Abstract
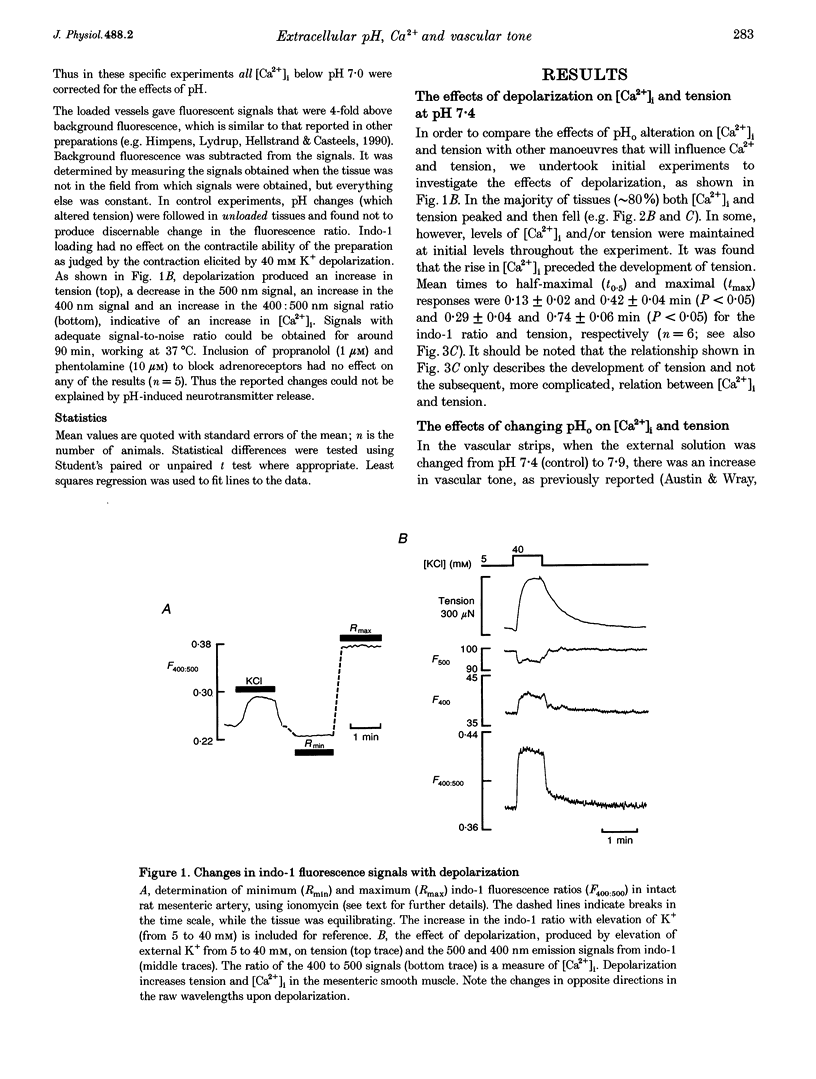
1. In order to investigate the mechanism whereby changes in external pH (pHo) alter tone in rat mesenteric resistance vessels, we have made simultaneous measurements of tension and intracellular Ca2+ [Ca2+]i. Strips of mesenteric artery were loaded with the Ca(2+)-sensitive indicator indo-1 and superfused with physiological salt solution at pH 7.4 and 37 degrees C. 2. An increase of pHo from 7.4 to 7.9 produced an increase in tension. This was accompanied by an increase in [Ca2+]i in resting and high-K(+)-depolarized vessels. Acidification to 6.9 reduced tension and was associated with a fall in [Ca2+]i. Over the pHi range examined, 6.6-7.9, parallel changes in [Ca2+]i and tension were found in K(+)-activated vessels. 3. In contrast to the relatively slow change in [Ca2+]i, pHi and tension with change of pHo, depolarization produced rapid changes in [Ca2+]i and tension, consistent with a more direct action on Ca2+ mobilization. 4. Reducing the external [Ca2+] below 1 mM produced a pronounced fall in [Ca2+]i and force. Changes in [Ca2+]i, produced by alteration of external [Ca2+] (Cao2+) were used to examine the relation between [Ca2+]i and tension. A linear relation was found. Alteration of pHo to 6.9 or 7.9 did not significantly change this relation. When the tension data were normalized to their own maxima, no shift in the tension-Ca2+ relation occurred, suggesting little or no effect of pH on the Ca2+ sensitivity of force production by the contractile proteins. 5. To determine further whether the changes in [Ca2+]i produced by alteration of pHo could account for all the changes observed in tension, [Ca2+]i was restored to control levels while maintaining an altered pHo. When this was done, restoration of [Ca2+]i led to restoration of force. Thus, in this preparation, the changes in [Ca2+]i produced by altering pHo in depolarized vessels can account for the changes in vascular tone.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalkjaer C., Hughes A. Chloride and bicarbonate transport in rat resistance arteries. J Physiol. 1991 May;436:57–73. doi: 10.1113/jphysiol.1991.sp018539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arheden H., Arner A., Hellstrand P. Calcium sensitivity and energetics of contraction in skinned smooth muscle of the guinea pig taenia coli at altered pH. Pflugers Arch. 1989 Mar;413(5):476–481. doi: 10.1007/BF00594176. [DOI] [PubMed] [Google Scholar]
- Austin C., Wray S. A quantitative study of the relation between intracellular pH and force in rat mesenteric vascular smooth muscle. Pflugers Arch. 1994 Jun;427(3-4):270–276. doi: 10.1007/BF00374534. [DOI] [PubMed] [Google Scholar]
- Austin C., Wray S. Changes of intracellular pH in rat mesenteric vascular smooth muscle with high-K+ depolarization. J Physiol. 1993 Sep;469:1–10. doi: 10.1113/jphysiol.1993.sp019800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C., Wray S. Extracellular pH signals affect rat vascular tone by rapid transduction into intracellular pH changes. J Physiol. 1993 Jul;466:1–8. [PMC free article] [PubMed] [Google Scholar]
- Batlle D. C., Peces R., LaPointe M. S., Ye M., Daugirdas J. T. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol. 1993 Apr;264(4 Pt 1):C932–C943. doi: 10.1152/ajpcell.1993.264.4.C932. [DOI] [PubMed] [Google Scholar]
- Bohlen H. G., Gore R. W. Comparison of microvascular pressures and diameters in the innervated and denervated rat intestine. Microvasc Res. 1977 Nov;14(3):251–264. doi: 10.1016/0026-2862(77)90024-3. [DOI] [PubMed] [Google Scholar]
- Crichton C. A., Taggart M. J., Wray S., Smith G. L. Effects of pH and inorganic phosphate on force production in alpha-toxin-permeabilized isolated rat uterine smooth muscle. J Physiol. 1993 Jun;465:629–645. doi: 10.1113/jphysiol.1993.sp019697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton C. A., Templeton A. G., Smith G. L. Effect of altered bathing pH on calcium activated force in alpha toxin permeabilised rat portal vein and human umbilical artery. Cardiovasc Res. 1994 Sep;28(9):1378–1384. doi: 10.1093/cvr/28.9.1378. [DOI] [PubMed] [Google Scholar]
- Gardner J. P., Diecke F. P. Influence of pH on isometric force development and relaxation in skinned vascular smooth muscle. Pflugers Arch. 1988 Aug;412(3):231–239. doi: 10.1007/BF00582502. [DOI] [PubMed] [Google Scholar]
- Gaskell W. H. On the Tonicity of the Heart and Blood Vessels. J Physiol. 1880 Aug;3(1):48–92.16. doi: 10.1113/jphysiol.1880.sp000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R. Effect of H+ and elevated PCO2 on membrane electrical properties of rat cerebral arteries. Pflugers Arch. 1982 Aug;394(2):182–185. doi: 10.1007/BF00582922. [DOI] [PubMed] [Google Scholar]
- Himpens B., Lydrup M. L., Hellstrand P., Casteels R. Free cytosolic calcium during spontaneous contractions in smooth muscle of the guinea-pig mesotubarium. Pflugers Arch. 1990 Dec;417(4):404–409. doi: 10.1007/BF00370660. [DOI] [PubMed] [Google Scholar]
- Himpens B., Matthijs G., Somlyo A. V., Butler T. M., Somlyo A. P. Cytoplasmic free calcium, myosin light chain phosphorylation, and force in phasic and tonic smooth muscle. J Gen Physiol. 1988 Dec;92(6):713–729. doi: 10.1085/jgp.92.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol. 1981 Nov;320:449–467. doi: 10.1113/jphysiol.1981.sp013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajikuri J., Kuriyama H. Characteristic features of noradrenaline-induced Ca2+ mobilization and tension in arterial smooth muscle of the rabbit. J Physiol. 1992 Nov;457:297–314. doi: 10.1113/jphysiol.1992.sp019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E., Hughes A., Boonen H. C., Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res. 1993 Aug;73(2):314–324. doi: 10.1161/01.res.73.2.314. [DOI] [PubMed] [Google Scholar]
- Kim B. K., Mitsui M., Karaki H. The long-term inhibitory effect of a Ca2+ channel blocker, nisoldipine, on cytosolic Ca2+ and contraction in vascular smooth muscle. Eur J Pharmacol. 1992 Nov 17;223(2-3):157–162. doi: 10.1016/0014-2999(92)94834-i. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J Gen Physiol. 1994 Apr;103(4):665–678. doi: 10.1085/jgp.103.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem Biophys Res Commun. 1990 Aug 31;171(1):102–108. doi: 10.1016/0006-291x(90)91362-v. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Martínez-Zaguilán R., Martínez G. M., Lattanzio F., Gillies R. J. Simultaneous measurement of intracellular pH and Ca2+ using the fluorescence of SNARF-1 and fura-2. Am J Physiol. 1991 Feb;260(2 Pt 1):C297–C307. doi: 10.1152/ajpcell.1991.260.2.C297. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrwa U., Achtig I., Ruegg J. C. Influences of calcium concentration and pH on the tension development and ATPase activity of the arterial actomyosin contractile system. Blood Vessels. 1974;11(5-6):277–286. doi: 10.1159/000158021. [DOI] [PubMed] [Google Scholar]
- Nagesetty R., Paul R. J. Effects of pHi on isometric force and [Ca2+]i in porcine coronary artery smooth muscle. Circ Res. 1994 Dec;75(6):990–998. doi: 10.1161/01.res.75.6.990. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y., Schwartz A. Possible control of intracellular calcium metabolism by [H+]: sarcoplasmic reticulum of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1970 Nov 25;41(4):830–836. doi: 10.1016/0006-291x(70)90157-9. [DOI] [PubMed] [Google Scholar]
- Rinaldi G. J., Amado Cattaneo E., Cingolani H. E. Interaction between calcium and hydrogen ions in canine coronary arteries. J Mol Cell Cardiol. 1987 Aug;19(8):773–784. doi: 10.1016/s0022-2828(87)80388-7. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Siskind M. S., McCoy C. E., Chobanian A., Schwartz J. H. Regulation of intracellular calcium by cell pH in vascular smooth muscle cells. Am J Physiol. 1989 Feb;256(2 Pt 1):C234–C240. doi: 10.1152/ajpcell.1989.256.2.C234. [DOI] [PubMed] [Google Scholar]
- Wahl M., Lucherini M. J., Gruenstein E. Intracellular Ca2+ measurement with Indo-1 in substrate-attached cells: advantages and special considerations. Cell Calcium. 1990 Aug;11(7):487–500. doi: 10.1016/0143-4160(90)90081-5. [DOI] [PubMed] [Google Scholar]
- West G. A., Leppla D. C., Simard J. M. Effects of external pH on ionic currents in smooth muscle cells from the basilar artery of the guinea pig. Circ Res. 1992 Jul;71(1):201–209. doi: 10.1161/01.res.71.1.201. [DOI] [PubMed] [Google Scholar]
- Ziegelstein R. C., Cheng L., Blank P. S., Spurgeon H. A., Lakatta E. G., Hansford R. G., Capogrossi M. C. Modulation of calcium homeostasis in cultured rat aortic endothelial cells by intracellular acidification. Am J Physiol. 1993 Oct;265(4 Pt 2):H1424–H1433. doi: 10.1152/ajpheart.1993.265.4.H1424. [DOI] [PubMed] [Google Scholar]


