Abstract
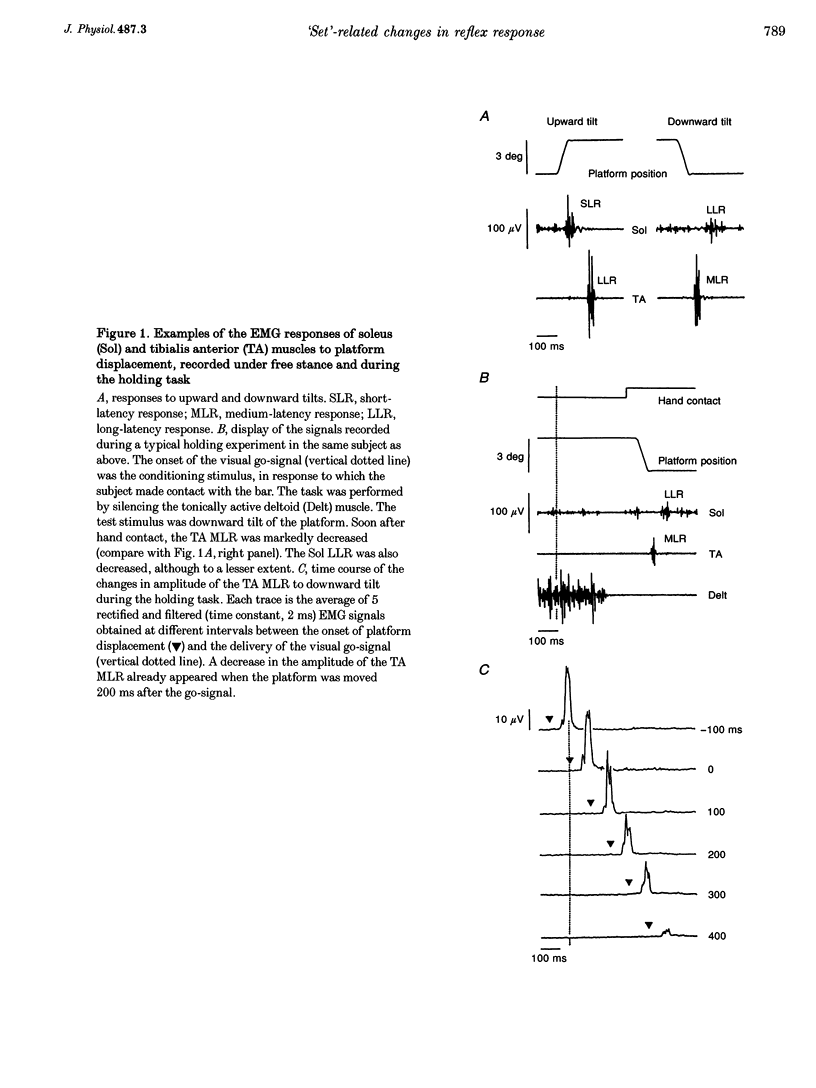
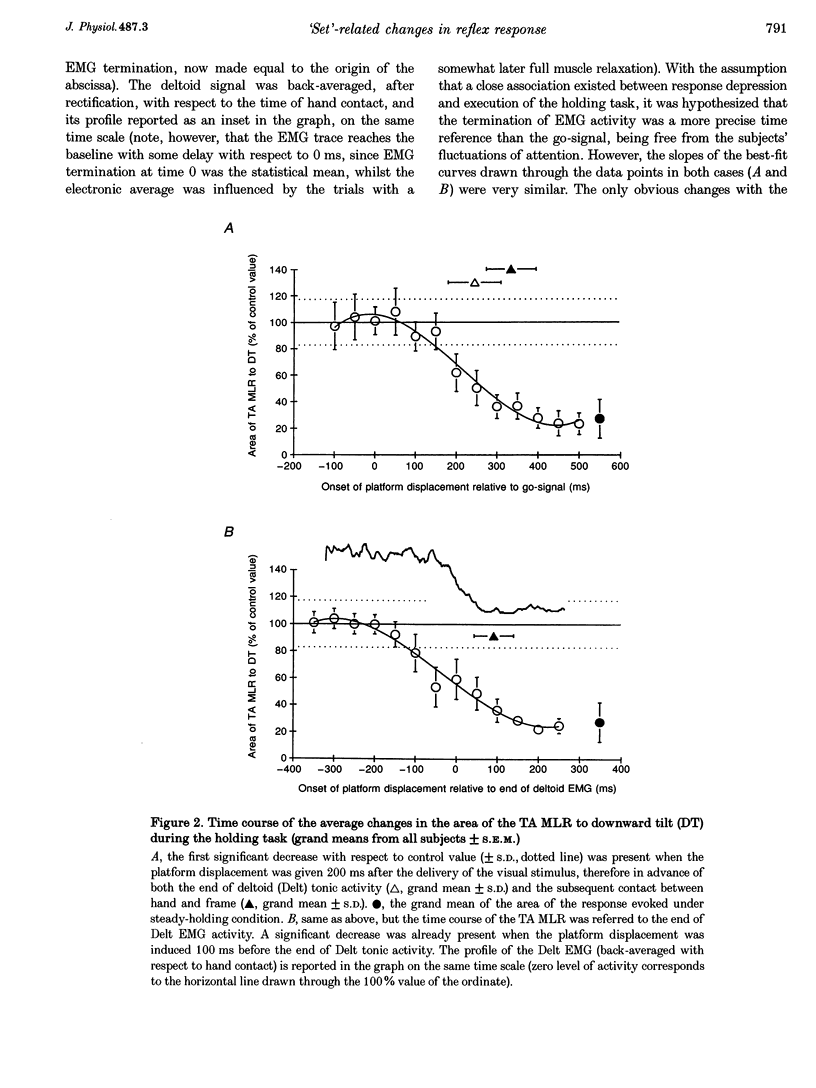
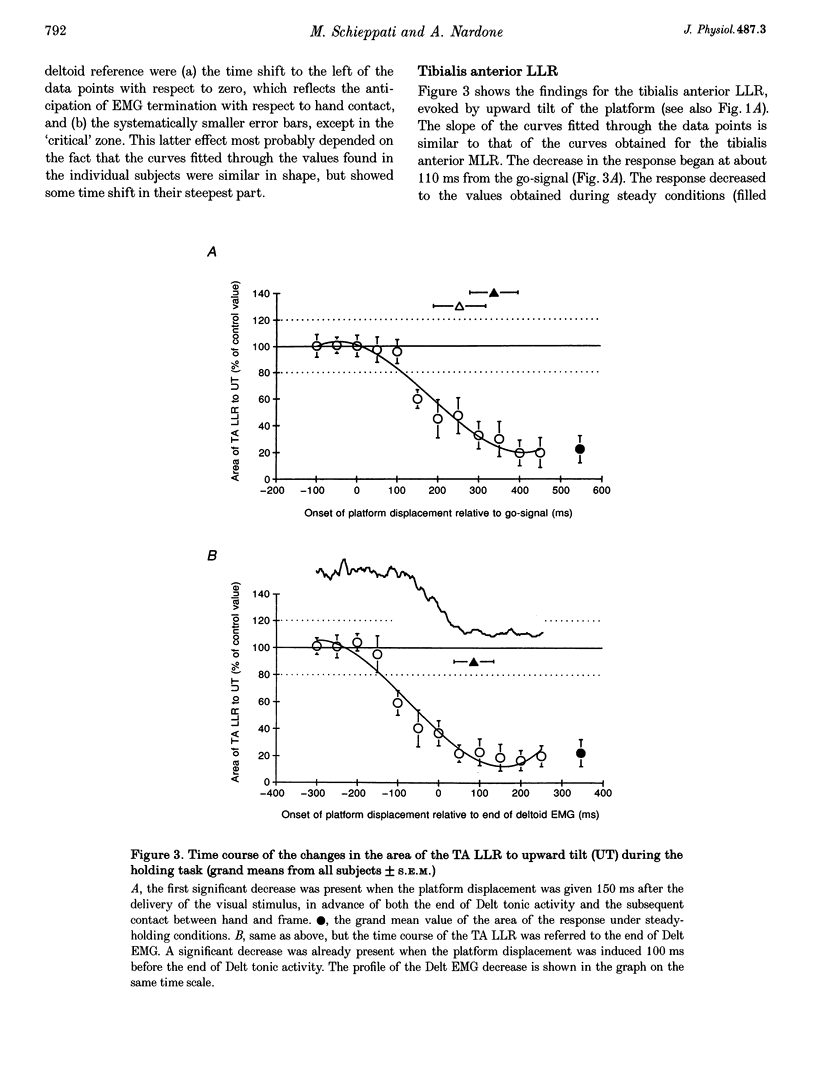
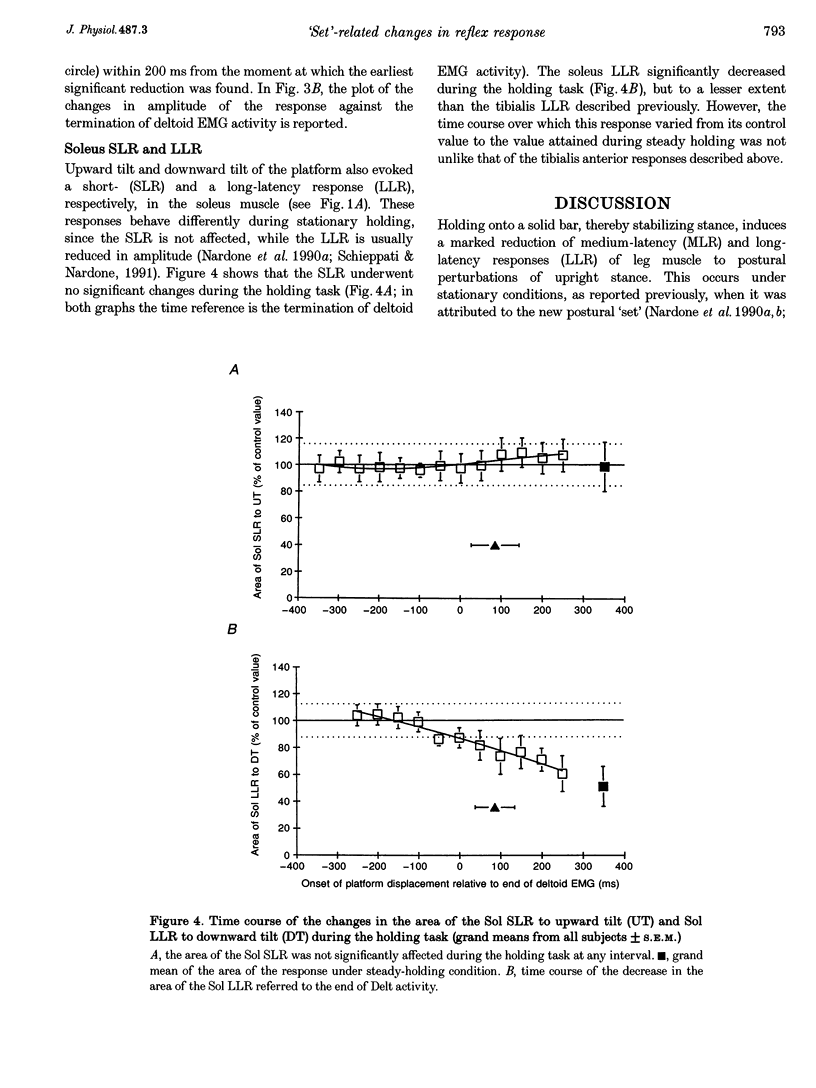
1. In standing subjects, toe-down rotation of a supporting platform elicits a medium-latency response (MLR) in tibialis anterior (TA) muscle and a long-latency response (LLR) in soleus (Sol). Toe-up rotation induces a short-latency response (SLR) in Sol and a LLR in TA. When subjects steadily hold onto a stable frame, all responses are decreased, except Sol SLR. The aim of this investigation was to assess whether the response modulation is dependent on information from the hand touching the frame, or whether it anticipates the holding task. 2. The time course of the changes in response amplitude was studied in a time interval centred around the act of holding, performed in a reaction-time mode. Subjects kept their extended arm close to the frame in front of them and brought the hand in contact with the frame in response to a visual go-signal. The platform was moved at different intervals prior to or after the go-signal. Surface EMGs of Sol, TA and deltoid (Delt) were recorded. 3. TA MLR began to decrease when the platform was displaced at an interval of 140 ms after the go-signal, about 200 ms before subjects touched the frame and 120 ms before termination of Delt EMG. Four hundred milliseconds after the go-signal the response reached and maintained maximal inhibition, similar to that occurring under the stationary holding condition. The time course of inhibition of Sol LLR and TA LLR was similar to that of TA MLR, except that LLRs began to decrease at an earlier interval. Due to the different response latency from the onset of the perturbations, the beginning of inhibition of both MLRs and LLRs occurred almost simultaneously. 4. The changes in amplitude of leg muscle responses are not triggered by the go-signal, contact with the frame, or arm motion, suggesting that the modulation is related to the transition to a new, stabilized postural 'set'. The similar extent and parallel time course of MLR and LLR suppression, possibly transmitted through different pathways, points to the spinal cord as the site of action. The lack of depression of the monosynaptic SLR suggests an effect at premotoneuronal level. On the basis of selectivity, latency and time course of the effect, we favour the hypothesis that a monoaminergic pathway from the brainstem is involved.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bras H., Cavallari P., Jankowska E., McCrea D. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Exp Brain Res. 1989;76(1):27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Capaday C., Stein R. B. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986 May;6(5):1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corna S., Grasso M., Nardone A., Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an alpha 2-agonist in humans. J Physiol. 1995 May 1;484(Pt 3):803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiol Rev. 1992 Jan;72(1):33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Afferent control of human stance and gait: evidence for blocking of group I afferents during gait. Exp Brain Res. 1985;61(1):153–163. doi: 10.1007/BF00235630. [DOI] [PubMed] [Google Scholar]
- Fellows S. J., Dömges F., Töpper R., Thilmann A. F., Noth J. Changes in the short- and long-latency stretch reflex components of the triceps surae muscle during ischaemia in man. J Physiol. 1993 Dec;472:737–748. doi: 10.1113/jphysiol.1993.sp019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J., Hömberg V. EMG responses in leg muscles to postural perturbations in Huntington's disease. J Neurol Neurosurg Psychiatry. 1990 Jan;53(1):55–62. doi: 10.1136/jnnp.53.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Nardone A., Corrà T., Schieppati M. Different activations of the soleus and gastrocnemii muscles in response to various types of stance perturbation in man. Exp Brain Res. 1990;80(2):323–332. doi: 10.1007/BF00228159. [DOI] [PubMed] [Google Scholar]
- Nardone A., Giordano A., Corrà T., Schieppati M. Responses of leg muscles in humans displaced while standing. Effects of types of perturbation and of postural set. Brain. 1990 Feb;113(Pt 1):65–84. doi: 10.1093/brain/113.1.65. [DOI] [PubMed] [Google Scholar]
- Nardone A., Schieppati M. Postural adjustments associated with voluntary contraction of leg muscles in standing man. Exp Brain Res. 1988;69(3):469–480. doi: 10.1007/BF00247301. [DOI] [PubMed] [Google Scholar]
- Noga B. R., Bras H., Jankowska E. Transmission from group II muscle afferents is depressed by stimulation of locus coeruleus/subcoeruleus, Kölliker-Fuse and raphe nuclei in the cat. Exp Brain Res. 1992;88(3):502–516. doi: 10.1007/BF00228180. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33(4):281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Schieppati M., Nardone A. Free and supported stance in Parkinson's disease. The effect of posture and 'postural set' on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain. 1991 Jun;114(Pt 3):1227–1244. doi: 10.1093/brain/114.3.1227. [DOI] [PubMed] [Google Scholar]
- Schieppati M., Nardone A., Musazzi M. Modulation of the Hoffmann reflex by rapid muscle contraction or release. Hum Neurobiol. 1986;5(1):59–66. [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28(4):345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Ugawa Y., Genba K., Shimpo T., Mannen T. Physiologic analysis of central motor pathways--simultaneous recording from multiple relaxed muscles. Eur Neurol. 1989;29(3):135–140. doi: 10.1159/000116396. [DOI] [PubMed] [Google Scholar]


