Abstract
Study objectives
Observational and cohort studies have associated Sjögren's syndrome (SS) with various types of cardiovascular disease (CVD), yet causal relationships have not been established. We employed Mendelian randomization (MR) to investigate potential causal links between SS and CVD in the general population.
Methods
We conducted a two-sample MR analysis using data from four distinct sources for 11 genome-wide significant single nucleotide polymorphisms (SNPs) associated with SS and data for 13 types of CVD sourced from FinnGen, IEU OpenGWAS, and GWAS catalog. The inverse variance weighted method was selected as the primary analytical approach, complemented by various sensitivity analyses.
Results
MR analyses provide evidence of a significantly increased risk of ischemic stroke associated with genetically predicted SS (odds ratio [OR], 1.0237; 95 % CI, 1.0096 to 1.0379; p = 0.0009), as well as suggestive evidence of a potential causal relationship between SS and an increased risk of chronic heart failure (OR, 1.0302; 95 % CI, 1.0020 to 1.0592; p = 0.0355). Sensitivity analyses reinforced these associations, demonstrating robustness and consistency across multiple statistical methods. The secondary analysis, conducted after outlier correction using MR-PRESSO and RadialMR methods, reaffirmed these associations and also indicated a suggestive causal link between SS and non-rheumatic valvular heart disease (OR, 1.0251; 95 % CI, 1.0021 to 1.0486; p = 0.0323).
Conclusions
This study demonstrates that genetically predicted SS is a potential causative risk factor for ischemic stroke, chronic heart failure, and non-rheumatic valvular heart disease on a large-scale population. However, further research incorporating ancestral diversity is required to confirm a causal relationship between SS and CVD.
Keywords: Sjögren's syndrome, Cardiovascular disease, Mendelian randomization, Single-nucleotide polymorphisms, FinnGen
1. Introduction
Cardiovascular disease (CVD) comprises a spectrum of disorders affecting the heart and blood vessels and it remains the leading cause of death globally. Epidemiological data indicate that CVD accounts for approximately 17.9 million deaths annually, representing about 31 % of all global deaths [1]. This significant burden is also accompanied by considerable economic implications, with direct medical costs expected to approximate $1 trillion by 2030. Notably, coronary heart disease and stroke are the principal contributors to this mortality [2,3].
Sjögren's syndrome (SS) is an autoimmune disorder that predominantly affects the exocrine glands, particularly the salivary and lacrimal glands. This condition manifests with the hallmark symptoms of dry mouth and dry eyes [4,5]. Recent estimates suggests that SS affects between 0.1 % to 4 % of the global population, with a significantly higher prevalence among women, particularly in postmenopausal age groups [6]. Emerging evidence increasingly supports the notion that SS serves as a significant independent risk factor for a wide range of CVD [[7], [8], [9], [10]]. The underlying pathophysiological mechanisms are believed to involve chronic inflammation, autoimmune responses, and endothelial dysfunction [11]. Research shows that patients with Sjögren's syndrome experience a higher incidence of major adverse cardiovascular events compared to the general population, with rates ranging from 34 % to 46 % [7,12].
Despite accumulating evidence of a robust connection between SS and increased CVD risk, the precise nature of this association remains unclear. This uncertainty largely arises from the dependency on observational studies, which are frequently confounded by factors such as age, gender, and coexisting autoimmune conditions, thereby blurring whether the relationship is causal or merely correlative. The presence of shared risk factors, including advanced age, female gender, and autoimmune comorbidities, frequently contribute to confounding bias. Consequently, establishing a causal connection between SS and specific CVD has been challenging due to these overlapping conditions and comorbidities [[13], [14], [15]]. A deeper understanding of the causal pathways linking SS to specific cardiovascular outcomes is essential for developing targeted interventions to mitigate these potential adverse effects.
Given the complexities and costs associated with randomized controlled trials (RCT), alternative approaches such as Mendelian randomization (MR) provide robust avenues for enhancing causal inference in epidemiological studies. MR utilizes genetic variants as instrumental variables, exploiting their random allocation during transmission from parents to offspring, which minimizes confounding effects and eliminate issues of reverse causation [16,17]. The strength of this method resides in its capacity to utilize genetic predispositions as proxies to deduce causal relationships between exposures and outcomes, free from biases often introduced by self-selected behaviors or unmeasured confounders [18]. We propose conducting a two-sample MR study to rigorously investigate the causal relationships between genetic predispositions to SS and various CVD outcomes. This study will encompass 13 conditions, including heart failure, myocardial infarction, peripheral arterial disease, ischemic stroke and venous thromboembolism.
2. Methods
2.1. Study design
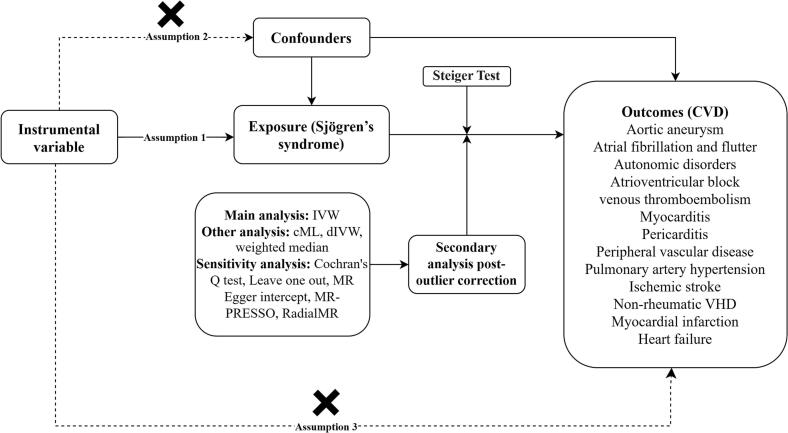
We conducted a MR analysis to investigate the potential causal relationship between SS with the susceptibility to 13 types of CVD. MR analysis utilizes genetic variants as instrumental variables, which estimate the causal impact of an exposure on disease development [19]. These genetic variants must satisfy three key assumptions to reliably indicate causality: [1] they are robustly associated with the exposure; [2] they are unrelated to any confounders that could influence the relationship between the exposure and the outcome; and [3] they impact the outcome exclusively through the exposure [20]. We performed univariable MR to assess the causal relationships between SS and 13 types of CVD. The genome-wide association study (GWAS) summary statistics used in this study were publicly available, and the original studies received ethical approvals from the relevant authorities. The detailed research design process is illustrated in Fig. 1.
Fig. 1.
Overview of the design and main results of this Mendelian randomization (MR) study on Sjögren's syndrome risk factors and cardiovascular disease. IVW, inverse variance weighted; cML, constrained maximum likelihood; dIVW, debiased inverse-variance weighted; CVD, cardiovascular disease; VHD, valvular heart disease.
2.2. Data sources and instrument variables
2.2.1. Sjögren's syndrome
In 2013, the Sjögren's Genetics Network published the first large-scale genomic study on SS in individuals of European descent [21]. Subsequently, in 2017, Li et al. discovered a novel susceptibility locus in European populations [22]. The Sjögren's International Collaborative Clinical Alliance conducted the first multiethnic GWAS on SS, finding no new loci in the European cohort [23]. More recently, Khatri et al. identified ten novel genetic loci linked to SS susceptibility in Europeans [24]. By integrating results from these four studies, we selected 11 single-nucleotide polymorphisms (SNPs) as instrumental variables, associated with SS traits in 29,804 individuals of European ancestry. The GWAS summary statistics encompassed 4977 SS cases and 24,827 controls from European populations. Diagnoses of SS were classified according to the International Classification of Diseases (ICD-10 M35.0) and PheCode 709.2, with statistical adjustments made using Firth and SPA corrections. Detailed information regarding the SNPs selected as instrumental variables is presented in Table 1.
Table 1.
Information on instrumental variables of Sjögren's syndrome.
| SNP | Chr | Position | Mapped genes | Effect allele | Other allele | EAF | Beta | SE | P |
|---|---|---|---|---|---|---|---|---|---|
| rs3135394 | 6 | 32,440,720 | HLA-DRA | G | A | 0.1100 | 1.2585 | 0.0557 | 5.00e-113 |
| rs3757387 | 7 | 128,936,032 | KCP/IRF5 | C | T | 0.4500 | 0.3646 | 0.0407 | 3.00e-19 |
| rs485497 | 3 | 160,001,345 | IL12A-AS1 | A | CG | 0.4800 | 0.2624 | 0.0406 | 1.00e-10 |
| rs7119038 | 11 | 118,867,572 | Y_RNA/CXCR5 | A | G | 0.7700 | 0.3011 | 0.0525 | 1.00e-08 |
| rs2293765 | 2 | 190,656,119 | NAB1 | A | C | 0.5586 | 0.2151 | 0.0286 | 5.53e-14 |
| rs2431697 | 5 | 160,452,971 | MIR3142HG | C | T | 0.4314 | −0.1863 | 0.0315 | 3.33e-09 |
| rs11250098 | 8 | 10,961,097 | XKR6 | A | G | 0.4851 | 0.1484 | 0.0216 | 6.89e-12 |
| rs7210219 | 17 | 45,941,153 | MAPT | C | T | 0.2406 | −0.2485 | 0.0392 | 2.40e-10 |
| rs8071514 | 17 | 80,990,283 | CHMP6 | A | C | 0.5477 | −0.1744 | 0.0309 | 1.64e-08 |
| rs11085725 | 19 | 10,351,837 | TYK2 | C | T | 0.7306 | −0.2485 | 0.0346 | 7.17e-13 |
| rs2069235 | 22 | 39,351,775 | SYNGR1 | A | G | 0.2992 | 0.1906 | 0.0307 | 5.06e-10 |
Chr, chromosome; EAF, effect allele frequency; SE, standard error; SNP, single nucleotide polymorphism; Beta value is equal to log (OR). GRCh38 is the RefSNP for anchor position of the above SNPs.
2.3. 13 types of CVD
We utilized summary-level data for 13 CVD outcomes primarily sourced from the FinnGen R10 version database (https://r10.finngen.fi/), supplemented by data from the IEU online GWAS database (https://gwas.mrcieu.ac.uk/), GWAS catalog database (https://www.ebi.ac.uk/gwas/) and the CARDIoGRAMplusC4D consortium [[25], [26], [27], [28]]. The GWAS study conducted by Sakaue et al. incorporates data from both the FinnGen and the UK Biobank [29]. The use of these combined data ensured comprehensive coverage while avoiding redundancy. These outcomes included aortic aneurysm, atrial fibrillation and flutter, autonomic disorders, atrioventricular block, venous thromboembolism, myocarditis, pericarditis, peripheral artery disease, pulmonary artery hypertension, ischemic stroke, non-rheumatic valvular heart disease, myocardial infarction, and chronic heart failure. Detailed information on the data sources for these outcomes is available in Supplementary Table S1.
2.4. Selection of the genetic instrumental variables
Instrumental variables were selected based on their significant genetic association with the exposure, defined by p-values <5 × 10−8. Each instrumental variable was assessed to ensure an r2 value <0.001, within a 10,000 kb window around each genetic variant. Linkage disequilibrium among these SNPs was evaluated using the 1000 Genomes Project European panels as the reference population [30]. Instrumental SNPs for the exposure absent in the outcome datasets were proxied using SNPs in high linkage disequilibrium (r2 > 0.8), where possible. To mitigate the risk of Type I errors, we established a threshold where p-values for the outcome association must exceed 5 × 10−5 [31]. This criterion helps ensure that our genetic instruments are valid and minimizes the risk of pleiotropic effects that could bias the causal estimates.
2.5. Strength of instrumental variables
The strength of the instrumental variables was assessed using the F-statistic, calculated with the formula: , where R2 represents the proportion of variance in the exposure that is explained by the instrumental variables, and n denotes the sample size [32]. An F-value >10 is generally considered indicative of a strong instrument, suggesting that our selected genetic variants provide reliable estimates of the causal effects [33]. The R2 was calculated using the formula: , where MAF is the minor allele frequency and β2 is the estimated effect size of the genetic variant on the exposure [34].
2.6. Statistical analysis
The inverse-variance weighted (IVW) model was chosen as the primary MR analysis method and was applied independently to each cohort [35]. The fixed-effects meta-analysis method was used to combine the odds ratios (OR) estimates from different sources for a single endpoint [36]. To enhance the rigor of our analysis, we conducted further tests under more stringent assumptions. First, we utilized the constrained maximum likelihood (cML) method, which maximizes the likelihood function while ensuring that most genetic variants serve as valid instruments, thus effectively minimizing the impact of pleiotropy [37]. Second, we used the debiased inverse-variance weighted (dIVW) method to adjust for biases. This method provides a more precise estimation of causal effects and corrects for any imbalances and heterogeneity observed among the instruments [38]. Third, the weighted median method in MR utilizes the median estimate of the causal effects from multiple genetic instruments. Each instrument's strength weights these estimates, offering a robust estimate that is less influenced by outliers or invalid instruments [39]. To account for the multiple hypothesis testing inherent in assessing 13 cardiovascular outcomes, a Bonferroni correction was applied to adjust the p-value threshold. The conventional significance threshold of 0.05 was divided by the number of outcomes, resulting in a corrected p-value threshold of 0.0038 [40]. This adjusted threshold was used to determine statistical significance across all outcome variables to control for the risk of Type I errors. We considered p-values between 0.0038 and 0.05 to be suggestive, while p-values below 0.0038 were considered statistically significant. In our MR analysis, we first calculated Cochran's Q statistic to quantify heterogeneity across instrumental variables [41]. To assess potential directional pleiotropy, we utilized the MR-Egger method. A statistically significant intercept in this analysis suggests a violation of the instrumental variable assumptions attributable to directional pleiotropy [42]. Additionally, we applied the MR pleiotropy residual sum and outlier (MR-PRESSO) method, specifically designed to reduce estimate heterogeneity by excluding SNPs that disproportionately contribute to it, setting the NbDistribution at 3000 [43]. Furthermore, we implemented the RadialMR method to systematically identify and exclude outliers that could distort causal inference [44]. To ensure the validity of our instruments, we conducted Steiger-filtering analysis, which identifies and removes genetic variants exhibiting a stronger association with the outcome than with the exposure, thus indicating potential reverse causality [45]. All analyses were performed using the TwoSampleMR (version 0.5.7), MendelianRandomization (version 0.9.0), MRPRESSO (1.0), RadialMR (1.1) and psych (2.3.9) package in R Software 4.3.1 (https://www.R-project.org).
3. Results
3.1. Initial causal analysis between SS and CVD
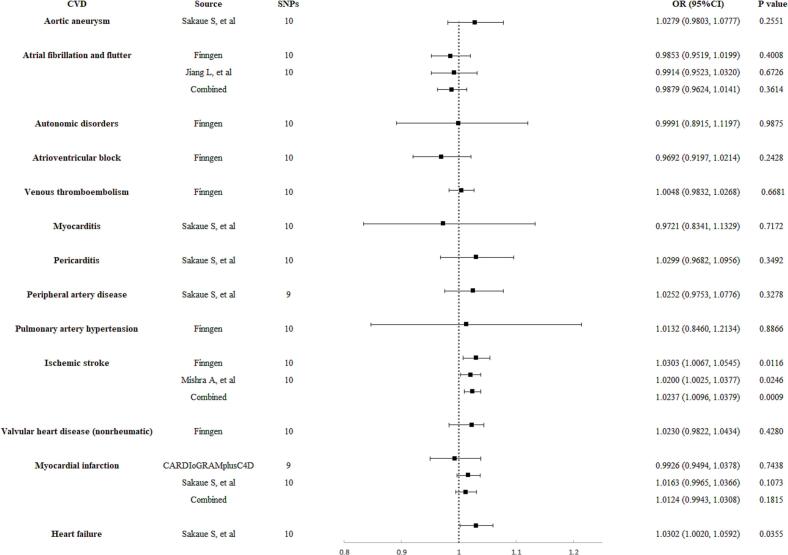
The relationships between SS and 13 CVD outcomes, as predicted by eleven selected SNPs, are demonstrated in Supplementary Table S3. Genetically predicted SS showed a significant causal association with ischemic stroke and a suggestive causal association with chronic heart failure. According to the IVW method results, a one-unit increase in the log-odds of genetically predicted SS was associated with increased OR for these conditions: 1.0237 (95 % CI 1.0096 to 1.0379, p = 0.0009) for ischemic stroke, and 1.0302 (95 % CI 1.0020 to 1.0592, p = 0.0355) for chronic heart failure (Fig. 2). Conversely, no significant causal associations were observed between genetically predicted SS and other types of CVD, including aortic aneurysm, atrial fibrillation and flutter, autonomic disorders, atrioventricular block, venous thromboembolism, myocarditis, pericarditis, pulmonary artery hypertension, peripheral artery disease, non-rheumatic valvular heart disease, and myocardial infarction (Fig. 2). The F-statistics for the instrumental variables used to estimate SS all exceeded 10, indicating a low likelihood of weak instrument bias.
Fig. 2.
Initial causal analysis of genetically predicted Sjögren's syndrome with risk of cardiovascular disease. OR, odds ratio; SNPs, single-nucleotide polymorphisms; CI, confidence interval; CVD, cardiovascular disease.
3.2. Sensitivity analysis
The application of cML, dIVW, and weighted median methods has strengthened the evidence for robust causal relationships between SS and several CVD outcomes. The IVW method revealed a significant causal relationship between SS and ischemic stroke, with the dIVW and weighted median methods showing consistent results (OR 1.0243, 95 % CI 1.0108 to 1.0379, p = 0.0004; OR 1.0291, 95 % CI 1.0135 to 1.0450, p = 0.0002, respectively). In contrast, the cML method only revealed a suggestive causal relationship (OR 1.0214, 95 % CI 1.0049 to 1.0381, p = 0.0107). For heart failure, the dIVW method showed results consistent with the IVW method, but both the cML and weighted median methods did not indicate a significant or suggestive causal relationship (OR 1.0266, 95 % CI 0.9992 to 1.0548, p = 0.0572; OR 1.0222, 95 % CI 0.9916 to 1.0537, p = 0.1572, respectively) (Supplementary Table S3).
The Cochran's Q test indicated significant heterogeneity in the SNP effect estimates for various cardiovascular conditions, with I2 values exceeding 30 % (Supplementary Table S4). These conditions include atrial fibrillation and flutter, atrioventricular block, myocarditis, ischemic stroke, non-rheumatic valvular heart disease, myocardial infarction, and heart failure. While most studies showed no directional pleiotropy in the MR-Egger analysis, the MR-PRESSO method identified three outlier SNPs (rs3135394, rs3757387, rs7210219) specifically associated with atrial fibrillation and flutter, indicating significant pleiotropy (Global Test p < 0.05). Further analysis with RadialMR across different studies pinpointed several outlier SNPs in conditions such as atrial fibrillation and flutter, atrioventricular block, venous thromboembolism, myocarditis, ischemic stroke, non-rheumatic valvular heart disease, myocardial infarction, and heart failure (Supplementary Table S5).
3.3. Secondary analysis between SS and CVD post-outlier correction
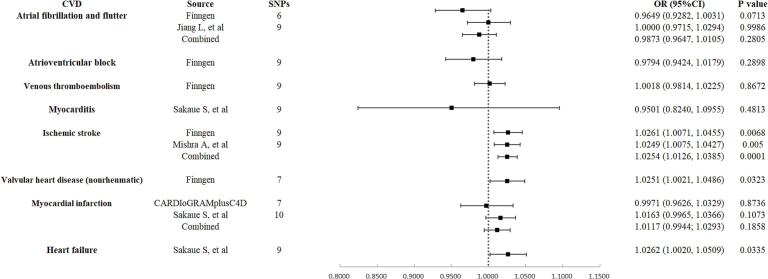
After removing outlier SNPs identified by MR-PRESSO and RadialMR methods in various studies, a secondary analysis was conducted to reaffirm the causal relationships between SS and CVD outcomes. Genetically predicted SS continued to demonstrate a significant causal association with ischemic stroke (Supplementary Table S6). Using the IVW method, it was found that a one-unit increase in the log-odds of genetically predicted SS was associated with increased ORs for ischemic stroke (OR 1.0254, 95 % CI 1.0126 to 1.0385, p = 0.0001). Similarly, genetically predicted SS also demonstrate a suggestive causal association with chronic heart failure (OR 1.0262, 95 % CI 1.0020 to 1.0509, p = 0.0335). These results are consistent with those from the initial analysis. The main distinction in this analysis is the suggestive association found between genetically predicted SS and non-rheumatic valvular heart disease (OR 1.0251, 95 % CI 1.0021 to 1.0486, p = 0.0323). No significant associations were observed between SS and other types of CVD (Fig. 2, Fig. 3).
Fig. 3.
Secondary causal analysis of genetically predicted Sjögren's syndrome with risk of cardiovascular disease post-outlier correction. OR, odds ratio; SNPs, single-nucleotide polymorphisms; CI, confidence interval; CVD, cardiovascular disease.
For ischemic stroke, results from cML, dIVW, and weighted median methods aligned with those from the IVW method, underscoring the robustness of the positive genetic associations. Unlike the IVW method, the cML and weighted median methods did not reveal a suggestive association with chronic heart failure (OR 1.0238, 95 % CI 0.9979 to 1.0504, p = 0.0717; OR 1.0209, 95 % CI 0.9920 to 1.0508, p = 0.1585, respectively). Similarly, the cML method did not identify a suggestive association with non-rheumatic valvular heart disease (OR 1.0213, 95 % CI 0.9915 to 1.0520, p = 0.1624). Across all studies, the Cochran's Q test showed no significant heterogeneity in SNP effects, and the MR-Egger analysis detected no evidence of directional pleiotropy, thus confirming the reliability of the genetic instruments used. Furthermore, for the three outcomes with positive genetic predictions, the Steiger-filtering analysis excluded the possibility of reverse causality between SS and these conditions, affirming the directionality of the associations from SS to these cardiovascular outcomes (Supplementary Table S7).
4. Discussion
Leveraging a comprehensive genetic consortium, this MR study explored the potential causal effects of SS on a range of CVD. The evidence from our preliminary analysis indicates that SS has a significant detrimental causal relationship with ischemic stroke and a suggestive detrimental causal relationship with chronic heart failure. After addressing heterogeneity and pleiotropy, the results for these two cardiovascular outcomes remain robust, with a strong causal link observed for ischemic stroke and a suggestive association for chronic heart failure. Additionally, SS was found to potentially exert a detrimental causal effect on non-rheumatic valvular heart disease. The outcomes are significantly robust, supported by strong instrumental variables and diverse MR analysis techniques that accommodate various scenarios of horizontal pleiotropy. Furthermore, we conducted the Steiger test to assess the positive causal effects of SS on the three cardiovascular outcomes, finding no evidence of reverse causality.
To our knowledge, this is the first MR investigating the causal relationship between genetically predicted SS and various types of CVD. Currently, associations between SS and CVD primarily rely on evidence from cohort and cross-sectional studies, which may be influenced by confounding factors. For instance, a retrospective cohort study by Cai X et al. indicated that patients with SS have a higher propensity for CVD. Their analysis of 367 SS patients and an equal number of age and gender-matched controls, revealing significantly higher rates of cardiovascular involvement in SS patients (61.6 % vs. 29.7 %; p < 0.01) [46]. Additionally, Santos CS et al. conducted a 20-year observational study on 102 SS patients, finding that at least 36 % developed one type of CVD, such as cerebrovascular disease, coronary artery disease, peripheral artery disease, and arrhythmias [47]. These studies offer preliminary evidence of a link between SS and specific types of CVD. Nevertheless, due to the low prevalence of SS and the associated costs, no RCTs have been conducted to further explore this relationship. In a multicenter cohort study, Bartoloni E et al. analyzed 788 SS patients and 4774 controls, discovering that SS patients were significantly more likely to suffer myocardial infarctions (1.0 % vs 0.4 %, p = 0.002) and cerebrovascular diseases (2.5 % vs 1.4 %, p = 0.005) compared to controls [7]. This study, which included a large cohort of SS patients, exhibited less bias, and its findings align with our MR study results. Furthermore, a meta-analysis by Beltai A et al., encompassing 14 studies and 67,124 SS patients, indicated that SS patients have a 2.54-fold increased risk of heart failure compared to controls. Although this risk rate is higher than that what was observed in our MR study, it supports the suggestive causal link between SS and heart failure [12]. However, current research linking SS with heart valve disease is limited; a case report suggests that SS may impact the mitral and aortic valves, yet robust clinical evidence remains scarce [48]. Our MR study, after adjusting for outlier SNPs, suggests a potential causal relationship between SS and non-rheumatic heart valve disease. However, due to limited clinical evidence, this conclusion must be approached with caution.
This MR study did not identify causal links between genetically predicted SS and additional ten types of CVD, contrasting with findings from earlier cohort and observational studies. For example, a nationwide cohort study reported that SS patients are at a significantly increased risk of aortic dissection or aneurysm, with those having secondary SS facing a higher risk (adjusted HR = 1.753, p = 0.042; secondary SS adjusted HR = 3.693, p < 0.001) compared to the general population [49]. Furthermore, studies suggests that SS patients are up to seven times more likely to develop venous thromboembolism, particularly in the first-year post-diagnosis when disease management may be suboptimal [[50], [51], [52]]. However, the risk of developing venous thromboembolism is not uniform among all SS patients. A meta-analysis revealed that SS patients exhibited the widest confidence interval for the incidence of venous thromboembolism compared to patients with other autoimmune diseases [52]. Lung involvement is not uncommon in patients with SS, with small airway and interstitial lung disease being the most frequently reported pulmonary manifestations [53]. Recent research further indicates that patients with SS experience a higher hospitalization rate for pulmonary hypertension compared to the general population, underscoring it as a rare but severe complication [14,52]. Additionally, dysfunction of the autonomic nervous system, which plays a crucial role in regulating the cardiovascular system, is prevalent among SS patients and significantly impacts their quality of life [54]. In a cohort study involving patients with SS, 35.7 % exhibited autonomic dysfunction as assessed by reduced heart rate variability [55]. Autoimmune diseases including SS have also been reported to develop other cardiovascular conditions such as atrial fibrillation, atrial flutter, atrioventricular block, pericarditis, and myocarditis [[56], [57], [58]]. However, it is important to emphasize that our MR study results do not confirm a causal relationship between SS and these types of CVD. This suggests that traditional cohort or observational studies may be influenced by numerous confounding factors, such as lifestyle, environmental influences, or unmeasured genetic predispositions, which could lead to an apparent increased incidence of certain cardiovascular conditions among SS patients.
From a pathophysiological perspective, SS can increase the risk of CVD through various pathways. As a chronic autoimmune disease, SS involves systemic immune dysregulation that contributes significantly to the pathogenesis of early arteriosclerosis [59,60]. Notably, anti-SSA/Ro and anti-SSB/La antibodies, well-known biomarkers of the disease, may contribute to endothelial dysfunction and increased intimal-medial thickness. These changes impair vascular contractile function and promote a pro-inflammatory state, thereby elevating the risk of myocardial infarction, stroke, and peripheral artery disease [61,62]. Moreover, in SS, activated T cells can infiltrate vascular and cardiac tissues, releasing pro-inflammatory cytokines. These cytokines not only directly damage myocardial and vascular cells but also exacerbate cardiovascular inflammation and injury by activating other immune cells, such as macrophages [63]. Furthermore, studies have indicated that patients with SS have elevated levels of ICAM-1 and VCAM-1 adhesion molecules, which facilitate leukocyte recruitment to the vascular wall. This recruitment leads to infiltration and atherosclerotic damage [61]. Hematological abnormalities, such as leukopenia and lymphopenia, are common in patients with SS and are considered biomarkers of disease activity. Specifically, leukopenia is associated with vascular injury in SS, characterized by large vessel endothelial-independent dysfunction and increased intimal-medial thickness [61]. Studies have shown that patients with leukopenia face a sixfold increased risk of angina, often in the absence of traditional cardiovascular risk factors [7]. Additionally, SS involves abnormal activation of B cells and T cells, which leads to increased production of various cytokines such as interleukin-1β (IL-1β) and IL-6, further perpetuating inflammation. Elevated levels of IL-1β, IL-6, and C-reactive protein are known to promote atherosclerosis [63]. Notably, IL-1β is crucial for understanding the cardiovascular risk in SS patients, as its levels are higher in those with metabolic syndrome [64]. In SS, chronic immune-mediated inflammatory responses in the arteries cause increased arterial stiffness, which contributes to cardiac diastolic dysfunction, a primary mechanism that leads to heart failure [65]. The causal relationships between SS and conditions such as ischemic stroke, chronic heart failure, and non-rheumatic valvular heart disease observed in this MR study can be attributed to the immune and inflammatory mechanisms of SS. It is crucial to recognize that the pathophysiological mechanisms of SS not only impact the vascular system but also directly affect the cardiac organ itself. Cardiac fibrosis, although less studied in SS, carries significant clinical implications. Autoantibodies in SS patients, particularly anti-myocardial antibodies, may directly damage myocardial cells, leading to cell death or dysfunction and promoting fibrosis. These autoantibodies might also interact with specific components within cardiac tissue, activating fibrosis-related signaling pathways. While direct clinical studies on SS and cardiac fibrosis are limited, findings such as enhanced late gadolinium enhancement on cardiac MRI exams in SS patients suggest the presence of cardiac fibrosis [66]. Furthermore, electrocardiographic abnormalities like bradycardia and conduction blocks, which are prevalent in SS patients, could be related to subtle changes in cardiac structure and function [67]. However, more common arrhythmias such as atrial fibrillation, atrial flutter, and atrioventricular block appear to result from multifactorial influences, and this MR study does not establish a causal association between SS and these arrhythmias. This summary of the pathophysiological mechanisms of SS and the results of this MR study emphasize the necessity of addressing early signs of arteriosclerosis in SS patients to prevent major cardiovascular events such as chronic heart failure, ischemic stroke.
Following the MR guidelines, this study conducted on a broad population provides strong or suggestive evidence of a direct causal link between SS and various cardiovascular conditions including ischemic stroke, heart failure, and non-rheumatic valvular heart disease [68]. After the removal of outlier SNPs and addressing heterogeneity and pleiotropy effects using MR-PRESSO and RadialMR methods, the associations remain significant. To ensure rigorous control of Type I errors, a Bonferroni correction was applied due to the assessment of multiple outcomes. Following correction, only the association between SS and ischemic stroke remained statistically significant. This highlights the robustness of this particular finding, while suggesting that the other associations, such as those with chronic heart failure and non-rheumatic valvular heart disease, may be weaker or more variable. Unlike conventional observational studies, which often focus on short-term impacts, MR designs estimate lifetime exposure to SS, yielding outcomes that are free from confounding factors and reverse causation. Adhering to MR guidelines, this study clarifies the causal connections between genetic predisposition to SS and several types of CVD, providing substantial research benefits. A principal advantage of the MR approach is its capacity to eliminate potential residual confounding and reverse causation from observational studies, thus enhancing the reliability of causal inferences. Additionally, by limiting our sample to Europeans, we minimized potential bias due to ethnic diversity. Another strength of this study lies in its use of data from diverse sources to estimate the causal links between genetically predicted SS and CVD. This approach ensures the authenticity and robustness of our findings and significantly reduces the effects of population stratification. Furthermore, we applied the Steiger test to strengthen the robustness of the instrumental variables, effectively ruling out potential reverse causality between the exposure and the outcomes.
It is undeniable that this MR study has numerous limitations, and its findings should be interpreted with caution within the context of these limitations and the broader framework of MR methodology. Firstly, although we selected instrumental variables closely related to SS, genetic variants only represent a portion of the disease complexity and are not fully representative of exposure. Secondly, the available GWAS data on SS is limited, the sample size for genetic prediction is small, and there is no differentiation between primary and secondary SS. Future MR studies should incorporate more SS GWAS summary statistics to improve the robustness of findings. Thirdly, given that SS is treated as a binary exposure, traditional MR analysis may yield indistinguishable relative risk values. This is because, although the risk boundaries can be established, precise causal effects might remain elusive [69]. Fourthly, the biological roles of the genetic instruments used are not fully understood, raising concerns about potential violations of the MR assumptions of independence and exclusion, particularly concerning pleiotropy. However, we have implemented various methods to validate the robustness of the causal estimations. Fifthly, while the RadialMR method employed removes outlier SNPs and enhances the robustness of positive findings, it may over-adjust the data, potentially eliminating genetic variations critical for establishing causality. This over-adjustment could dilute genuine signals, introducing biases [44]. Sixthly, MR analysis reflects the effects of lifelong exposure to SS, which might have a more pronounced impact than that observed in time-limited observational studies. Therefore, further verification of the causal link between SS and CVD through RCTs is necessary. Finally, since our findings predominantly involve individuals of European ancestry, they may not be applicable to other populations.
5. Conclusion
This two-sample MR study supports the hypothesis that SS may be associated with the development of certain types of CVD. We found significant evidence of an adverse causal relationship between SS and ischemic stroke, along with suggestive evidence of a possible adverse causal relationship between SS and chronic heart failure, as well as non-rheumatic valvular heart disease. However, further research is required to confirm these associations and explore the relationship between SS and other types of CVD.
Ethical statement
This Mendelian randomization study uses publicly available summary-level data from previously published genome-wide association studies (GWAS), including data from the FinnGen study. All original GWAS received ethical approval from their respective institutional review boards and participants provided informed consent. No new data collection was performed specifically for this study. Therefore, additional ethical approval and informed consent were not required.
Funding
This work was supported by the Changzhou Sci & Tech Program (CJ20220165), Young Talent Development Plan of Changzhou Health Commission (CZQM2022027), and Changzhou Longcheng Medical Star Health Youth Sci & Tech Talent Support Project.
CRediT authorship contribution statement
Chen Su: Writing – original draft. Xiaobo Zhu: Software, Formal analysis, Data curation. Qiang Wang: Software, Methodology. Feng Jiang: Software, Methodology. Junjie Zhang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We want to acknowledge the participants and investigators of the FinnGen study. Data on myocardial infarction have been contributed by CARDIoGRAMplusC4D investigators and have been downloaded from www.CARDIOGRAMPLUSC4D.ORG. We also express our gratitude to Lessard CJ, et al., Li H, et al., Taylor KE, et al., Khatri B, et al., Sakaue S, et al., Jiang L, et al., and Mishra A, et al. for making the data used in this study publicly available.
Footnotes
Supplementary 1: Table S1. GWAS summary statistics for Sjögren's syndrome and cardiovascular disease. Supplementary 2: Definition/Diagnostic Criteria of 13 types of outcomes. Supplementary 3: Table S3. Initial causal analysis of cardiovascular disease on Sjögren's syndrome. Supplementary 4: Table S4. The results of heterogeneity analysis on Mendelian randomization. Supplementary 5: Table S5. The results of horizontal pleiotropy analysis on Mendelian randomization. Supplementary 6: Table S6. Secondary causal analysis of cardiovascular disease on Sjögren's syndrome post-outlier correction. Supplementary 7: Table S7. The Steiger-filtering analysis of Sjögren's syndrome and cardiovascular disease with positive causality. Supplementary 8: Fig. S1. Scatter plots depicting the results of four Mendelian randomization analysis methods used to evaluate the positive causal relationship between Sjögren's syndrome and cardiovascular disease. Supplementary 9: Fig. S2. Forest plots depicting the sensitivity outcomes of leave one out analysis of positive causal relationship between Sjögren's syndrome and cardiovascular disease. Supplementary 10: Fig. S3. Funnel plots depicting the distribution of SNPs from harmonized data in cardiovascular disease with positive causal associations with Sjögren's syndrome. Supplementary 11: Fig. S4. Radial plots depicting outlier SNPs analyzed using the Inverse Variance Weighted and MR-Egger methods in cardiovascular disease with positive causal associations with Sjögren's syndrome. Supplementary data to this article can be found online at [doi:https://doi.org/10.1016/j.ahjo.2024.100482].
Appendix A. Supplementary data
Supplementary material
Data availability
Information on how to obtain summary-level data from the FinnGen consortium is available at https://finngen.gitbook.io/documentation/. Summary statistics were downloaded from the NHGRI-EBI GWAS Catalog for study GCST004879, GCST012796, GCST90043977, GCST90104540. The CARDIoGRAMplusC4D Consortium is available at https://www.cardiogramplusc4d.org/data-downloads/. Other summary statistics from the IEU OpenGWAS project is available at https://gwas.mrcieu.ac.uk/datasets/.
References
- 1.Thomas H., Diamond J., Vieco A., Chaudhuri S., Shinnar E., Cromer S., et al. Global atlas of cardiovascular disease 2000-2016: the path to prevention and control. Glob. Heart. 2018;13(3):143–163. doi: 10.1016/j.gheart.2018.09.511. [DOI] [PubMed] [Google Scholar]
- 2.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmis A., Townsend N., Gale C.P., Torbica A., Lettino M., Petersen S.E., et al. European Society of Cardiology: cardiovascular disease statistics 2019. Eur. Heart J. 2020;41(1):12–85. doi: 10.1093/eurheartj/ehz859. [DOI] [PubMed] [Google Scholar]
- 4.Stefanski A.L., Tomiak C., Pleyer U., Dietrich T., Burmester G.R., Dorner T. The diagnosis and treatment of Sjogren’s syndrome. Dtsch. Arztebl. Int. 2017;114(20):354–361. doi: 10.3238/arztebl.2017.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolstad A.I., Skarstein K. Epidemiology of Sjogren’s syndrome-from an oral perspective. Curr. Oral Health Rep. 2016;3(4):328–336. doi: 10.1007/s40496-016-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izmirly P.M., Buyon J.P., Wan I., Belmont H.M., Sahl S., Salmon J.E., et al. The incidence and prevalence of adult primary Sjogren’s syndrome in New York County. Arthritis Care Res. 2019;71(7):949–960. doi: 10.1002/acr.23707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartoloni E., Baldini C., Schillaci G., Quartuccio L., Priori R., Carubbi F., et al. Cardiovascular disease risk burden in primary Sjogren’s syndrome: results of a population-based multicentre cohort study. J. Intern. Med. 2015;278(2):185–192. doi: 10.1111/joim.12346. [DOI] [PubMed] [Google Scholar]
- 8.Atzeni F., Gozza F., Cafaro G., Perricone C., Bartoloni E. Cardiovascular involvement in Sjogren’s syndrome. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.879516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X.F., Huang J.Y., Chiou J.Y., Chen H.H., Wei J.C., Dong L.L. Increased risk of coronary heart disease among patients with primary Sjogren’s syndrome: a nationwide population-based cohort study. Sci. Rep. 2018;8(1):2209. doi: 10.1038/s41598-018-19580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melissaropoulos K., Bogdanos D., Dimitroulas T., Sakkas L.I., Kitas G.D., Daoussis D. Primary Sjogren’s syndrome and cardiovascular disease. Curr. Vasc. Pharmacol. 2020;18(5):447–454. doi: 10.2174/1570161118666200129125320. [DOI] [PubMed] [Google Scholar]
- 11.Beydon M., McCoy S., Nguyen Y., Sumida T., Mariette X., Seror R. Epidemiology of Sjogren syndrome. Nat. Rev. Rheumatol. 2024;20(3):158–169. doi: 10.1038/s41584-023-01057-6. [DOI] [PubMed] [Google Scholar]
- 12.Beltai A., Barnetche T., Daien C., Lukas C., Gaujoux-Viala C., Combe B., et al. Cardiovascular morbidity and mortality in primary Sjogren’s syndrome: a systematic review and meta-analysis. Arthritis Care Res. 2020;72(1):131–139. doi: 10.1002/acr.23821. [DOI] [PubMed] [Google Scholar]
- 13.Yong W.C., Sanguankeo A., Upala S. Association between primary Sjogren’s syndrome, cardiovascular and cerebrovascular disease: a systematic review and meta-analysis. Clin. Exp. Rheumatol. 2018;36 Suppl 112(3):190–197. [PubMed] [Google Scholar]
- 14.Goulabchand R., Roubille C., Montani D., Fesler P., Bourdin A., Malafaye N., et al. Cardiovascular events, sleep apnoea, and pulmonary hypertension in primary Sjogren’s syndrome: data from the French health insurance database. J. Clin. Med. 2021;10(21) doi: 10.3390/jcm10215115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alunno A., Carubbi F., Mariani F.M., Martini C., Campanozzi E., Ferri C. The interplay between cardiovascular risk, cardiovascular events, and disease activity in primary Sjogren’s syndrome: is uric acid the missing link? Nutrients. 2023;15(7) doi: 10.3390/nu15071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J., Baird D., Borges M.C., Bowden J., Hemani G., Haycock P., et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson E., Glymour M.M., Holmes M.V., Kang H., Morrison J., Munafo M.R., et al. Mendelian randomization. Nat Rev Methods Primers. 2022:2. doi: 10.1038/s43586-021-00092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K., Lim C.Y. Mendelian randomization analysis in observational epidemiology. J Lipid Atheroscler. 2019;8(2):67–77. doi: 10.12997/jla.2019.8.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S., Small D.S., Thompson S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017;26(5):2333–2355. doi: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emdin C.A., Khera A.V., Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 21.Lessard C.J., Li H., Adrianto I., Ice J.A., Rasmussen A., Grundahl K.M., et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat. Genet. 2013;45(11):1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., Reksten T.R., Ice J.A., Kelly J.A., Adrianto I., Rasmussen A., et al. Identification of a Sjogren’s syndrome susceptibility locus at OAS1 that influences isoform switching, protein expression, and responsiveness to type I interferons. PLoS Genet. 2017;13(6) doi: 10.1371/journal.pgen.1006820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor K.E., Wong Q., Levine D.M., McHugh C., Laurie C., Doheny K., et al. Genome-wide association analysis reveals genetic heterogeneity of Sjogren’s syndrome according to ancestry. Arthritis Rheum. 2017;69(6):1294–1305. doi: 10.1002/art.40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khatri B., Tessneer K.L., Rasmussen A., Aghakhanian F., Reksten T.R., Adler A., et al. Genome-wide association study identifies Sjogren’s risk loci with functional implications in immune and glandular cells. Nat. Commun. 2022;13(1):4287. doi: 10.1038/s41467-022-30773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurki M.I., Karjalainen J., Palta P., Sipila T.P., Kristiansson K., Donner K.M., et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–518. doi: 10.1038/s41586-022-05473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsworth B, Lyon M, Alexander T, Liu Y, Matthews P, Hallett J, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020:2020.08.10.244293.
- 27.Sollis E., Mosaku A., Abid A., Buniello A., Cerezo M., Gil L., et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2023;51(D1) doi: 10.1093/nar/gkac1010. D977-D85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schunkert H., Konig I.R., Kathiresan S., Reilly M.P., Assimes T.L., Holm H., et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011;43(4):333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaue S., Kanai M., Tanigawa Y., Karjalainen J., Kurki M., Koshiba S., et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021;53(10):1415–1424. doi: 10.1038/s41588-021-00931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke L., Zheng-Bradley X., Smith R., Kulesha E., Xiao C., Toneva I., et al. The 1000 genomes project: data management and community access. Nat. Methods. 2012;9(5):459–462. doi: 10.1038/nmeth.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng Y., Tan Z., Su Y., Li L., Chen C. Causal association between common rheumatic diseases and glaucoma: a Mendelian randomization study. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1227138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess S., Thompson S.G., Collaboration CCG Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011;40(3):755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 33.Palmer T.M., Lawlor D.A., Harbord R.M., Sheehan N.A., Tobias J.H., Timpson N.J., et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012;21(3):223–242. doi: 10.1177/0962280210394459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv X., Hu Z., Liang F., Liu S., Gong H., Du J., et al. Causal relationship between ischemic stroke and its subtypes and frozen shoulder: a two-sample Mendelian randomization analysis. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1178051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess S., Scott R.A., Timpson N.J., Davey Smith G., Thompson S.G., Consortium E.-I. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015;30(7):543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue H., Shen X., Pan W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects. Am. J. Hum. Genet. 2021;108(7):1251–1269. doi: 10.1016/j.ajhg.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye T., Shao J., Kang H. Debiased inverse-variance weighted estimator in two-sample summary-data Mendelian randomization. Ann. Stat. 2021;49(4) doi: 10.1002/sim.10245. 2079-100, 22. [DOI] [PubMed] [Google Scholar]
- 39.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedgwick P. STATISTICAL QUESTION multiple hypothesis testing and Bonferroni’s correction. Bmj-Brit Med J. 2014:349. doi: 10.1136/bmj.g6284. [DOI] [PubMed] [Google Scholar]
- 41.Burgess S., Butterworth A., Thompson S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowden J., Spiller W., Del Greco M.F., Sheehan N., Thompson J., Minelli C., et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int. J. Epidemiol. 2018;47(4):1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemani G., Tilling K., Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11) doi: 10.1371/journal.pgen.1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai X., Luo J., Wei T., Qin W., Wang X., Li X. Risk of cardiovascular involvement in patients with primary Sjogren’s syndrome: a large-scale cross-sectional cohort study. Acta Reumatol. Port. 2019;44(1):71–77. [PubMed] [Google Scholar]
- 47.Santos C.S., Salgueiro R.R., Morales C.M., Castro C.A., Alvarez E.D. Risk factors for cardiovascular disease in primary Sjogren’s syndrome (pSS): a 20-year follow-up study. Clin. Rheumatol. 2023;42(11):3021–3031. doi: 10.1007/s10067-023-06686-6. [DOI] [PubMed] [Google Scholar]
- 48.Bridge K., Farivar R.S. A case of Sjogren’s syndrome leading to mitral and aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2010;139(6):e139–e140. doi: 10.1016/j.jtcvs.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 49.Tsai Y.D., Chien W.C., Tsai S.H., Chung C.H., Chu S.J., Chen S.J., et al. Increased risk of aortic aneurysm and dissection in patients with Sjogren’s syndrome: a nationwide population-based cohort study in Taiwan. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2018-022326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avina-Zubieta J.A., Jansz M., Sayre E.C., Choi H.K. The risk of deep venous thrombosis and pulmonary embolism in primary Sjogren syndrome: a general population-based study. J. Rheumatol. 2017;44(8):1184–1189. doi: 10.3899/jrheum.160185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung W.S., Lin C.L., Sung F.C., Hsu W.H., Chen Y.F., Kao C.H. Increased risks of deep vein thrombosis and pulmonary embolism in Sjogren syndrome: a nationwide cohort study. J. Rheumatol. 2014;41(5):909–915. doi: 10.3899/jrheum.131345. [DOI] [PubMed] [Google Scholar]
- 52.Lee J.J., Pope J.E. A meta-analysis of the risk of venous thromboembolism in inflammatory rheumatic diseases. Arthritis Res. Ther. 2014;16(5):435. doi: 10.1186/s13075-014-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flament T., Bigot A., Chaigne B., Henique H., Diot E., Marchand-Adam S. Pulmonary manifestations of Sjogren’s syndrome. Eur. Respir. Rev. 2016;25(140):110–123. doi: 10.1183/16000617.0011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies K., Ng W.F. Autonomic nervous system dysfunction in primary Sjogren’s syndrome. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.702505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh J.H., Kwok S.K., Lee J., Park S.H. Autonomic dysfunction in primary Sjogren’s syndrome: a prospective cohort analysis of 154 Korean patients. Korean J. Intern. Med. 2017;32(1):165–173. doi: 10.3904/kjim.2015.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehtesham M., Fortune K., Shabbir M.A., Peredo-Wende R. Sjogren syndrome presenting as atrioventricular block in an adult. BMJ Case Rep. 2022;15(4) doi: 10.1136/bcr-2021-247337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bucci T., Cardamone C., Triggiani M., Ames P.R.J., Lip G.Y.H. Risk of death, thrombotic and hemorrhagic events in anticoagulated patients with atrial fibrillation and systemic autoimmune diseases: an analysis from a global federated dataset. Clin. Res. Cardiol. 2024;113(6):942–950. doi: 10.1007/s00392-024-02426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tilly M.J., Geurts S., Zhu F., Bos M.M., Ikram M.A., de Maat M.P.M., et al. Autoimmune diseases and new-onset atrial fibrillation: a UK Biobank study. Europace. 2023;25(3):804–811. doi: 10.1093/europace/euac244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 60.Shoenfeld Y., Sherer Y., Harats D. Artherosclerosis as an infectious, inflammatory and autoimmune disease. Trends Immunol. 2001;22(6):293–295. doi: 10.1016/s1471-4906(01)01922-6. [DOI] [PubMed] [Google Scholar]
- 61.Gerli R., Vaudo G., Bocci E.B., Schillaci G., Alunno A., Luccioli F., et al. Functional impairment of the arterial wall in primary Sjogren’s syndrome: combined action of immunologic and inflammatory factors. Arthritis Care Res. 2010;62(5):712–718. doi: 10.1002/acr.20117. [DOI] [PubMed] [Google Scholar]
- 62.Vaudo G., Bocci E.B., Shoenfeld Y., Schillaci G., Wu R., Del Papa N., et al. Precocious intima-media thickening in patients with primary Sjogren’s syndrome. Arthritis Rheum. 2005;52(12):3890–3897. doi: 10.1002/art.21475. [DOI] [PubMed] [Google Scholar]
- 63.Chivasso C., Sarrand J., Perret J., Delporte C., Soyfoo M.S. The involvement of innate and adaptive immunity in the initiation and perpetuation of Sjogren’s syndrome. Int. J. Mol. Sci. 2021;22(2) doi: 10.3390/ijms22020658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Augusto K.L., Bonfa E., Pereira R.M., Bueno C., Leon E.P., Viana V.S., et al. Metabolic syndrome in Sjogren’s syndrome patients: a relevant concern for clinical monitoring. Clin. Rheumatol. 2016;35(3):639–647. doi: 10.1007/s10067-015-3072-1. [DOI] [PubMed] [Google Scholar]
- 65.Berger M., Fesler P., Roubille C. Arterial stiffness, the hidden face of cardiovascular risk in autoimmune and chronic inflammatory rheumatic diseases. Autoimmun. Rev. 2021;20(9) doi: 10.1016/j.autrev.2021.102891. [DOI] [PubMed] [Google Scholar]
- 66.Nishiwaki A., Kobayashi H., Ikumi N., Kobayashi Y., Yokoe I., Sugiyama K., et al. Salivary gland focus score is associated with myocardial fibrosis in primary Sjogren syndrome assessed by a cardiac magnetic resonance approach. J. Rheumatol. 2021;48(6):859–866. doi: 10.3899/jrheum.200352. [DOI] [PubMed] [Google Scholar]
- 67.Tam W.K., Hsu H.C., Hsieh M.H., Yeh J.S., Tam W.C. Association of anti-Ro/Sjogren’s syndrome type a antibodies and complete atrioventricular block in an adult with Sjogren’s syndrome. Arch Rheumatol. 2018;33(2):225–229. doi: 10.5606/ArchRheumatol.2018.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burgess S., Davey Smith G., Davies N.M., Dudbridge F., Gill D., Glymour M.M., et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019;4:186. doi: 10.12688/wellcomeopenres.15555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burgess S., Labrecque J.A. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur. J. Epidemiol. 2018;33(10):947–952. doi: 10.1007/s10654-018-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Information on how to obtain summary-level data from the FinnGen consortium is available at https://finngen.gitbook.io/documentation/. Summary statistics were downloaded from the NHGRI-EBI GWAS Catalog for study GCST004879, GCST012796, GCST90043977, GCST90104540. The CARDIoGRAMplusC4D Consortium is available at https://www.cardiogramplusc4d.org/data-downloads/. Other summary statistics from the IEU OpenGWAS project is available at https://gwas.mrcieu.ac.uk/datasets/.