Abstract
Background
This study aimed to evaluate the prognostic value of the newly revised International Association for the Study of Lung Cancer (IASLC) grading system (2020) on the 5-year overall survival (OS) and recurrence-free survival (RFS) in patients with lung adenocarcinoma (LADC).
Methods
Clinical studies that investigated the prognostic value of revised IASLC staging system in patients with LADC were retrieved from the PubMed, Web of Science, ScienceDirect, and Cochrane Library databases. This study was conducted in accordance to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklists.
Results
Based on inclusion and exclusion criteria, we included 12 studies for analysis. The grade of LADC was assessed by revised IASLC system, which included three grades. Compared to Grade 3 LADC, grade 1 (total [95% CI]: 1.38 [1.19, 1.60]) and grade 2 (total [95% CI]: 1.29 [1.15, 1.44]) LADC had higher 5-year OS rates. Similarly, Grade 1 (total [95% CI]: 1.76 [1.42, 2.18]) and Grade 2 (total [95% CI]: 1.51 [1.28, 1.77]) had higher 5-year RFS rates Grade 3 LADC. However, 5-year OS and RFS had no significant difference between Grade 1 and Grade 2 patients.
Conclusion
This systematic review and meta-analysis provides evidence that the newly revised IASLC grading system is significantly associated with the prognosis of patients with LADC, where Grade 3 indicated unfavorable prognosis.
Keywords: Lung adenocarcinoma, IASLC grading system, Prognosis, Systematic review, Meta-analysis
Introduction
Lung adenocarcinoma (LADC) is the major histological subtype of non-small cell lung cancer (NSCLC) with high heterogeneity [1, 2]. Tumor grading, assessed based on histological manifestation, is crucial for predicting patient prognosis [3]. In 2015, the World Health Organization (WHO) categorize LADC into low grade (lepidic predominant), intermediate grade (acinar or papillary predominant), and high grade (solid or micropapillary predominant) [4]. However, this histology-based system does not include other prognostic factors, such as variant subtypes, and does not establish clear prognostic stratifications. To address these shortcomings, the International Association for the Study of Lung Cancer (IASLC) Pathology Committee introduced a novel grading system (2020) for invasive LADC [5, 6] (Table 1). This system incorporates key histologic subtypes and high-grade patterns, establishing clear cut-off values for each grade. Specifically, tumors exhibiting ≥ 20% high-grade patterns are classified as poorly differentiated. The revised IASLC grading system (2020) offers a more precise prediction of patients’ outcomes.
Table 1.
The categories of IASLC grade
| Grade | Differentiation | Pattern (2020) | Pattern (2015) |
|---|---|---|---|
| 1 | Well differentiated | Lepidic predominant tumors wiht <20% of high-grade patters | Lepidic predominant |
| 2 | Moderately differentiated | Acinar or papillary predominant tumors with <20% of high-grade patters | Acinar or papillary predominant |
| 3 | Poorly differentiated | Any tumor with ≥ 20% of high-grade patterns (solid, micropapillary, complex glandular) | Solid or micropapillary predominant |
The inclusion of high-grade patterns distinguishes IASLC grading system (2020) from other systems. High-grade patterns, including solid, micropapillary, and complex glandular patterns, are closely associated with the prognosis, similar to that observed in solid-predominant and micropapillary-predominant LADC [7–9]. Overlooking the identification of complex glandular patterns may underestimate the heterogeneity of acinar-predominant adenocarcinoma, thereby diminishing the prognostic discriminatory power of grading system in acinar pattern-dominant LADC [10, 11]. Therefore, determining the appropriate percentage threshold for high-grade patterns is the key for this revised grading system. While several studies have linked high-grade patterns to adverse prognosis [12, 13], the percentage threshold of high-grade patterns as a determinant of tumor recurrence and mortality has not been well established. Therefore, compared to traditional grading systems utilizing mitotic count, nuclear grade, and cytologic grading, the IASLC grading system appears to provide a more accurate prediction of the prognosis of patients with LADC.
Several studies have failed to observe significant differences in recurrence-free survival (RFS) and overall survival (OS) in LADC patients stratified into different grades [14, 15], which warrants further identification of prognostic grading systems. Herein, the present study aimed to conduct a systematic review and meta-analysis to assess the predictive value of the new IASLC grading system (2020) in the 5-year OS and RFS rates of patients with invasive LADC.
Methods
We conducted a systematic review and meta-analysis following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement [16]. The study protocol was registered with the PROSPERO International Prospective Register of Systematic Reviews (CRD42024531113).
Search strategy
A comprehensive literature search was conducted by two independent investigators from four scientific databases: PubMed, Web of Science, ScienceDirect, and the Cochrane Library. To extract the publication incorporating IASLC grading system, we selected literature that was published from October 2020 to April 2024. The search term used across all databases was: (“lung adenocarcinoma” OR “adenocarcinoma of the lung”) AND (“prognosis” OR “outcome”) AND (“micropapillary” OR “solid”) AND (“classification” OR “subtype”) AND (“The Pathology Committee of the International Association for the Study of Lung Cancer” OR “IASLC”).
Selection and eligibility criteria
Although both randomized controlled trials (RCTs) and non-randomized controlled studies were eligible for our analysis, only non-randomized studies were identified and included in the meta-analysis. The inclusion criteria were as follows: (1) patients diagnosed with primary LADC and aged 18 years or older; (2) studies focusing on the IASLC grading system (2020) for LADC; (3) studies that included OS and RFS or disease-free interval (DFI), recurrence-free probability (RFP), and disease-free survival (DFS) as prognostic indicators; and (4) original research articles published in peer-reviewed journals after October 2020. The exclusion criteria were as follows: (1) study types were case reports, commentaries, editorials, conference abstracts, or other non-original research; (2) articles not written in English; and (3) articles unavailable or inaccessible for full-text access. OS was defined as the time from surgery to the date of death from any cause, or the date of the last follow-up. RFS, DFI, RFP, and DFS were defined as the time from surgery to the date of regional recurrence, distant metastasis, or last follow-up, respectively.
Duplicate screening was performed by two independent reviewers (Yingding Ruan and JianWei Han) based on title, publication year, and author labels. After duplicate removal, two independent reviewers (Yingding Ruan and JianWei Han) review the title, abstract, and full text. Any disagreements were resolved by third-party consultation (Ting Zhang).
Data extraction
Data extraction from eligible studies was performed by two independent reviewers (Yingding Ruan and JianWei Han). The extracted data included article title, first author, publication year, TNM stages, RFS, DFS, DFI, RFP, OS, follow-up time, number and percentage of patients in different grades, distribution of sex, age, vascular and lymphovascular invasion, pleural involvement, spread through air space (STAS), percentage of patients actively smoking cigarettes at the time of surgery, and patients who underwent surgery. Furthermore, hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and RFS were calculated by multivariate analyses. Any discrepancies were resolved by a third investigator (Ting Zhang) to guarantee accuracy and consistency.
Assessment of bias
The Newcastle-Ottawa Scale (NOS) tool, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions, was used to assess nonrandomized controlled studies [17]. The NOS scoring system consists of a maximum score of nine points, covering the selection of the study population (four points), comparability (two points), and assessment of exposure or outcome (three points). A NOS score of ≥ 5 points indicated moderate to high quality. Assessment of bias was conducted by two independent reviewers (Yingding Ruan and JianWei Han) to evaluate the quality of the included studies, and any disagreements were resolved by a third reviewer (Ting Zhang).
Statistical analysis
The meta-analysis was conducted using Stata 12.0, and Review Manager software 5.4.1. Heterogeneity of the included studies was assessed using a quantitative I² test combined with a qualitative Cochrane’s Q test, with α = 0.1 as the test criterion. When P > 0.1 and I²≤ 50%, low heterogeneity was considered, and the fixed-effects model (FEM) was employed for meta-analysis. Conversely, P < 0.1 and I²> 50% indicated significant heterogeneity among studies, where random effects model (REM) was adopted for meta-analysis. Publication bias of the included studies was evaluated using funnel plot analysis. A funnel plot that was visually symmetric or P > 0.05 indicated a low likelihood of publication bias. Sensitivity analysis was performed to assess the stability of the results. The stability of the meta-analysis findings was indicated by effect sizes falling within the 95% CI range, and the consistency of the results was confirmed by consistent results after excluding each study.
Results
Characteristics of the included studies
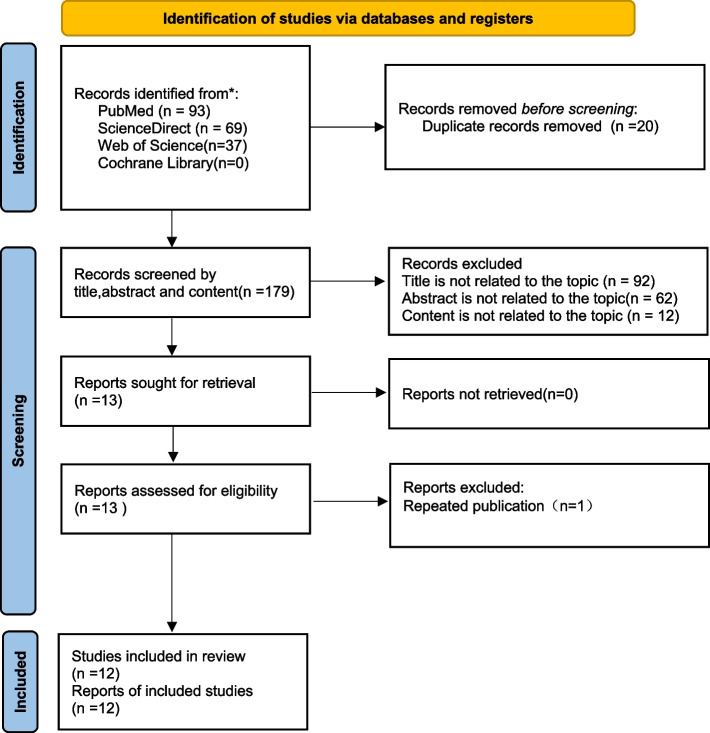
The PRISMA flow diagram (Fig. 1) shows the details of the selection process. A total of 199 records were identified from four databases. After removing duplicates and performing eligibility assessment, 12 retrospective studies were included in the meta-analysis [14, 15, 18–27] with moderate-to-high quality (Table 2). Characteristics of the included studies are shown in Table 3. A total of 8725 patients were included in the analysis, where 1246, 4020, and 3459 patients belonged to IASLC Grades 1, 2, and 3, respectively. OS data were available in all studies, whereas RFS data were available in eight of them [15, 18–21, 23–25]. DFI, progression-free survival (PFS), RFP, and DFS were described in previous publications [14, 22, 27, 28]. The number of patients at each IASLC Grade with 5-year follow-up was provided in eight studies [14, 15, 18–21, 24–26]. Nine studies conducted multivariate analysis [14, 15, 20–22, 24–27].
Fig. 1.
The PRISMA flow diagram displays the details of the selection process. *From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
Table 2.
The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in our study
| Study | Year | Country | Type of Article | The Newcastle-Ottawa Scale (NOS) | ||
|---|---|---|---|---|---|---|
| Selection | Comparability | Exposure | ||||
| Deng et al. [16] | 2021 | China | Single-center, retrospective case-control study | * * * * | * | * * * |
| Fan et al. [17] | 2023 | China | Multi-center, prospective and retrospective case-control study | * * * * | * * * | |
| Fujikawa et al. [18] | 2022 | Japan | Single-center, retrospective case-control study | * * * * | * | * * |
| Hou et al. [19] | 2022 | China | Multi-center, retrospective case-control study | * * * * | * | * * * |
| Jeon et al. [12] | 2021 | Korea | Multi-center, retrospective case-control study | * * * * | * | * * * |
| Mikubo et al. [13] | 2023 | Japan | Single-center, retrospective case-control study | * * * * | * * * | |
| Weng et al. [20] | 2020 | China | Single-center, retrospective case-control study | * * * * | * | * |
| Woo et al. [21] | 2022 | Korea | Multi-center, retrospective case-control study | * * * * | * * | * ** |
| Xu et al. [22] | 2022 | China | Single-center, retrospective case-control study | * * * * | * | * * * |
| Yanagawa et al. [23] | 2022 | Japan | Single-center, retrospective case-control study | * * * * | * * * | |
| Yoshida et al. [24] | 2022 | Japan | Single-center, retrospective case-control study | * * * * | * | * * |
| Zhang et al. [25] | 2022 | China | Single-center, retrospective case-control study | * * * * | * | * * |
*:1 point
Table 3.
Patient characteristics, pathology, and survival outcomes summary
| Study | Year | Country | Type of Article | Total(n) | Report Outcome | IASLC grade(n) | 5 years OS(n) | 5 years RFS or DFS(n) | Male(n,%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade1 | Grade2 | Grade3 | Grade1 | Grade2 | Grade1+2 | Grade3 | Grade1 | Grade2 | Grade1+2 | Grade3 | |||||||
| Deng et al. [16] | 2021 | China | Single-center, retrospective case-control study | 950 | OS,RFS | 34 | 285 | 631 | 24 | 146 | 170 | 221 | 24 | 128 | 152 | 139 | 421(44.3) |
| Fan et al. [17] | 2023 | China | Multi-center, prospective and retrospective case-control study | 373 | OS,RFS | 40 | 174 | 159 | 34 | 149 | 183 | 121 | 33 | 138 | 171 | 103 | 169(45.3) |
| Fujikawa et al. [18] | 2022 | Japan | Single-center, retrospective case-control study | 781 | OS,RFS | 114 | 438 | 229 | 12 | 40 | 52 | 18 | 12 | 38 | 50 | 7 | 373(47.8) |
| Hou et al. [19] | 2022 | China | Multi-center, retrospective case-control study | 1852 | OS,RFS | 238 | 862 | 700 | 115 | 379 | 494 | 283 | 111 | 356 | 467 | 247 | 850(45.9) |
| Jeon et al. [12] | 2021 | Korea | Multi-center, retrospective case-control study | 429 | OS, DFI | 191 | 200 | 38 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 187(43.6) |
| Mikubo et al. [13] | 2023 | Japan | Single-center, retrospective case-control study | 648 | OS,RFS | 125 | 341 | 182 | 78 | 199 | 277 | 89 | 78 | 175 | 253 | 67 | 329(47.8) |
| Weng et al. [20] | 2020 | China | Single-center, retrospective case-control study | 136 | OS,PFS | 7 | 74 | 55 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 65(47.8) |
| Woo et al. [21] | 2022 | Korea | Multi-center, retrospective case-control study | 1358 | OS,RFS | 181 | 755 | 422 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 707(52.1) |
| Xu et al. [22] | 2022 | China | Single-center, retrospective case-control study | 753 | OS,RFS | 73 | 293 | 387 | 64 | 243 | 307 | 267 | 61 | 219 | 280 | 222 | 538(71.4) |
| Yanagawa et al. [23] | 2022 | Japan | Single-center, retrospective case-control study | 395 | OS,RFS | 66 | 216 | 113 | 27 | 110 | 137 | 36 | 25 | 83 | 108 | 25 | 190(48.1) |
| Yoshida et al. [24] | 2022 | Japan | Single-center, retrospective case-control study | 471 | OS, RFP | 88 | 202 | 181 | 79 | 173 | 252 | 92 | 80 | 167 | 247 | 81 | 255(54.1) |
| Zhang et al. [25] | 2022 | China | Single-center, retrospective case-control study | 631 | OS,DFS | 89 | 180 | 362 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | 280(44.4) |
| Study | Age | Smoking(n,%) | Eexcent of surgery(n,%) | TNM stage(n,%) | Lymphatic invasion(n,%) | Vascular incasion(n,%) | Pleural invasion(n,%) | STAS | Adjuvant therapy(n,%) | Follow-up(month) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sublobar resection#1 | Lobectomy#2 | Pneumonectomy | I | II | III | IV | |||||||||
| Deng et al. [16] | 60.5±10.1* | 239(25.2) | 45(4.7) | 888(93.5) | 17(1.8) | 635(66.8) | 73(7.7) | 242(25.5) | 0(0) | 137(14.5) | 198(19.) | 317(33.4) | n.d | 317(33.4) | n.d |
| Fan et al. [17] | <65:261 ; ≥65:112 | 112(30) | 25(6.7)#1 | 348(93.3) | 0(0) | 373(100) | n.d | n.d | n.d | n.d | n.d | 94(25.2) | 91(24.4) | n.d | n.d |
| Fujikawa et al. [18] | n.d | 429(54.9) | 226(28.9)#1 | 551(70.6) | 4(0.5) | 585(74.9) | 96(12.3) | 100(12.8) | 0(0) | 191(24.5) | 309(40) | 174(22.3) | n.d | 102(13.1) | 42.5(median) |
| Hou et al. [19] | <60:1298 ; ≥60:554 | 440(23.8) | 122(6.6) | 1730(93.4) | 0(0) | 1852(100) | n.d | n.d | n.d | 188(10.1) | 264(14.3) | n.d | n.d | n.d | n.d |
| Jeon et al. [12] | n.d | 113(26.3) | 45(10.5)#1 | 384(89.5)#2 | 0(0) | 429(100) | n.d | n.d | n.d | 80(18.6) | n.d | n.d | n.d | 7(1.6) | n.d |
| Mikubo et al. [13] | n.d | 376(58) | 74(11.4)#1 | 571(88.1) | 3(0.5) | 475(73.3) | 89(13.7) | 84(13) | 0(0) | 187(28.9) | 230(35.5) | 156(24.1) | n.d | 215(33.2) | 61(median) |
| Weng et al. [20] | ≤70:87;>70:49 | 44(27.9) | n.d | n.d | n.d | n.d | n.d | 17(12.5) | 119(87.5) | n.d | n.d | n.d | n.d | 109(80.1) | n.d |
| Woo et al. [21] | n.d | 520(38.3) | 136(10.)# | 1216(89.5)#2 | 6(0.5) | 1054(77.6) | 146(10.8) | 158(11.6) | 0(0) | 237(17.5) | 285(21) | 13(1) | 230(16.9) | n.d | 59.1[43.3,74.1]$ |
| Xu et al. [22] | <65:350 ; ≥65:403 | 236(31.3) | 73(9.7)#1 | 680(90.3) | 0(0) | n.d | n.d | n.d | n.d | n.d | n.d | 137(18.2) | 276(36.7) | n.d | n.d |
| Yanagawa et al. [23] | ≤68:180 ; ≥69:215 | 202(51.1) | 10(2.5) | 377(95.4)#2 | 8(2.1) | 267(67.6) | 32(8.1) | 74(18.7) | 0(0) | 70(17.7) | 86(21.8) | 117(29.6) | n.d | 209(52.9) | 60.3[6.4,137.9]$ |
| Yoshida et al. [24] | ≤65:163 ; >65:308 | n.d | 43(9.1) | 428(90.9) | 0(0) | 357(75.8) | 50(10.6) | 64(13.6) | 0(0) | 189(40.1%) | 179(38) | n.d | 221(46.9) | n.d | n.d |
| Zhang et al. [25] | ≤60:304 ; >60:327 | 272(43.1) | 46(7.3) | n.d | n.d | 472(74.8) | 0(0) | 0(0) | 0(0) | n.d | 150(23.8) | 166(26.3) | n.d | 186(29.5) | 71(56,89)$ |
OS Overall survival, RFS Recurrence-free Survival, DFI Disease-free Interval, RFP Recurrence-free Probability, DFS Disease-free Survival, IASLC International Association for the Study of Lung Cancer, n.d No data
*Mean±Standard deviation;$Median IQR;#1:Segmentectomy+Wedge;#2:Lobectomy+Bilobectomy;STAS, Spread through air space;$:Median(25th percentile,75th percentile)
Quantitative synthesis of 5-year OS and RFS
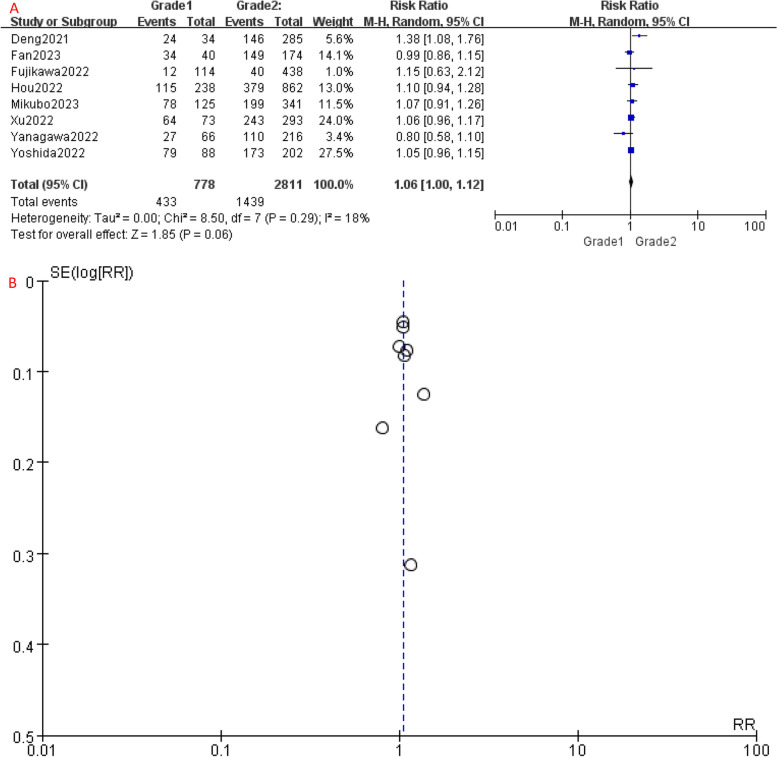
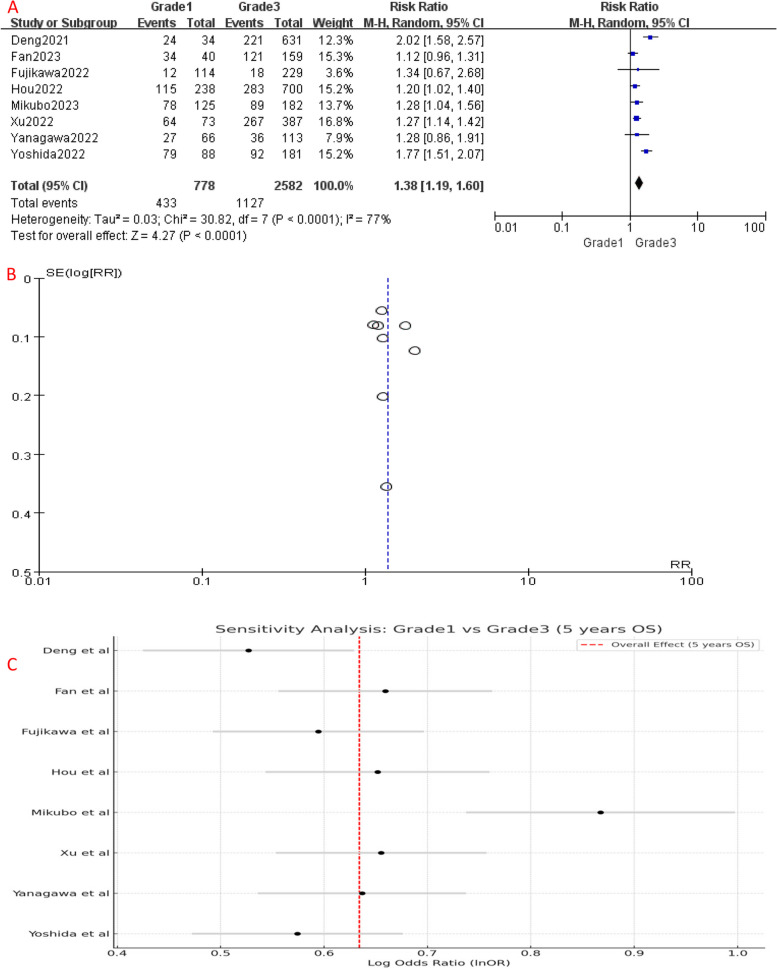
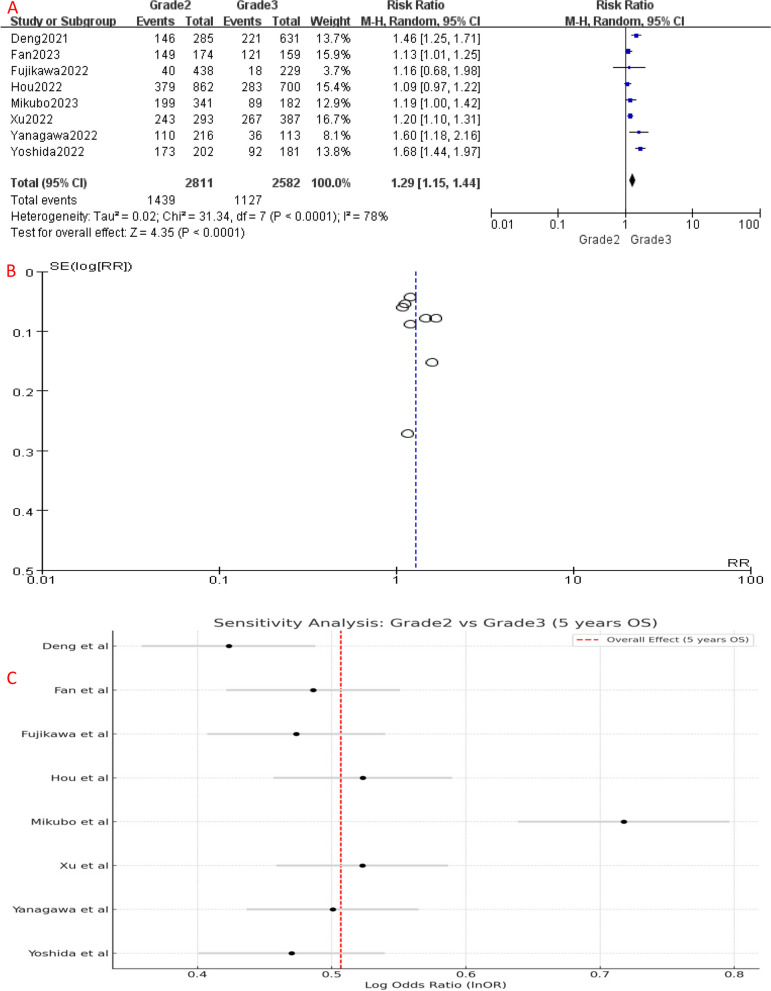
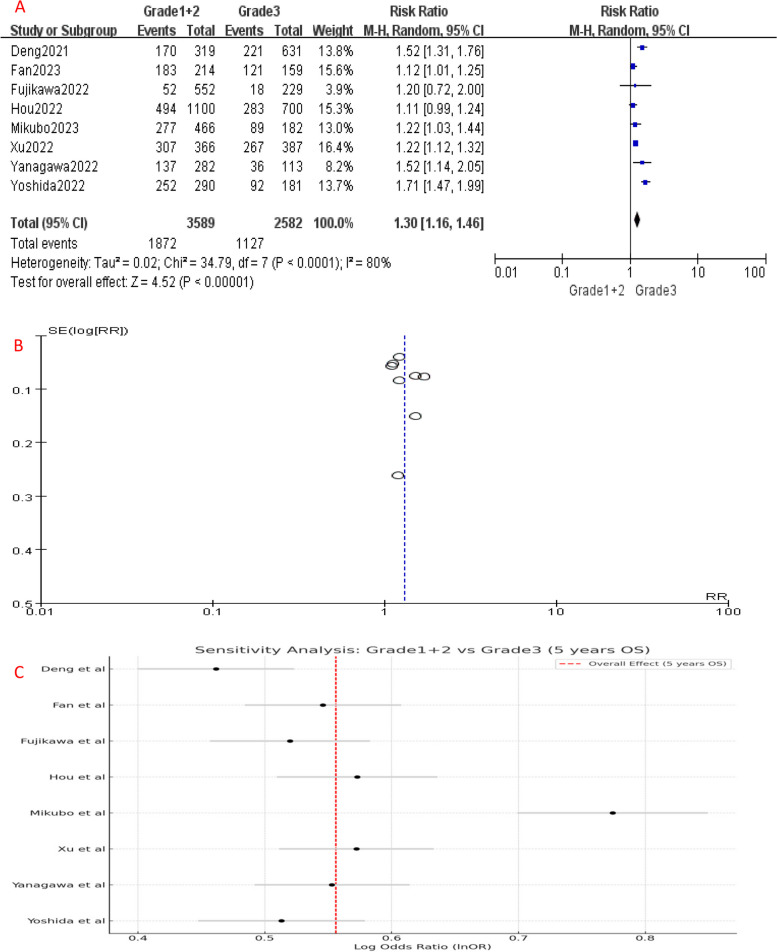
Comparing to Grade 1, the 5-year OS of Grade 2 patients had no significant difference (P = 0.06), with an HR of 1.06 (95%CI: 1.00-1.12), which suggests a slightly increased risk of unfavorable prognosis in Grade 2 patients (Fig. 2A). However, the 5-year OS of Grade 3 patients was significantly worse than Grade 1 (HR [95% CI] = 1.38 [1.19–1.60], P < 0.001) (Fig. 4A) and Grade 2 (HR [95% CI] = 1.29 [1.15–1.44], P < 0.001) patients (Fig. 6A), showing a significantly higher risk of mortality. Since Grade 1 and Grade 2 patients had similar prognosis, we combined their OS data and found that Grade 3 still had a significant poorer survival than combined group (HR [95% CI] = 1.30 [1.16–1.46], P < 0.001) (Fig. 8A).
Fig. 2.
A Forest plot of studies that compared 5-year OS of Grade 1 and Grade 2 LADC. B Funnel plot of the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; OS, over survival; M-H, mantel-haenszel; SE, standard error
Fig. 4.
A Forest plot of included studies that compared 5-year OS of Grade 1 and Grade 3 LADC. B Funnel plot of the included studies. C Sensitivity analysis of the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; OS, over survival; M-H, mantel-haenszel; SE, standard error)
Fig. 6.
A Forest plot of included studies that compared 5-year OS of Grade 2 and Grade 3 LADC. B Funnel plot of the included studies C Sensitivity analysis the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; OS, over survival; M-H, mantel-haenszel; SE, standard error
Fig. 8.
A Forest plot of the included studies that compared 5-year OS of Grade 1 + 2 and Grade 3 LADC. B Funnel plot of the included studies. C Sensitivity analysis of the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; OS, over survival; M-H, mantel-haenszel; SE, standard error)
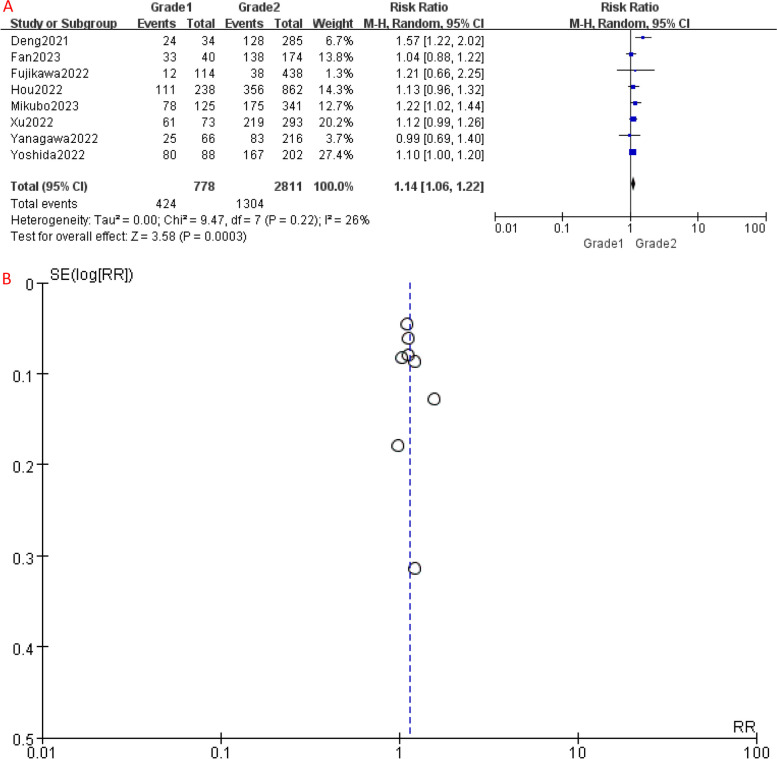
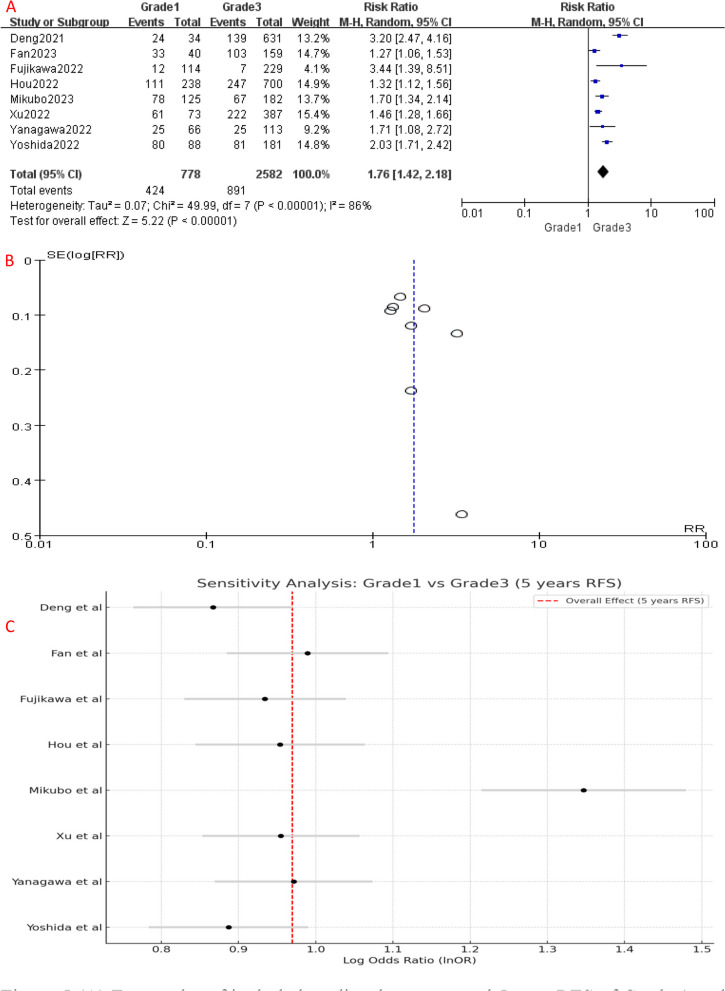
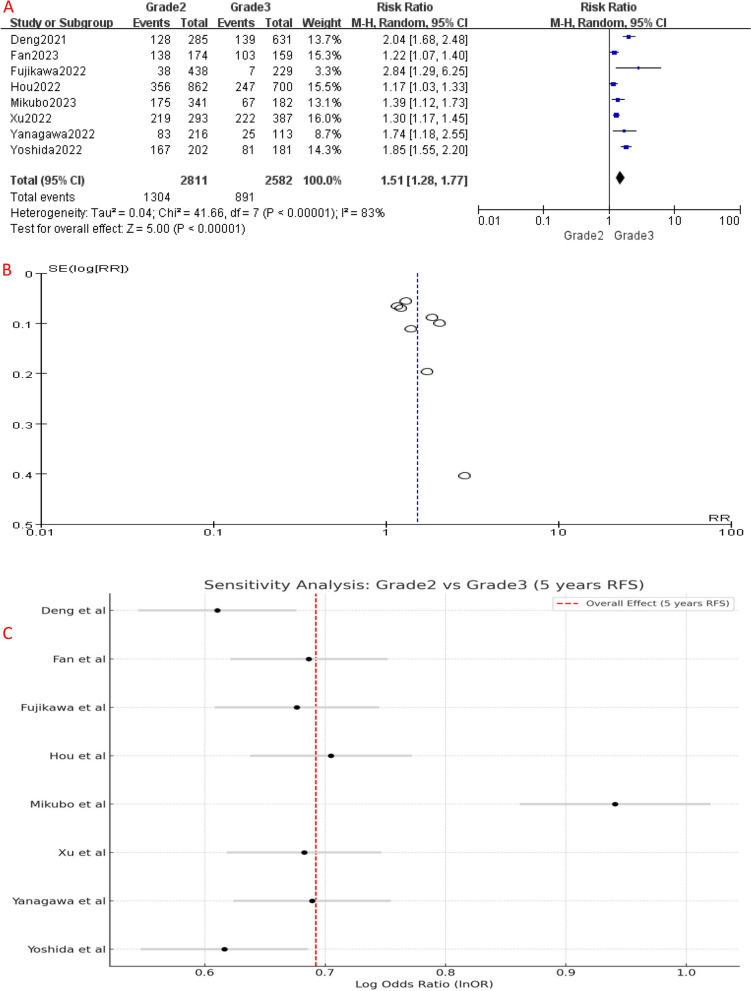
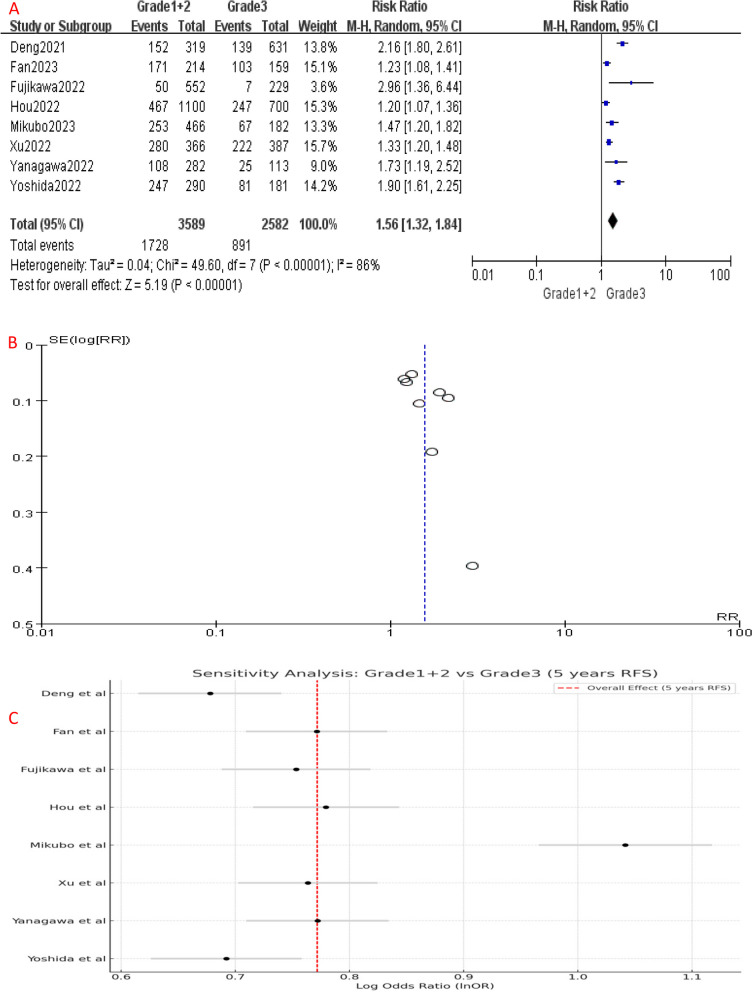
Similarly, RFS analysis demonstrated significant differences between the different IASLC grade groups. Compared to Grade 1, Grade 2 had a higher risk of disease recurrence or progression (HR [95% CI] = 1.14 [1.06–1.22], P = 0.006) (Fig. 3A). Similarly, the 5-year RFS of Grade 3 patients was significantly worse than Grade 1 (HR [95% CI] = 1.76 [1.42–2.18], P < 0.001) (Fig. 5A) and Grade 2 (HR [95% CI] = 1.51 [1.28–1.77], P < 0.001) patients (Fig. 7A), indicating a higher risk of disease recurrence or progression in Grade 3 patients. Additionally, when combining RFS data of Grade 1 and Grade 2 patients, Grade 3 group still had a shorter RFS (HR [95% CI] = 1.56 [1.32–1.84], P < 0.001) (Fig. 9A). Overall, these results indicate that IASLC grades could clearly stratify patients with different expected outcomes, where Grade 3 had the worst OS and RFS.
Fig. 3.
A Forest plot of included studies that compared 5-year RFS of Grade 1 and Grade 2 LADC. B Funnel plot of the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; RFS, recurrence-free survival; M-H, mantel-haenszel; SE, standard error
Fig. 5.
A Forest plot of included studies that compared 5-year RFS of Grade 1 and Grade 3 LADC. B Funnel plot of the included studies. C Sensitivity analysis of included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; RFS, recurrence-free survival; M-H, mantel-haenszel; SE, standard error
Fig. 7.
A Forest plot of included studies that compared 5-year RFS of Grade 2 and Grade 3 LADC. B Funnel plot of the included studies. C Sensitivity analysis of the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; RFS, recurrence-free survival; M-H, mantel-haenszel; SE, standard error
Fig. 9.
A Forest plot of included studies that compared 5-year RFS of Grade 1 + 2 and Grade 3 LADC. B Funnel plot of the included studies. C Sensitivity analysis of the included studies. CI, confidence interval; HR, hazard ratio; RR, risk ratio; RFS, recurrence-free survival; M-H, mantel-haenszel; SE, standard error)
Sensitivity analysis and bias assessment
Significant heterogeneity was detected among the included studies (Figs. 4, 5, 6, 7, 8 and 9A). Leave-one-out analysis revealed that the study by Mikubo [15] had a relatively large impact on results, suggesting that it may be potential source of study heterogeneity (Figs. 4, 5, 6, 7, 8 and 9C). Furthermore, funnel plot showed a symmetrical distribution (Figs. 2, 3, 4, 5, 6, 7, 8 and 9B), indicating a low likelihood of publication bias and, thus, a relatively high reliability of results.
Discussion
Our analysis revealed that LADC classified as Grade 1 and Grade 2, according to the IASLC grading system (2020), exhibited significantly better 5-year OS and RFS rates than Grade 3 patients, while no significant differences in 5-year OS and RFS were observed between the Grade 1 and Grade 2 groups, indicating that the IASLC grading system may not be able to stratify the prognosis of patients with these two grades.
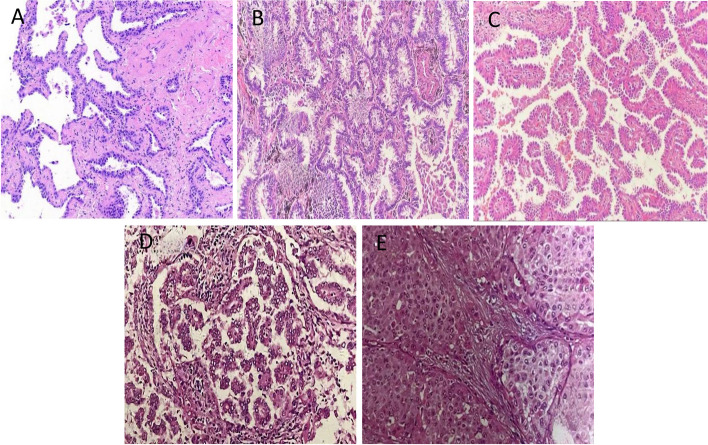
Figure 10 illustrates the growth patterns of prevalence of invasive non-mucinous adenocarcinomas, which are closely associated with DFS and OS [29]. This grading based on growth patterns has been incorporated into the World Health Organization (WHO) classification of lung tumors [30]. Specifically, micropapillary adenocarcinoma grows in clusters, filaments, or elongated papillae lacking a fibrovascular core, usually adhering to alveolar walls or floating within alveolar spaces, exhibiting high aggressiveness, metastatic potential, and recurrence rates, leading to the poorest prognosis. Solid adenocarcinoma, on the other hand, presents as polygonal cells forming solid sheets of nests without lumina or secretions, characterized by rapid growth and a propensity for metastasis and recurrence. In contrast, acinar adenocarcinoma grows in an acinar pattern with a relatively low malignancy and good response to treatment, and a favorable prognosis. Papillary adenocarcinoma contains a fibrovascular core surrounded by cancer cells and has a prognosis inferior to that of acinar adenocarcinoma [31, 32]. Notably, squamous-predominant adenocarcinomas appear to have the most favorable prognosis, while micropapillary and solid adenocarcinomas have the worst [31–33]. In summary, LADC with different growth patterns demonstrate significant variations in prognosis, which underscores the necessity of IASLC staging system to comprehensively assess patient’s prognosis.
Fig. 10.
The major histopathological patterns of non-mucinous pulmonary adenocarcinoma. A Lepidic; B Acinar; C Papillary; D Micropapillary; E Solid
Previous studies have reported no survival differences of LADC patients between different IASLC grades. Weng et al. [22] investigated the association between the IASLC grading system and survival outcomes in advanced-stage LADC patients. The study included 136 cases, with 7 cases classified as Grade 1 (5.1%), 74 cases as Grade 2 (54.4%), and 55 cases as Grade 3 (40.5%) LADC. The results showed a statistically significant difference in PFS among the different IASLC grades (P = 0.013), but no difference in OS (P = 0.154). However, this study had a relatively small sample size, and the unequal distribution of cases across different grades may have introduced selection bias, leading to nonsignificant findings. A systematic review and meta-analysis by Hegedűs F et al. [34] found that Grade 1 LADC had higher 5-year OS rates than Grade 3 one in both univariate (HR = 0.19, 95% CI: 0.05–0.66, P = 0.009) and multivariate analyses (HR = 0.21, 95% CI: 0.12–0.38, P < 0.001). Grade 3 LADC had a worse RFS compared to Grade 1 in multivariate analysis (HR: 0.22, 95% CI: 0.14–0.35, P < 0.001). Similarly, our study, using different databases and recent research, showed that Grade 1 and Grade 2 LADCs had better 5-year OS rates than Grade 3. Grade 3 also had lower 5-year RFS rates than Grade 1 and Grade 2. There were no significant differences in 5-year OS or RFS between Grade 1 and 2 LADCs. Our findings provide supportive evidence for the prognostic value of the IASLC grading system and highlight the importance of continually developing and refining the grading system for LADCs.
In another study [14] on stage IA LADC, significant difference of DFI was detected between Grade 1 and 2 (P = 0.001), as well as between Grade 1 and 3 (P < 0.001). However, there was no significant difference in OS between Grades 2 and 3 (P = 0.128), and Grade 3 was not a prognostic factor for OS. These findings could be attributed to non-cancer-related deaths in stage IA LADC, while a substantial number of deaths unrelated to cancer during the follow-up period (53.1%). According to another study [22] on patients with stage III and IV lung cancer, a total of 136 patients were included, with 17 in stage III and 119 in stage IV. According to the latest IASLC guidelines, there was no significant difference in OS among patients with stage III and IV regardless of IASLC grades. Grade 3 patients showed the worst PFS compared to Grade 2 in patients receiving chemotherapy. However, there were no significant differences between Grade 1 and Grade 2 (P = 0.948) or Grade 1 and Grade 3 (P = 0.125). In another study [20], analysis of Grade 2 and Grade 3 lung cancer in stages I, II, and III revealed that stage II (P < 0.001) and stage III (P < 0.001) were independent risk factors for RFS, and stage III (P < 0.001) was an independent risk factor for OS. Yet, adjuvant chemotherapy cannot significantly improve OS and RFS in Grade 2 and Grade 3 patients. These studies collectively emphasize the complexity of prognostic factors in LADC, highlighting stage- and grade-specific differences in DFS and OS, and suggest that non-cancer-related deaths play a significant role in certain stages, while advanced-stage cancer consistently shows poor survival outcomes regardless of grades.
The high expression of driver gene mutations in certain subtypes may also affect the prognosis [15, 18, 20, 22]. Deng et al. [18] found that EGFR mutations was linked to a notably high incidence of Grade 2 (P < 0.001), while KRAS mutations (P = 0.041) and ALK rearrangements (P = 0.021) were commonly observed in Grade 3. Exon 19 deletions are more frequently detected Grade 3 adenocarcinomas compared to those with the L858R mutation (P = 0.027). However, another study [15] suggested that EGFR mutations were more prevalent in tumors of low to moderate grades, accounting for 64.3%, 56.3%, and 46.4% in Grade 1, 2, and 3. This disparity might stem from the genomic diversity of Grade 3 tumor and varying proportions of histologic subtypes across studies. On the contrary, a study indicated that mutations in EGFR, KRAS, and ALK genes are not associated with the IASLC grading system [27]. Moreover, patients with EGFR mutated LADC may receive tyrosine kinase inhibitors (TKIs) as the first-line therapeutic choice, which will further induce bias in prognosis evaluation. Collectively, these studies reveal a complex relationship between gene mutations and tumor grading, while the discrepancies of their findings require additional investigation to delineate the correlation between gene mutation and tumor grading systems.
Our meta-analysis yielded similar results, demonstrating no statistically significant differences between Grade 1 and Grade 2 for 5-year OS and RFS. Even when we combined the survival data of Grade 1 and Grade 2, Grade 3 remained significantly the worst. However, it is worth noting that high heterogeneity among included studies when comparing Grade 3 with the other groups was observed during analysis, which may account for varied results. Sensitivity analysis identified a potential source of this heterogeneity, Mikubo’s study [15]. Future research should apply strict inclusion criteria and standardized assessment methods to reduce study heterogeneity. Nonetheless, our findings were conclusive.
The IASLC grading system, which incorporates pathological pattern recognition with histopathology manifestation, is considered as a practical and convenient assessment method. Numerous studies have demonstrated that the IASLC grading system offers improved prognostic accuracy compared to assessment by the predominant pattern [5, 18, 23, 25, 26, 28, 35]. Yanagawa et al. [25] compared the architectural (Arch) [4], Sica’s grading [12], and the IASLC grading system [5] for the prognostic assessment of patients with LADC. Their findings indicated that although three grading systems could predict patients’ outcomes across all stages, the IASLC grading system exhibited superior performance over the Arch and Sica’s grading systems in distinguishing OS (IASLC vs. Arch vs. Sica’s: HR = 3.77 vs. 3.03 vs. 2.63) and RFS (HR = 4.25 vs. 2.69 vs. 2.4) of Grade 3 from Grade 1 LADC.
Our study has several limitations. First, our data did not have sufficient information such as clinicopathological information, which increases the risk of confounding bias. Furthermore, the limited number of eligible studies restricts the expansion of our findings, and heterogeneity of included studies affects the reliability of the results. The inclusion of more data from RCTs would enhance the reliability of the findings, reduce bias, and allow for a more comprehensive assessment of survival differences of different IASLC grades. Additionally, our analysis only analyzed the difference of OS and RFS, while that in other prognostic indicators remains to be further determined. Furthermore, the comparisons between Grades 1, Grade 2, and Grade 3 were performed based on a limited number of studies, which may introduce potential bias due to small sample sizes.
Despite these limitations, our study had several strengths. We conducted a comprehensive meta-analysis that underscored the value of the IASLC grading system for clinical management and prognosis assessment. This finding can facilitate physicians make clinical decisions and design appropriate treatment regimens.
In conclusion, our meta-analysis shows the significant discriminative ability of IASLC grading system in predicting the prognosis of patients with LADC, highlighting the value of IASLC grading system as supplement tool. Further research is required to validate the prognostic stratification between the Grade 1 and Grade 2 groups.
Acknowledgments
AI and AI-assisted technologies
In the preparation of this work, the authors did not utilize AI technology to edit the manuscript. The authors take full responsibility for the contents of this manuscript.
Abbreviations
- IASLC
International Association for the Study of Lung Cancer
- OS
Overall Survival
- RFS
Recurrence-Free Survival
- LADC
Lung Adenocarcinoma
- DFI
Disease-free Interval
- RFP
Recurrence-free Probability
- DFS
Disease-free Survival
- STAS
Spread Through Air Space
- WHO
World Health Organization
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NSCLC
Non-small cell lung cancer
- BMI
Body mass index
- TNM
Tumor, node, and metastasis
- ECG
Electrocardiography
- RR
Relative Risk, Hazard Ratio
- CI
Confidence Interval
- NOS
The Newcastle-Ottawa Scale
- FEM
The Fixed Effects Model
- REM
The Random Effects Models
Authors' contributions
Yingding Ruan: Conceptualization, Methodology, Investigation, Data curation, Writing– original draft, writing– review & editing. Wenjun Cao: Investigation, Data curation, Writing– original draft. Jianwei Han: Methodology, Investigation, Data curation. Aiming Yang: Methodology, Investigation, Data curation. Jincheng Xu: Methodology, Investigation, Data curation. Ting Zhang: Conceptualization, Supervision, Writing – review & editing.
Funding
This research was supported by the Jiande Municipal Science and Technology Bureau (Grant No. 2023YW05 and Grant No. 2023SJZX22.
Data availability
Any researchers interested in this study could contact Yingding Ruan (E-mail: ruanyingding@sina.com) to request the data.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The First People’s Hospital of Jiande. Written informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yingding Ruan and Wenjun Cao contributed equally to this work.
References
- 1.Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M, Desai AP, Dilling TJ, Dowell J, Durm GA, Gettinger S, Grotz TE, Gubens MA, Juloori A, Lackner RP, Lanuti M, Lin J, Loo BW, Lovly CM, Maldonado F, Massarelli E, Morgensztern D, Mullikin TC, Ng T, Owen D, Owen DH, Patel SP, Patil T, Polanco PM, Riess J, Shapiro TA, Singh AP, Stevenson J, Tam A, Tanvetyanon T, Yanagawa J, Yang SC, Yau E, Gregory KM, Hang L. Non-small cell lung cancer, version 4 2024. J Natl Compr Canc Netw. 2024;22(4):249–74. 10.6004/jnccn.2204.0023. PMID: 38754467. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 2024;46(3):221–31. 10.3760/cma.j.cn112152-20240119-00035. Chinese. PMID: 38468501. [DOI] [PubMed] [Google Scholar]
- 3.Kuang M, Shen X, Yuan C, Hu H, Zhang Y, Pan Y, Cheng C, Zheng D, Cheng L, Zhao Y, Tao X, Li Y, Chen H, Sun Y. Clinical significance of complex glandular patterns in lung adenocarcinoma: clinicopathologic and molecular study in a large series of cases. Am J Clin Pathol. 2018;150(1):65–73. 10.1093/ajcp/aqy032. PMID: 29746612; PMCID: PMC5978020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I, WHO Panel. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. 10.1097/JTO.0000000000000630. PMID: 26291008. [DOI] [PubMed] [Google Scholar]
- 5.Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y, Cooper WA, Yoshida A, Bubendorf L, Papotti M, Pelosi G, Lopez-Rios F, Kunitoki K, Ferrari-Light D, Sholl LM, Beasley MB, Borczuk A, Botling J, Brambilla E, Chen G, Chou TY, Chung JH, Dacic S, Jain D, Hirsch FR, Hwang D, Lantuejoul S, Lin D, Longshore JW, Motoi N, Noguchi M, Poleri C, Rekhtman N, Tsao MS, Thunnissen E, Travis WD, Yatabe Y, Roden AC, Daigneault JB, Wistuba II, Kerr KM, Pass H, Nicholson AG, Mino-Kenudson M. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15(10):1599–610. 10.1016/j.jtho.2020.06.001. Epub 2020 Jun 17. PMID: 32562873; PMCID: PMC8362286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S, Noguchi M, Papotti M, Rekhtman N, Scagliotti G, van Schil P, Sholl L, Yatabe Y, Yoshida A, Travis WD. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17(3):362–87. [DOI] [PubMed] [Google Scholar]
- 7.Kadota K, Kushida Y, Kagawa S, Ishikawa R, Ibuki E, Inoue K, Go T, Yokomise H, Ishii T, Kadowaki N, Haba R. Cribriform subtype is an independent predictor of recurrence and survival after adjustment for the eighth edition of TNM staging system in patients with resected lung adenocarcinoma. J Thorac Oncol. 2019;14(2):245–54. 10.1016/j.jtho.2018.09.028. Epub 2018 Oct 15. PMID: 30336325. [DOI] [PubMed] [Google Scholar]
- 8.Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Pääkkö P, Lappi-Blanco E, Kaarteenaho R. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology. 2017;71(3):425–36. 10.1111/his.13236. Epub 2017 Jun 16. PMID: 28401582. [DOI] [PubMed] [Google Scholar]
- 9.Warth A, Muley T, Kossakowski C, Stenzinger A, Schirmacher P, Dienemann H, Weichert W. Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol. 2015;10(4):638–44. 10.1097/JTO.0000000000000490. PMID: 25634008. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Zhang Z. The self-distillation trained multitask dense-attention network for diagnosing lung cancers based on CT scans. Med Phys. 2024;51(3):1738–53. 10.1002/mp.16736. Epub 2023 Sep 16. PMID: 37715993. [DOI] [PubMed] [Google Scholar]
- 11.Lin G, Li H, Kuang J, Tang K, Guo Y, Han A, Xie C. Acinar-predominant pattern correlates with poorer prognosis in invasive mucinous adenocarcinoma of the lung. Am J Clin Pathol. 2018;149(5):373–8. 10.1093/ajcp/aqx170. PMID: 29538611. [DOI] [PubMed] [Google Scholar]
- 12.Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, Rusch VW, Travis WD, Moreira AL. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34(8):1155–62. 10.1097/PAS.0b013e3181e4ee32. PMID: 20551825. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wang R, Cai D, Li Y, Pan Y, Hu H, Wang L, Li H, Ye T, Luo X, Zhang Y, Li B, Shen L, Sun Y, Chen H. A comprehensive investigation of molecular features and prognosis of lung adenocarcinoma with micropapillary component. J Thorac Oncol. 2014;9(12):1772–8. 10.1097/JTO.0000000000000341. PMID: 25226429. [DOI] [PubMed] [Google Scholar]
- 14.Jeon HW, Kim YD, Sim SB, Moon MH. Significant difference in recurrence according to the proportion of high grade patterns in stage IA lung adenocarcinoma. Thorac Cancer. 2021;12(13):1952–8. 10.1111/1759-7714.13984. Epub 2021 May 25. PMID: 34037324; PMCID: PMC8258359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikubo M, Tamagawa S, Kondo Y, Hayashi S, Sonoda D, Naito M, Shiomi K, Ichinoe M, Satoh Y. Micropapillary and solid components as high-grade patterns in IASLC grading system of lung adenocarcinoma: clinical implications and management. Lung Cancer. 2024;187:107445. 10.1016/j.lungcan.2023.107445. Epub 2023 Dec 16. PMID: 38157805. [DOI] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. PMID: 33782057; PMCID: PMC8005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Chichester: Cochrane; 2022. Available at: www.training.cochrane.org/handbook.
- 18.Deng C, Zheng Q, Zhang Y, Jin Y, Shen X, Nie X, Fu F, Ma X, Ma Z, Wen Z, Wang S, Li Y, Chen H. Validation of the novel International Association for the study of Lung Cancer grading system for invasive pulmonary adenocarcinoma and association with common driver mutations. J Thorac Oncol. 2021;16(10):1684–93. 10.1016/j.jtho.2021.07.006. Epub 2021 Jul 22. PMID: 34302987. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Yao J, Si H, Xie H, Ge T, Ye W, Chen J, Yin Z, Zhuang F, Xu L, Su H, Zhao S, Xie X, Zhao D, Wu C, Zhu Y, Ren Y, Xu N, Chen C. Surgical Thoracic Alliance of Rising Star Group. Frozen sections accurately predict the IASLC proposed grading system and prognosis in patients with invasive lung adenocarcinomas. Lung Cancer. 2023;178:123–30. 10.1016/j.lungcan.2023.02.010. Epub 2023 Feb 17. PMID: 36822017. [DOI] [PubMed] [Google Scholar]
- 20.Fujikawa R, Muraoka Y, Kashima J, Yoshida Y, Ito K, Watanabe H, Kusumoto M, Watanabe SI, Yatabe Y. Clinicopathologic and genotypic features of lung adenocarcinoma characterized by the international association for the study of lung cancer grading system. J Thorac Oncol. 2022;17(5):700–7. 10.1016/j.jtho.2022.02.005. Epub 2022 Feb 25. PMID: 35227909. [DOI] [PubMed] [Google Scholar]
- 21.Hou L, Wang T, Chen D, She Y, Deng J, Yang M, Zhang Y, Zhao M, Zhong Y, Ma M, Zhao G, Chen Y, Xie D, Zhu Y, Chen Q, Wu C, Chen C, Multi-omics Classifier for Pulmonary Nodules (MISSION) Collaborative Group. Prognostic and predictive value of the newly proposed grading system of invasive pulmonary adenocarcinoma in Chinese patients: a retrospective multicohort study. Mod Pathol. 2022;35(6):749–56. 10.1038/s41379-021-00994-5. Epub 2022 Jan 10. PMID: 35013526. [DOI] [PubMed] [Google Scholar]
- 22.Weng CF, Huang CJ, Huang SH, Wu MH, Tseng AH, Sung YC, Lee HH, Ling TY. New International Association for the Study of Lung Cancer (IASLC) Pathology Committee grading system for the prognostic outcome of advanced lung adenocarcinoma. Cancers (Basel). 2020;12(11):3426. 10.3390/cancers12113426. Erratum in: Cancers (Basel). 2021 Aug 10;13(16): PMID: 33218158; PMCID: PMC7698816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo W, Yang YH, Cha YJ, Moon DH, Shim HS, Cho A, Kim BJ, Kim HE, Park BJ, Lee JG, Kim DJ, Paik HC, Lee S, Lee CY. Prognosis of resected invasive mucinous adenocarcinoma compared with the IASLC histologic grading system for invasive nonmucinous adenocarcinoma: surgical database study in the TKIs era in Korea. Thorac Cancer. 2022;13(23):3310–21. 10.1111/1759-7714.14687. Epub 2022 Nov 7. PMID: 36345148; PMCID: PMC9715870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Su H, Hou L, Wang F, Xie H, She Y, Gao J, Zhao S, Dai C, Xie D, Zhu Y, Wu C, Zhao D, Chen C, Surgical Thoracic Alliance of Rising Star Group. The IASLC proposed grading system accurately predicts prognosis and mediastinal nodal metastasis in patients with clinical stage I lung adenocarcinoma. Am J Surg Pathol. 2022;46(12):1633–41. 10.1097/PAS.0000000000001876. Epub 2022 Oct 13. PMID: 36224092. [DOI] [PubMed] [Google Scholar]
- 25.Yanagawa N, Sugai M, Shikanai S, Sugimoto R, Osakabe M, Uesugi N, Saito H, Maemondo M, Sugai T. The new IASLC grading system for invasive non-mucinous lung adenocarcinoma is a more useful indicator of patient survival compared with previous grading systems. J Surg Oncol. 2023;127(1):174–82. 10.1002/jso.27091. Epub 2022 Sep 13. PMID: 36098331. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida C, Yokomise H, Ibuki E, Go T, Haba R, Kadota K. High-grade tumor classified by new system is a prognostic predictor in resected lung adenocarcinoma. Gen Thorac Cardiovasc Surg. 2022;70(5):455–62. 10.1007/s11748-021-01758-3. Epub 2022 Jan 20. PMID: 35050467. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang Y, Hu Y, Zhang S, Zhu M, Hu B, Guo X, Lu J, Zhang Y. Validation of the novel International Association for the study of Lung Cancer grading system and prognostic value of filigree micropapillary and discohesive growth pattern in invasive pulmonary adenocarcinoma. Lung Cancer. 2023;175:79–87. 10.1016/j.lungcan.2022.11.022. Epub 2022 Dec 5. PMID: 36481678. [DOI] [PubMed] [Google Scholar]
- 28.Rokutan-Kurata M, Yoshizawa A, Ueno K, Nakajima N, Terada K, Hamaji M, Sonobe M, Menju T, Date H, Morita S, Haga H. Validation study of the International Association for the Study of Lung Cancer histologic grading system of invasive lung adenocarcinoma. J Thorac Oncol. 2021;16(10):1753–8. 10.1016/j.jtho.2021.04.008. Epub 2021 Apr 24. PMID: 33905897. [DOI] [PubMed] [Google Scholar]
- 29.Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, Park BJ, Rusch VW, Travis WD. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24(5):653–64. 10.1038/modpathol.2010.232. Epub 2011 Jan 21. PMID: 21252858. [DOI] [PubMed] [Google Scholar]
- 30.Travis WD, World Health Organization, International Agency for Research on Cancer, International Association for the Study of Lung Cancer, International Academy of Pathology. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon and Oxford: IARC Press, Oxford University Press (distributor); 2015. [Google Scholar]
- 31.Kuhn E, Morbini P, Cancellieri A, Damiani S, Cavazza A, Comin CE. Adenocarcinoma classification: patterns and prognosis. Pathologica. 2018;110(1):5–11 PMID: 30259909. [PubMed] [Google Scholar]
- 32.Zabeck H, Dienemann H, Hoffmann H, Pfannschmidt J, Warth A, Schnabel PA, Muley T, Meister M, Sültmann H, Fröhlich H, Kuner R, Lasitschka F. Molecular signatures in IASLC/ATS/ERS classified growth patterns of lung adenocarcinoma. PLoS One. 2018;13(10):e0206132. 10.1371/journal.pone.0206132. PMID: 30352093; PMCID: PMC6198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85. 10.1097/JTO.0b013e318206a221. PMID: 21252716; PMCID: PMC4513953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegedűs F, Zombori-Tóth N, Kiss S, Lantos T, Zombori T. Prognostic impact of the IASLC grading system of lung adenocarcinoma: a systematic review and meta-analysis. Histopathology. 2024;85(1):51–61. 10.1111/his.15172. Epub 2024 Mar 14. PMID: 38485464. [DOI] [PubMed] [Google Scholar]
- 35.Lucà S, Zannini G, Morgillo F, Della Corte CM, Fiorelli A, Zito Marino F, Campione S, Vicidomini G, Guggino G, Ronchi A, Accardo M, Franco R. The prognostic value of histopathology in invasive lung adenocarcinoma: a comparative review of the main proposed grading systems. Expert Rev Anticancer Ther. 2023;23(3):265–77. 10.1080/14737140.2023.2179990. Epub 2023 Feb 20. PMID: 36772823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any researchers interested in this study could contact Yingding Ruan (E-mail: ruanyingding@sina.com) to request the data.