Chan SM, Chiu PWY, Sung JJY, Zhang L. Magnetic microswarm and fluoroscopy-guided platform for biofilm eradication in biliary stents. Adv Mater. 2022;34(34): e2201888. [DOI] [PubMed] [Google Scholar]
- 209.Shi T, Hou X, Guo S, Zhang L, Wei C, Peng T, Hu X. Nanohole-boosted electron transport between nanomaterials and bacteria as a concept for nano–bio interactions. Nat Commun. 2021;12(1):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu W, Wang R, Vedarethinam V, Huang L, Qian K. Advanced materials for precise detection and antibiotic-free inhibition of bacteria. Mater Today Adv. 2022;13: 100204. [Google Scholar]
- 211.Chen SW, Xie J, Weng SH, Meng WY, Zheng JH, Huang BX, Zhan RM, Zhang WA, Tian J. A supramolecular photosensitizer for combating multiple antibiotic resistance via photodynamic biofilm dispersion. Chem Eng J. 2024;496: 153951. [Google Scholar]
- 212.Zeng Y, Hu X, Cai Z, Qiu D, Ran Y, Ding Y, Shi J, Cai X, Pan Y. Photodynamic and nitric oxide therapy-based synergistic antimicrobial nanoplatform: an advanced root canal irrigation system for endodontic bacterial infections. J Nanobiotechnol. 2024;22(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tse Sum Bui B, Auroy T, Haupt K. Fighting antibiotic-resistant bacteria: promising strategies orchestrated by molecularly imprinted polymers. Angew Chem Int Ed Engl. 2022;61(8):e202106493. [DOI] [PubMed] [Google Scholar]
- 214.Lopez JG, Piletska EV, Whitcombe MJ, Czulak J, Piletsky SA. Application of molecularly imprinted polymer nanoparticles for degradation of the bacterial autoinducer-hexanoyl homoserine lactone. Chem Commun. 2019;55(18):2664–7. [DOI] [PubMed] [Google Scholar]
- 215.Han J, Poma A. Molecular targets for antibody-based anti-biofilm therapy in infective endocarditis. Polymers. 2022. 10.3390/polym14153198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen Y, Gao Y, Huang Y, Jin Q, Ji J. Inhibiting quorum sensing by active targeted ph-sensitive nanoparticles for enhanced antibiotic therapy of biofilm-associated bacterial infections. ACS Nano. 2023;17(11):10019–32. [DOI] [PubMed] [Google Scholar]
- 217.Ndayishimiye J, Kumeria T, Popat A, Falconer JR, Blaskovich MAT. Nanomaterials: the new antimicrobial magic bullet. ACS Infect Dis. 2022;8(4):693–712. [DOI] [PubMed] [Google Scholar]
- 218.Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xu XC, Zhang J, Liu S, Wang CH, Wang HY, Fan HH, Tong YG, Liu HY, Zhou DS. New advances in nanomaterial-based antiviral strategies. Small Struct. 2022;3(7):2200021. [Google Scholar]
- 220.Ye SY, Shao K, Li ZH, Guo N, Zuo YP, Li Q, Lu ZC, Chen L, He QG, Han HY. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015;7(38):21571–9. [DOI] [PubMed] [Google Scholar]
- 221.Tong T, Hu H, Zhou J, Deng S, Zhang X, Tang W, Fang L, Xiao S, Liang J. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16(13): e1906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rafiei S, Rezatofighi SE, Roayaei Ardakani M, Rastegarzadeh S. Gold nanoparticles impair foot-and-mouth disease virus replication. IEEE Trans Nanobiosci. 2016;15(1):34–40. [DOI] [PubMed] [Google Scholar]
- 224.Sankarakumar N, Tong YW. Preventing viral infections with polymeric virus catchers: a novel nanotechnological approach to anti-viral therapy. J Mater Chem B. 2013;1(15):2031–7. [DOI] [PubMed] [Google Scholar]
- 225.Mandal S, Prathipati PK, Belshan M, Destache CJ. A potential long-acting bictegravir loaded nano-drug delivery system for HIV-1 infection: a proof-of-concept study. Antiviral Res. 2019;167:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine. 2014;10(1):177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Donalisio M, Leone F, Civra A, Spagnolo R, Ozer O, Lembo D, Cavalli R. Acyclovir-loaded chitosan nanospheres from nano-emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics. 2018;10(2):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jhawat V, Gulia M, Gupta S, Maddiboyina B, Dutt R. Integration of pharmacogenomics and theranostics with nanotechnology as quality by design (QbD) approach for formulation development of novel dosage forms for effective drug therapy. J Control Releas. 2020;327:500–11. [DOI] [PubMed] [Google Scholar]
- 229.Soltani M, Moradi Kashkooli F, Souri M, Zare Harofte S, Harati T, Khadem A, Haeri Pour M, Raahemifar K. Enhancing clinical translation of cancer using nanoinformatics. Cancers. 2021;13(10):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44(10):1029–38. [DOI] [PubMed] [Google Scholar]
- 231.Murar M, Albertazzi L, Pujals S. Advanced optical imaging-guided nanotheranostics towards personalized cancer drug delivery. Nanomaterials. 2022;12(3):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv Drug Deliv Rev. 2012;64(13):1394–416. [DOI] [PubMed] [Google Scholar]
- 233.Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2(7):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014. 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 235.Shetty Y, Prabhu P, Prabhakar B. Emerging vistas in theranostic medicine. Int J Pharm. 2019;558:29–42. [DOI] [PubMed] [Google Scholar]
- 236.Shende P, Jain S. Polymeric nanodroplets: an emerging trend in gaseous delivery system. J Drug Target. 2019;27(10):1035–45. [DOI] [PubMed] [Google Scholar]
- 237.dos Santos J, de Oliveira RS, de Oliveira TV, Velho MC, Konrad MV, da Silva GS, Deon M, Beck RC. 3D printing and nanotechnology: a multiscale alliance in personalized medicine. Adv Funct Mater. 2021;31(16):2009691. [Google Scholar]
- 238.Agrahari V, Agrahari V, Chou ML, Chew CH, Noll J, Burnouf T. Intelligent micro-/nanorobots as drug and cell carrier devices for biomedical therapeutic advancement: promising development opportunities and translational challenges. Biomaterials. 2020;260: 120163. [DOI] [PubMed] [Google Scholar]
- 239.Sainz V, Conniot J, Matos AI, Peres C, Zupancic E, Moura L, Silva LC, Florindo HF, Gaspar RS. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–10. [DOI] [PubMed] [Google Scholar]
- 240.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–87. [DOI] [PubMed] [Google Scholar]
- 241.Gresham G. ClinicalTrials.gov. In: Piantadosi S, Meinert CL, editors. Principles and practice of clinical trials. Cham: Springer International Publishing; 2020. p. 1–18. [Google Scholar]
- 242.Thapa RK, Kim JO. Nanomedicine-based commercial formulations: current developments and future prospects. J Pharm Investig. 2023;53(1):19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.P. Research, nanomedicine market (by modality: treatments, diagnostics; by application: drug delivery, diagnostic imaging, vaccines, regenerative medicine, implants, others; by nanomolecule type: nanoparticles, nanoshells, nanotubes, nanodevices; by indication: oncological diseases, infectious diseases, cardiovascular diseases, others)—global industry analysis, size, share, growth, trends, regional outlook, and forecast 2023–2032. 2023. https://www.precedenceresearch.com/nanomedicine-market. Accessed 25 Feb 2024.
- 244.M. Intelligence, nanomedicine market size & share analysis—growth trends & forecasts (2024–2029). 2023. https://www.mordorintelligence.com/industry-reports/healthcare-nanotechnology-nanomedicine-market. Accessed 25 Feb 2024.
- 245.Roco MC. National nanotechnology initiative at 20 years: enabling new horizons. J Nanopart Res. 2023;25(10):197. [Google Scholar]
- 246.G.V. Research, nanomedicine market size, share & trends analysis report by application (drug delivery), by indication (clinical oncology, infectious diseases), by molecule type, by region, and segment forecasts, 2023–2030, 2023. https://www.grandviewresearch.com/industry-analysis/nanomedicine-market. Accessed 21 Feb 2024.
- 247.Kumar R, Dkhar DS, Kumari R, Divya S, Mahapatra VK, Dubey P. Chandra. Lipid based nanocarriers: production techniques, concepts, and commercialization aspect. J Drug Deliv Sci Technol. 2022;74:103526. [Google Scholar]
- 248.Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154–155:102–22. [DOI] [PubMed] [Google Scholar]
- 249.Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12(7):3028–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Halwani AA. Development of pharmaceutical nanomedicines: from the bench to the market. Pharmaceutics. 2022;14(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang C, Xie J, Dong X, Mei L, Zhao M, Leng Z, Hu H, Li L, Gu Z, Zhao Y. Clinically approved carbon nanoparticles with oral administration for intestinal radioprotection via protecting the small intestinal crypt stem cells and maintaining the balance of intestinal flora. Small. 2020;16(16): e1906915. [DOI] [PubMed] [Google Scholar]
- 252.Jones AD 3rd, Mi G, Webster TJ. A status report on FDA approval of medical devices containing nanostructured materials. Trend Biotechnol. 2019;37(2):117–20. [DOI] [PubMed] [Google Scholar]
- 253.Darrow JJ, Avorn J, Kesselheim AS. FDA regulation and approval of medical devices: 1976–2020. JAMA. 2021;326(5):420–32. [DOI] [PubMed] [Google Scholar]
- 254.FDA. 510(k) premarket notification database. 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm. Accessed 20 June 2024.
- 255.FDA. Premarket approval (PMA) database. 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm. Accessed 20 June 2024.
- 256.Cornelissen A, Sakamoto A, Sato Y, Kawakami R, Mori M, Kawai K, Kutyna M, Fernandez R, Ghosh S, Barakat M, Virmani R, Finn A. COBRA PzF coronary stent in clinical and preclinical studies: setting the stage for new antithrombotic strategies? Fut Cardiol. 2022;18(3):207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.NMPA. National medical products administration database. 2024. https://www.nmpa.gov.cn/datasearch/home-index.html#category=ylqx. Accessed 20 June 2024.
- 258.Kunrath MF, Diz FM, Magini R, Galarraga-Vinueza ME. Nanointeraction: the profound influence of nanostructured and nano-drug delivery biomedical implant surfaces on cell behavior. Adv Colloid Interface Sci. 2020;284: 102265. [DOI] [PubMed] [Google Scholar]
- 259.Mavioglu I. Internal thoracic artery harvesting with a LigaSure device. Asian Cardiovasc Thorac Ann. 2019;27(8):707–9. [DOI] [PubMed] [Google Scholar]
- 260.Selvakumar J, Radhika B, Shamini S, Mahendra J, Preethi R, Shyma M, Poorani R. Emerging nanotechnologies in dentistry: a review. World J Pharmaceutical Res. 2019;8(13):599–620. [Google Scholar]
- 261.Maak TG, Wylie JD. Medical device regulation: a comparison of the United States and the European Union. J Am Acad Orthop Surg. 2016;24(8):537–43. [DOI] [PubMed] [Google Scholar]
- 262.EUDAMED. European database on medical devices. 2024. https://ec.europa.eu/tools/eudamed/#/screen/home. Accessed 20 June 2024.
- 263.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Amato SF. Regulatory strategies for biomaterials and medical devices in the USA: classification, design, and risk analysis. In: Amato SF, Ezzell RM, editors. Regulatory affairs for biomaterials and medical devices. Cambridge: Woodhead publishing; 2015. p. 27–46. [Google Scholar]
- 265.Aronson JK, Heneghan C, Ferner RE. Medical devices: definition, classification, and regulatory implications. Drug Saf. 2020;43(2):83–93. [DOI] [PubMed] [Google Scholar]
- 266.Neifert N, Martini ML, Yuk F, McNeill IT, Caridi JM, Steinberger J, Oermann EK. Predicting trends in cervical spinal surgery in the United States from 2020 to 2040. World Neurosurg. 2020;141:e175–81. [DOI] [PubMed] [Google Scholar]
- 267.Winkelmann J, Gomez Rossi J, van Ginneken E. Oral health care in Europe: financing, access and provision. Health Syst Transit. 2022;24(2):1–176. [PubMed] [Google Scholar]
- 268.Jiang H, Zhang P, Gu K, Gong Y, Peng P, Shi Y, Ai D, Chen W, Fu C. Cost-effectiveness analysis of a community-based colorectal cancer screening program in Shanghai, China. Front Public Health. 2022;10: 986728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Singh AV, Bhardwaj P, Upadhyay AK, Pagani A, Upadhyay J, Bhadra J, Tisato V, Thakur M, Gemmati D, Mishra R, Zamboni P. Navigating regulatory challenges in molecularly tailored nanomedicine. Explor BioMat-X. 2024;1(2):124–34. [Google Scholar]
- 270.Ma X, Poma A. Clinical translation and envisioned impact of nanotech for infection control: economy, government policy and public awareness. In: Poma A, Rizzello L, editors. Nanotechnology tools for infection control. Amsterdam: Elsevier; 2025. p. 299–392. [Google Scholar]
- 271.Denny KH. Acute, subacute, subchronic, and chronic general toxicity testing for preclinical drug development. In: Faqi AS, editor. A comprehensive guide to toxicology in nonclinical drug development. Amsterdam: Academic Press; 2024. p. 149–71. [Google Scholar]
- 272.Vrbanac J, Slauter R. Overview of ADME science. In: Faqi AS, editor. A comprehensive guide to toxicology in nonclinical drug development. Amsterdam: Academic Press; 2024. p. 49–82. [Google Scholar]
- 273.Hasirci V, Hasirci N. Design and manufacturing requirements for medical devices. In: Hasirci V, Hasirci N, editors. Fundamentals of biomaterials. Cham: Springer International Publishing; 2024. p. 363–88. [Google Scholar]
- 274.Webster TJ. Safety of nanoparticles. Berlin: Springer; 2008. [Google Scholar]
- 275.Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14(1):93–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Moore TJ, Heyward J, Anderson G, Alexander GC. Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015–2017: a cross-sectional study. BMJ Open. 2020;10(6): e038863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8(17):4653–64. [DOI] [PubMed] [Google Scholar]
- 278.Patel DM, Patel NN, Patel JK. Nanomedicine scale-up technologies: feasibilities and challenges. In: Patel JK, Pathak YV, editors. Emerging technologies for nanoparticle manufacturing. Cham: Springer International Publishing; 2021. p. 511–39. [Google Scholar]
- 279.Sharma A, Gamta V, Luthra G. The importance of good manufacturing practices (GMP) in the healthcare industry. J Pharm Res Int. 2023;35(18):75–90. [Google Scholar]
- 280.Lin PC, Lin S, Wang PC, Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32(4):711–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Malik S, Muhammad K, Waheed Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules. 2023. 10.3390/molecules28186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Atalla K, et al. Ethical, privacy, and intellectual property issues in nanomedicine. Wirel Comput Med Nano Cloud Ethical Legal Implic. 2016; 567–600. 10.1002/9781118993620.ch20.
- 283.Ioan BG, Hanganu B. Third-party sharing of genetic information. In: Hostiuc S, editor. Clinical ethics at the crossroads of genetic and reproductive technologies. Amsterdam: Academic Press; 2023. p. 401–29. [Google Scholar]
- 284.Uskokovic V. Nanomedicine for the poor: a lost cause or an idea whose time has yet to come? Nanomedicine. 2021;16(14):1203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Okazaki Y. Asbestos-induced mesothelial injury and carcinogenesis: involvement of iron and reactive oxygen species. Pathol Int. 2022;72(2):83–95. [DOI] [PubMed] [Google Scholar]
- 286.Lin RT, Chien LC, Jimba M, Furuya S, Takahashi K. Implementation of national policies for a total asbestos ban: a global comparison. Lancet Planet Health. 2019;3(8):e341–8. [DOI] [PubMed] [Google Scholar]
- 287.Morawska L, Buonanno G. The physics of particle formation and deposition during breathing. Nat Rev Phys. 2021;3(5):300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schulz H, Harder V, Ibald-Mulli A, Khandoga A, Koenig W, Krombach F, Radykewicz R, Stampfl A, Thorand B, Peters A. Cardiovascular effects of fine and ultrafine particles. J Aerosol Med. 2005;18(1):1–22. [DOI] [PubMed] [Google Scholar]
- 289.Tsuji JS, Maynard AD, Howard PC, James JT, Lam CW, Warheit DB, Santamaria AB. Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicol Sci. 2006;89(1):42–50. [DOI] [PubMed] [Google Scholar]
- 290.Romeo D, Hischier R, Nowack B, Jolliet O, Fantke P, Wick P. In vitro-based human toxicity effect factors: challenges and opportunities for nanomaterial impact assessment. Environ Sci Nano. 2022;9(6):1913–25. [Google Scholar]
- 291.Gambardella C, Pinsino A. Nanomaterial ecotoxicology in the terrestrial and aquatic environment: a systematic review. Toxics. 2022;10(7):393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.John AT, Wadhwa S, Mathur A. Nanotoxicology: exposure, mechanism, and effects on human health. New Front Environ Toxicol. 2022. 10.1007/978-3-030-72173-2_5. [Google Scholar]
- 293.DeLoid GM, Wang Y, Kapronezai K, Lorente LR, Zhang R, Pyrgiotakis G, Konduru NV, Ericsson M, White JC, De La Torre-Roche R, Xiao H, McClements DJ, Demokritou P. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part Fibre Toxicol. 2017;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shi JH, Axson JL, Bergin IL, Ault AP. Nanoparticle digestion simulator reveals pH-dependent aggregation in the gastrointestinal tract. Anal Chem. 2020;92(18):12257–64. [DOI] [PubMed] [Google Scholar]
- 295.Card JW, Zeldin DC, Bonner JC, Nestmann ER. Pulmonary applications and toxicity of engineered nanoparticles. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Vogt A, Rancan F, Ahlberg S, Nazemi B, Choe CS, Darvin ME, Hadam S, Blume-Peytavi U, Loza K, Diendorf J, Epple M, Graf C, Ruhl E, Meinke MC, Lademann J. Interaction of dermatologically relevant nanoparticles with skin cells and skin. Beilstein J Nanotechnol. 2014;5(1):2363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Cao M, Li B, Guo M, Liu Y, Zhang L, Wang Y, Hu B, Li J, Sutherland DS, Wang L, Chen C. In vivo percutaneous permeation of gold nanomaterials in consumer cosmetics: implication in dermal safety assessment of consumer nanoproducts. Nanotoxicology. 2021;15(1):131–44. [DOI] [PubMed] [Google Scholar]
- 298.Zhang Y, Zhang Y, Wu J, Liu J, Kang Y, Hu C, Feng X, Liu W, Luo H, Chen A, Chen L, Shao L. Effects of carbon-based nanomaterials on vascular endothelia under physiological and pathological conditions: interactions, mechanisms and potential therapeutic applications. J Contr Releas. 2021;330:945–62. [DOI] [PubMed] [Google Scholar]
- 299.Feng L, Yang X, Liang S, Xu Q, Miller MR, Duan J, Sun Z. Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway. Part Fibre Toxicol. 2019;16(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Dan M, Tseng MT, Wu P, Unrine JM, Grulke EA, Yokel RA. Brain microvascular endothelial cell association and distribution of a 5 nm ceria engineered nanomaterial. Int J Nanomed. 2012;7:4023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;2(sup1):55–60. [DOI] [PubMed] [Google Scholar]
- 302.Wang HT, Liu S, Song YK, Zhu BW, Tan MQ. Universal existence of fluorescent carbon dots in beer and assessment of their potential toxicity. Nanotoxicology. 2019;13(2):160–73. [DOI] [PubMed] [Google Scholar]
- 303.Lee JH, Kim YS, Song KS, Ryu HR, Sung JH, Park JD, Park HM, Song NW, Shin BS, Marshak D, Ahn K, Lee JE, Yu IJ. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats. Part Fibre Toxicol. 2013;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kielbik P, Kaszewski J, Dabrowski S, Faudnez R, Witkowski BS, Wachnicki L, Zhydachevskyy Y, Sapierzynski R, Gajewski Z, Godlewski M, Godlewski MM. Transfer of orally administered ZnO: Eu nanoparticles through the blood-testis barrier: the effect on kinetic sperm parameters and apoptosis in mice testes. Nanotechnology. 2019;30(45): 455101. [DOI] [PubMed] [Google Scholar]
- 305.Sundarraj K, Manickam V, Raghunath A, Periyasamy M, Viswanathan MP, Perumal E. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environ Toxicol. 2017;32(2):594–608. [DOI] [PubMed] [Google Scholar]
- 306.Zhang W, Yang L, Kuang H, Yang P, Aguilar ZP, Wang A, Fu F, Xu H. Acute toxicity of quantum dots on late pregnancy mice: effects of nanoscale size and surface coating. J Hazard Mater. 2016;318:61–9. [DOI] [PubMed] [Google Scholar]
- 307.N’Dea S, Nelson KM, Dang MN, Gleghorn JP, Day ES. Gold nanoparticle biodistribution in pregnant mice following intravenous administration varies with gestational age, nanomedicine: nanotechnology. Biol Med. 2021;36: 102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Farkas A, Balashazy I, Szocs K. Characterization of regional and local deposition of inhaled aerosol drugs in the respiratory system by computational fluid and particle dynamics methods. J Aerosol Med. 2006;19(3):329–43. [DOI] [PubMed] [Google Scholar]
- 309.Zhang M, Xu C, Jiang L, Qin J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol Res. 2018;7(6):1048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lu X, Zhu T, Chen C, Liu Y. Right or left: the role of nanoparticles in pulmonary diseases. Int J Mol Sci. 2014;15(10):17577–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhou X, Jin W, Ma J. Lung inflammation perturbation by engineered nanoparticles. Front Bioeng Biotechnol. 2023;11:1199230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Heringa MB, Peters RJB, Bleys R, van der Lee MK, Tromp PC, van Kesteren PCE, van Eijkeren JCH, Undas AK, Oomen AG, Bouwmeester H. Detection of titanium particles in human liver and spleen and possible health implications. Part Fibre Toxicol. 2018;15(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Li Y, Xu M, Zhang Z, Halimu G, Li Y, Li Y, Gu W, Zhang B, Wang X. In vitro study on the toxicity of nanoplastics with different charges to murine splenic lymphocytes. J Hazard Mater. 2022;424(Pt B): 127508. [DOI] [PubMed] [Google Scholar]
- 314.Li H, Huang T, Wang Y, Pan B, Zhang L, Zhang Q, Niu Q. Toxicity of alumina nanoparticles in the immune system of mice. Nanomedicine. 2020;15(9):927–46. [DOI] [PubMed] [Google Scholar]
- 315.Nabeh M, Taalab Y, Abd El Wahab D, Asker S, Elbedwehy A, El Harouny M. Silver nanotoxicity on kidneys and ovaries of female albino rats. Mansoura J Forensic Med Clin Toxicol. 2020;28(2):1–14. [Google Scholar]
- 316.Ryabova YV, Minigalieva IA, Sutunkova MP, Klinova SV, Tsaplina AK, Valamina IE, Petrunina EM, Tsatsakis AM, Mamoulakis C, Stylianou K, Kuzmin SV, Privalova LI, Katsnelson BA. Toxic kidney damage in rats following subchronic intraperitoneal exposure to element oxide nanoparticles. Toxics. 2023;11(9):791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yousef MI, Roychoudhury S, Jafaar KS, Slama P, Kesari KK, Kamel MA. Aluminum oxide and zinc oxide induced nanotoxicity in rat brain, heart, and lung. Physiol Res. 2022;71(5):677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cheng Y, Chen Z, Yang S, Liu T, Yin L, Pu Y, Liang G. Nanomaterials-induced toxicity on cardiac myocytes and tissues, and emerging toxicity assessment techniques. Sci Total Environ. 2021;800: 149584. [DOI] [PubMed] [Google Scholar]
- 319.Babiker F, Benter IF, Akhtar S. Nanotoxicology of dendrimers in the mammalian heart: ex vivo and in vivo administration of G6 PAMAM nanoparticles impairs recovery of cardiac function following ischemia-reperfusion injury. Int J Nanomed. 2020;15:4393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hussain Z, Thu HE, Elsayed I, Abourehab MAS, Khan S, Sohail M, Sarfraz RM, Farooq MA. Nano-scaled materials may induce severe neurotoxicity upon chronic exposure to brain tissues: a critical appraisal and recent updates on predisposing factors, underlying mechanism, and future prospects. J Contr Releas. 2020;328:873–94. [DOI] [PubMed] [Google Scholar]
- 321.Boyes WK, van Thriel C. Neurotoxicology of nanomaterials. Chem Res Toxicol. 2020;33(5):1121–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Bailey MR, Ansoborlo E, Guilmette RA, Paquet F. Updating the ICRP human respiratory tract model. Radiat Prot Dosim. 2007;127(1–4):31–4. [DOI] [PubMed] [Google Scholar]
- 323.Furuyama A, Kanno S, Kobayashi T, Hirano S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch Toxicol. 2009;83(5):429–37. [DOI] [PubMed] [Google Scholar]
- 324.Waheed S, Li Z, Zhang F, Chiarini A, Armato U, Wu J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J Nanobiotechnol. 2022;20(1):395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Wu J, Zhu Z, Liu W, Zhang Y, Kang Y, Liu J, Hu C, Wang R, Zhang M, Chen L, Shao L. How nanoparticles open the paracellular route of biological barriers: mechanisms. Appl Prospect ACS Nano. 2022;16(10):15627–52. [DOI] [PubMed] [Google Scholar]
- 326.Jia J, Wang Z, Yue T, Su G, Teng C, Yan B. Crossing biological barriers by engineered nanoparticles. Chem Res Toxicol. 2020;33(5):1055–60. [DOI] [PubMed] [Google Scholar]
- 327.Figueroa-Espada CG, Hofbauer S, Mitchell MJ, Riley RS. Exploiting the placenta for nanoparticle-mediated drug delivery during pregnancy. Adv Drug Deliv Rev. 2020;160:244–61. [DOI] [PubMed] [Google Scholar]
- 328.Egorova KS, Ananikov VP. Toxicity of metal compounds: knowledge and myths. Organometallics. 2017;36(21):4071–90. [Google Scholar]
- 329.Zhang A, Meng K, Liu Y, Pan Y, Qu W, Chen D, Xie S. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv Colloid Interface Sci. 2020;284: 102261. [DOI] [PubMed] [Google Scholar]
- 330.Rocker C, Potzl M, Zhang F, Parak WJ, Nienhaus GU. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat Nanotechnol. 2009;4(9):577–80. [DOI] [PubMed] [Google Scholar]
- 331.Walczak AP, Hendriksen PJ, Woutersen RA, van der Zande M, Undas AK, Helsdingen R, van den Berg HH, Rietjens IM, Bouwmeester H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J Nanopart Res. 2015;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Karmali PP, Simberg D. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin Drug Deliv. 2011;8(3):343–57. [DOI] [PubMed] [Google Scholar]
- 333.Yacobi NR, Malmstadt N, Fazlollahi F, DeMaio L, Marchelletta R, Hamm-Alvarez SF, Borok Z, Kim KJ, Crandall ED. Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. Am J Respir Cell Mol Biol. 2010;42(5):604–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Singh AV, Bansod G, Mahajan M, Dietrich P, Singh SP, Rav K, Thissen A, Bharde AM, Rothenstein D, Kulkarni S, Bill J. Digital transformation in toxicology: improving communication and efficiency in risk assessment. ACS Omega. 2023;8(24):21377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–50. [DOI] [PubMed] [Google Scholar]
- 336.Kumar V, Dasgupta N, Ranjan S. Nanotoxicology: toxicity evaluation, risk assessment and management. Boca Raton: CRC Press; 2018. [Google Scholar]
- 337.Otani T, Furuse M. Tight junction structure and function revisited. Trend Cell Biol. 2020;30(10):805–17. [DOI] [PubMed] [Google Scholar]
- 338.Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1(2): a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Compr Physiol. 2012;2(3):1981–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121(Pt 3):298–305. [DOI] [PubMed] [Google Scholar]
- 341.Lee M, Ni N, Tang H, Li Y, Wei W, Kakinen A, Wan X, Davis TP, Song Y, Leong DT, Ding F, Ke PC. A framework of paracellular transport via nanoparticles-induced endothelial leakiness. Adv Sci. 2021;8(21):e2102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rosas-Hernandez H, Escudero-Lourdes C, Ramirez-Lee MA, Cuevas E, Lantz SM, Imam SZ, Majeed W, Bourdo SE, Paule MG, Biris AS, Ali SF. Cytotoxicity profile of pristine graphene on brain microvascular endothelial cells. J Appl Toxicol. 2019;39(7):966–73. [DOI] [PubMed] [Google Scholar]
- 343.Skalska J, Dabrowska-Bouta B, Frontczak-Baniewicz M, Sulkowski G, Struzynska L. A low dose of nanoparticulate silver induces mitochondrial dysfunction and autophagy in adult rat brain. Neurotox Res. 2020;38(3):650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lynch I, Dawson KA. Protein–nanoparticle interactions. Nano Enabled Med Appl. 2020; 231–250.
- 345.Demir E. A review on nanotoxicity and nanogenotoxicity of different shapes of nanomaterials. J Appl Toxicol. 2021;41(1):118–47. [DOI] [PubMed] [Google Scholar]
- 346.Yuan H, Li J, Bao G, Zhang S. Variable nanoparticle-cell adhesion strength regulates cellular uptake. Phys Rev Lett. 2010;105(13): 138101. [DOI] [PubMed] [Google Scholar]
- 347.Akcan R, Aydogan HC, Yildirim MS, Tastekin B, Saglam N. Nanotoxicity: a challenge for future medicine. Turk J Med Sci. 2020;50(4):1180–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Williams Y, Sukhanova A, Nowostawska M, Davies AM, Mitchell S, Oleinikov V, Gun’ko Y, Nabiev I, Kelleher D, Volkov Y. Probing cell-type-specific intracellular nanoscale barriers using size-tuned quantum dots. Small. 2009;5(22):2581–8. [DOI] [PubMed] [Google Scholar]
- 349.Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc Natl Acad Sci U S A. 2013;110(30):12295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G, Wang T, Yuan H, Ye C, Zhao F, Chai Z, Zhu C, Fang X, Ma B, Wan L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163(2):109–20. [DOI] [PubMed] [Google Scholar]
- 351.Meng H, Chen Z, Xing G, Yuan H, Chen C, Zhao F, Zhang C, Zhao Y. Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano-copper particles. Toxicol Lett. 2007;175(1–3):102–10. [DOI] [PubMed] [Google Scholar]
- 352.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7. [DOI] [PubMed] [Google Scholar]
- 353.Unfried K, Albrecht C, Klotz LO, Von Mikecz A, Grether-Beck S, Schins RPF. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1(1):52–71. [Google Scholar]
- 354.Moller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44(1):1–46. [DOI] [PubMed] [Google Scholar]
- 355.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. [DOI] [PubMed] [Google Scholar]
- 356.Meng H, Xia T, George S, Nel AE. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano. 2009;3(7):1620–7. [DOI] [PubMed] [Google Scholar]
- 357.Kohl Y, Runden-Pran E, Mariussen E, Hesler M, El Yamani N, Longhin EM, Dusinska M. Genotoxicity of nanomaterials: advanced in vitro models and high throughput methods for human hazard assessment-a review. Nanomaterials. 2020;10(10):1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M, Ahmad I. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276(2):95–102. [DOI] [PubMed] [Google Scholar]
- 359.Akhtar MJ, Ahamed M, Fareed M, Alrokayan SA, Kumar S. Protective effect of sulphoraphane against oxidative stress mediated toxicity induced by CuO nanoparticles in mouse embryonic fibroblasts BALB 3T3. J Toxicol Sci. 2012;37(1):139–48. [DOI] [PubMed] [Google Scholar]
- 360.Auffan M, Rose J, Wiesner MR, Bottero JY. Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut. 2009;157(4):1127–33. [DOI] [PubMed] [Google Scholar]
- 361.Yoshida T, Yoshikawa T, Nabeshi H, Tsutsumi Y. Relation analysis between intracellular distribution of nanomateriarls, ROS generation and DNA damage. Yakugaku Zasshi. 2012;132(3):295–300. [DOI] [PubMed] [Google Scholar]
- 362.Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22(1):64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Fan Z, Lu JG. Zinc oxide nanostructures: synthesis and properties. J Nanosci Nanotechnol. 2005;5(10):1561–73. [DOI] [PubMed] [Google Scholar]
- 364.Ma HJ, Zhao JJ, Meng HB, Hu DM, Zhou Y, Zhang XY, Wang CR, Li J, Yuan JY, Wei Y. Carnosine-modified fullerene as a highly enhanced ROS scavenger for mitigating acute oxidative stress. ACS Appl Mater Interfaces. 2020;12(14):16104–13. [DOI] [PubMed] [Google Scholar]
- 365.Tsaioun K, Blaauboer BJ, Hartung T. Evidence-based absorption, distribution, metabolism, excretion (ADME) and its interplay with alternative toxicity methods. Altex. 2016;33(4):343–58. [DOI] [PubMed] [Google Scholar]
- 366.Bourquin J, Milosevic A, Hauser D, Lehner R, Blank F, Petri-Fink A, Rothen-Rutishauser B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv Mater. 2018;30(19): e1704307. [DOI] [PubMed] [Google Scholar]
- 367.Sharma R, Sharma PK, Malviya R. Modulation of shape and size-dependent characteristics of nanoparticles. Curr Nanomed Former Recent Pat Nanomed. 2019;9(3):210–5. [Google Scholar]
- 368.Sung JH, Ji JH, Song KS, Lee JH, Choi KH, Lee SH, Yu IJ. Acute inhalation toxicity of silver nanoparticles. Toxicol Ind Health. 2011;27(2):149–54. [DOI] [PubMed] [Google Scholar]
- 369.Da Silva RGT. The effect of silver nanoparticles: a chronic in vivo study for the evaluation of hepatic mitochondrial toxicity. Coimbra: Universidade de Coimbra; 2014. [Google Scholar]
- 370.Assad N, Sood A, Campen MJ, Zychowski KE. Metal-induced pulmonary fibrosis. Curr Environ Health Rep. 2018;5(4):486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Srinivasa DR, Miranda RN, Kaura A, Francis AM, Campanale A, Boldrini R, Alexander J, Deva AK, Gravina PR, Medeiros LJ, Nast K, Butler CE, Clemens MW. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017;139(5):1029–39. [DOI] [PubMed] [Google Scholar]
- 372.Whaley RD, Aldrees R, Dougherty RE, Prieto Granada C, Badve SS, Al Diffalha S. Breast implant capsule-associated squamous cell carcinoma: report of 2 patients. Int J Surg Pathol. 2022;30(8):900–7. [DOI] [PubMed] [Google Scholar]
- 373.Schoberleitner I, Faserl K, Tripp CH, Pechriggl EJ, Sigl S, Brunner A, Zelger B, Hermann-Kleiter N, Baier L, Steinkellner T, Sarg B, Egle D, Brunner C, Wolfram D. Silicone implant surface microtopography modulates inflammation and tissue repair in capsular fibrosis. Front Immunol. 2024;15:1342895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Chen L, Wang D, Peng F, Qiu J, Ouyang L, Qiao Y, Liu X. Nanostructural surfaces with different elastic moduli regulate the immune response by stretching macrophages. Nano Lett. 2019;19(6):3480–3489. 10.1021/acs.nanolett.9b00237. [DOI] [PubMed]
- 375.Noris M, Remuzzi G. Overview of complement activation and regulation, seminars in nephrology. Amsterdam: Elsevier; 2013. p. 479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Thangaraju P, Varthya SB. ISO 10993: biological evaluation of medical devices, medical device guidelines and regulations handbook. Berlin: Springer; 2022. p. 163–87. [Google Scholar]
- 377.Evans SJ, Clift MJ, Singh N, de Oliveira Mallia J, Burgum M, Wills JW, Wilkinson TS, Jenkins GJ, Doak SH. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis. 2017;32(1):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Pfuhler S, Downs TR, Allemang AJ, Shan Y, Crosby ME. Weak silica nanomaterial-induced genotoxicity can be explained by indirect DNA damage as shown by the OGG1-modified comet assay and genomic analysis. Mutagenesis. 2017;32(1):5–12. [DOI] [PubMed] [Google Scholar]
- 379.Doak SH, Dusinska M. Nano genotoxicology: present and the future. Mutagenesis. 2017;32(1):1–4. [DOI] [PubMed] [Google Scholar]
- 380.Kazimirova A, El Yamani N, Rubio L, Garcia-Rodriguez A, Barancokova M, Marcos R, Dusinska M. Effects of titanium dioxide nanoparticles on the Hprt gene mutations in V79 hamster cells. Nanomaterials. 2020;10(3):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Bartek J, Mistrik M, Bartkova J. Long-distance inflammatory and genotoxic impact of cancer in vivo. Proc Natl Acad Sci U S A. 2010;107(42):17861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Uygur B, Craig G, Mason MD, Ng AK. Cytotoxicity and genotoxicity of silver nanomaterials. Nanotech Confer Expo Technical Proceed. 2009;2:383. [Google Scholar]
- 383.Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–50. [DOI] [PubMed] [Google Scholar]
- 384.Singh S, D’Britto V, Prabhune AA, Ramana CV, Dhawan A, Prasad BLV. Cytotoxic and genotoxic assessment of glycolipid-reduced and -capped gold and silver nanoparticles. New J Chem. 2010;34(2):294–301. [Google Scholar]
- 385.Kim YJ, Yang SI, Ryu JC. Cytotoxicity and genotoxicity of nano-silver in mammalian cell lines. Mol Cell Toxicol. 2010;6(2):119–25. [Google Scholar]
- 386.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–90. [DOI] [PubMed] [Google Scholar]
- 387.Rossi EM, Pylkkanen L, Koivisto AJ, Vippola M, Jensen KA, Miettinen M, Sirola K, Nykasenoja H, Karisola P, Stjernvall T, Vanhala E, Kiilunen M, Pasanen P, Makinen M, Hameri K, Joutsensaari J, Tuomi T, Jokiniemi J, Wolff H, Savolainen K, Matikainen S, Alenius H. Airway exposure to silica-coated TiO2 nanoparticles induces pulmonary neutrophilia in mice. Toxicol Sci. 2010;113(2):422–33. [DOI] [PubMed] [Google Scholar]
- 388.Tuama AN, Alzubaidi LH, Jameel MH, Abass KH, Bin Mayzan MZH, Salman ZN. Impact of electron-hole recombination mechanism on the photocatalytic performance of ZnO in water treatment: a review. J Sol Gel Sci Technol. 2024;110(3):792–806. [Google Scholar]
- 389.Pesci FM, Wang G, Klug DR, Li Y, Cowan AJ. Efficient suppression of electron-hole recombination in oxygen-deficient hydrogen-treated TiO(2) nanowires for photoelectrochemical water splitting. J Phys Chem C Nanomater Interfaces. 2013;117(48):25837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Andrews JPM, Joshi SS, Tzolos E, Syed MB, Cuthbert H, Crica LE, Lozano N, Okwelogu E, Raftis JB, Bruce L, Poland CA, Duffin R, Fokkens PHB, Boere AJF, Leseman D, Megson IL, Whitfield PD, Ziegler K, Tammireddy S, Hadjidemetriou M, Bussy C, Cassee FR, Newby DE, Kostarelos K, Miller MR. First-in-human controlled inhalation of thin graphene oxide nanosheets to study acute cardiorespiratory responses. Nat Nanotechnol. 2024;19(5):705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Firouzamandi M, Hejazy M, Mohammadi A, Shahbazfar AA, Norouzi R. In vivo toxicity of oral administrated nano-SiO2: can food additives increase apoptosis? Biol Trace Elem Res. 2023;201(10):4769–78. [DOI] [PubMed] [Google Scholar]
- 392.Zahmatkesh S, Hajiaghaei-Keshteli M, Bokhari A, Sundaramurthy S, Panneerselvam B, Rezakhani Y. Wastewater treatment with nanomaterials for the future: a state-of-the-art review. Environ Res. 2023;216(Pt 3): 114652. [DOI] [PubMed] [Google Scholar]
- 393.Younis SA, Maitlo HA, Lee J, Kim KH. Nanotechnology-based sorption and membrane technologies for the treatment of petroleum-based pollutants in natural ecosystems and wastewater streams. Adv Coll Interface Sci. 2020;275: 102071. [DOI] [PubMed] [Google Scholar]
- 394.Yadav N, Garg VK, Chhillar AK, Rana JS. Detection and remediation of pollutants to maintain ecosustainability employing nanotechnology: a review. Chemosphere. 2021;280: 130792. [DOI] [PubMed] [Google Scholar]
- 395.Bobori D, Dimitriadi A, Karasiali S, Tsoumaki-Tsouroufli P, Mastora M, Kastrinaki G, Feidantsis K, Printzi A, Koumoundouros G, Kaloyianni M. Common mechanisms activated in the tissues of aquatic and terrestrial animal models after TiO(2) nanoparticles exposure. Environ Int. 2020;138: 105611. [DOI] [PubMed] [Google Scholar]
- 396.Park HG, Yeo MK. Comparison of gene expression changes induced by exposure to Ag, Cu-TiO2, and TiO2 nanoparticles in zebrafish embryos. Mol Cell Toxicol. 2013;9(2):129–39. [Google Scholar]
- 397.Handy RD, Owen R, Valsami-Jones E. The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology. 2008;17(5):315–25. [DOI] [PubMed] [Google Scholar]
- 398.Mitrano DM, Barber A, Bednar A, Westerhoff P, Higgins CP, Ranville JF. Silver nanoparticle characterization using single particle ICP-MS (SP-ICP-MS) and asymmetrical flow field flow fractionation ICP-MS (AF4-ICP-MS). J Anal At Spectrom. 2012;27(7):1131–42. [Google Scholar]
- 399.Poda AR, Bednar AJ, Kennedy AJ, Harmon A, Hull M, Mitrano DM, Ranville JF, Steevens J. Characterization of silver nanoparticles using flow-field flow fractionation interfaced to inductively coupled plasma mass spectrometry. J Chromatogr A. 2011;1218(27):4219–25. [DOI] [PubMed] [Google Scholar]
- 400.Spurgeon DJ, Lahive E, Schultz CL. Nanomaterial transformations in the environment: effects of changing exposure forms on bioaccumulation and toxicity. Small. 2020;16(36): e2000618. [DOI] [PubMed] [Google Scholar]
- 401.Adam V, Caballero-Guzman A, Nowack B. Considering the forms of released engineered nanomaterials in probabilistic material flow analysis. Environ Poll. 2018;243(Pt A):17–27. [DOI] [PubMed] [Google Scholar]
- 402.Aleksandra Z, Beatriz C, Ferreira MV, Diogo M, Louros J, Alessandra D, Massimo L, Piotr E, Chaud MV, Margreet M. Nanotoxicology and nanosafety: safety-by-design and testing at a glance. Int J Environ Res Public Health. 2020;17:4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Zhao J, Lin M, Wang Z, Cao X, Xing B. Engineered nanomaterials in the environment: are they safe? Crit Rev Environ Sci Technol. 2020;51(14):1443–78. [Google Scholar]
- 404.Liu J, Pennell KG, Hurt RH. Kinetics and mechanisms of nanosilver oxysulfidation. Environ Sci Technol. 2011;45(17):7345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Levard C, Hotze EM, Colman BP, Dale AL, Truong L, Yang XY, Bone AJ, Brown GE Jr, Tanguay RL, Di Giulio RT, Bernhardt ES, Meyer JN, Wiesner MR, Lowry GV. Sulfidation of silver nanoparticles: natural antidote to their toxicity. Environ Sci Technol. 2013;47(23):13440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wang Y, Dimkpa C, Deng C, Elmer WH, Gardea-Torresdey J, White JC. Impact of engineered nanomaterials on rice (Oryza sativa L.): a critical review of current knowledge. Environ Pollut. 2022;297:118738. [DOI] [PubMed] [Google Scholar]
- 407.Frazier EA, Patil RP, Mane CB, Sanaei D, Asiri F, Seo SS, Sharifan H. Environmental exposure and nanotoxicity of titanium dioxide nanoparticles in irrigation water with the flavonoid luteolin. RSC Adv. 2023;13(21):14110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Tufenkji N, Elimelech M. Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ Sci Technol. 2004;38(2):529–36. [DOI] [PubMed] [Google Scholar]
- 409.Praetorius A. Development of environmental fate models for engineered nanoparticles. ETH Zurich. 2014. [DOI] [PubMed]
- 410.Mech A, Rauscher H, Babick F, Hodoroaba VD, Wohlleben W, Marvin H, Weigel S, Brüngel R, Friedrich CM. The Nanodefine methods manual-part 1: the nanodefiner framework and tools. Luxembourg: Publications Office of the European Union; 2020. [Google Scholar]
- 411.Mech A, Rauscher H, Rasmussen K, Babick F, Hodoroaba V-D, Ghanem A, Wohlleben W, Marvin H, Brüngel R, Friedrich CM. The nanodefine methods manual-part 2: evaluation of methods. Luxembourg: Publications Office of the European Union; 2020. [Google Scholar]
- 412.Mech A, Babick F, Hodoroaba VD, Ghane AM, Wohlleben W, Marvin H, Löschner K, Brüngel R, Friedrich CM. The nanodefine methods manual. Part 3: standard operating procedures (SOPs): JRC117501. Luxembourg: European Union; 2020. [Google Scholar]
- 413.Liu Y, Zhu S, Gu Z, Chen C, Zhao Y. Toxicity of manufactured nanomaterials. Particuology. 2022;69:31–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.