Abstract
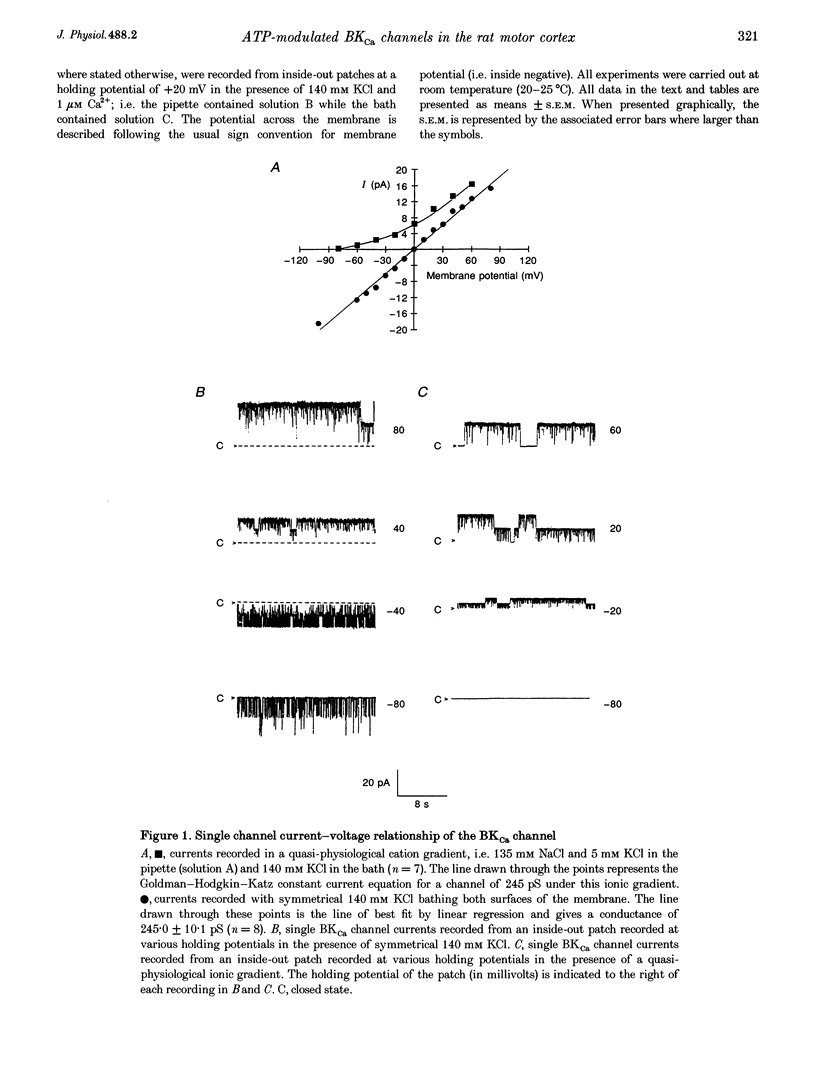
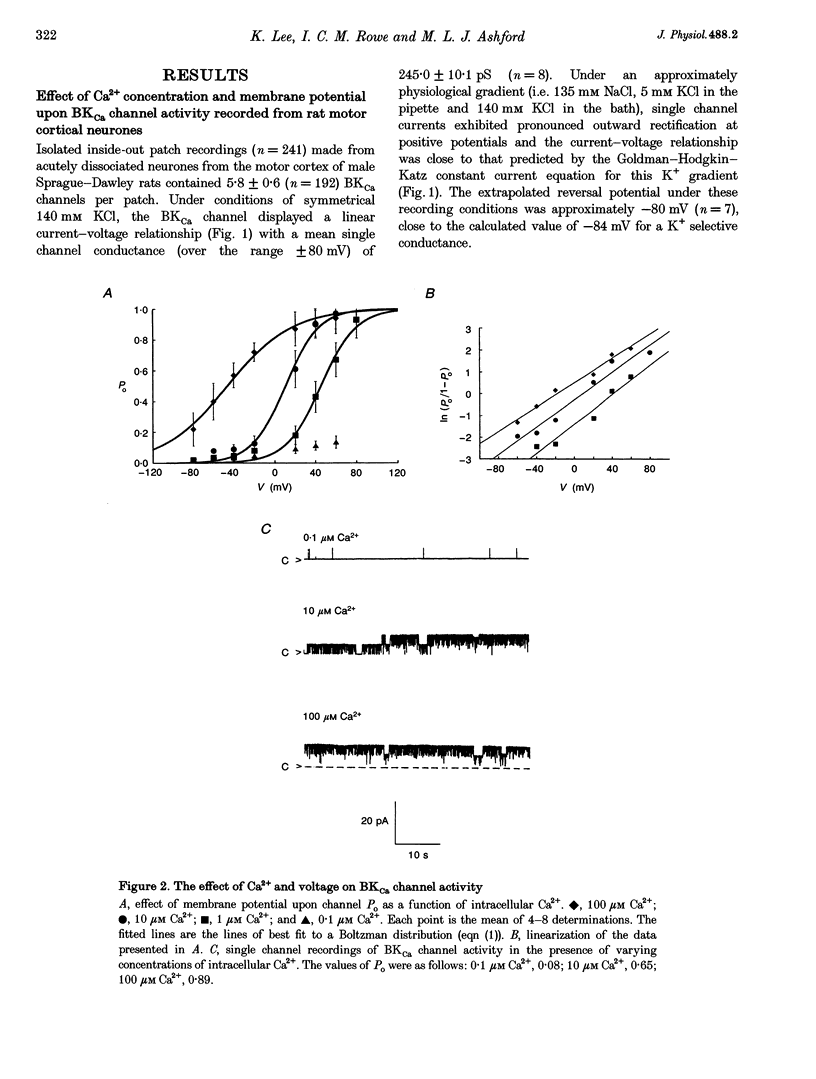
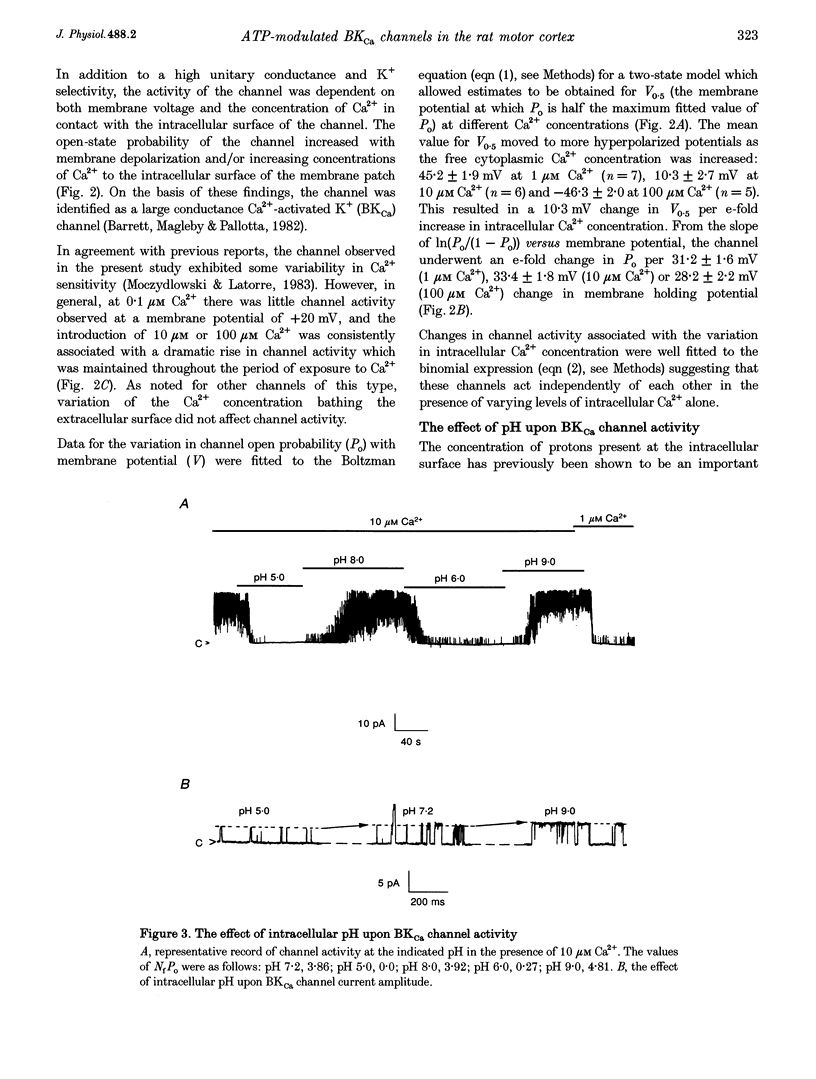
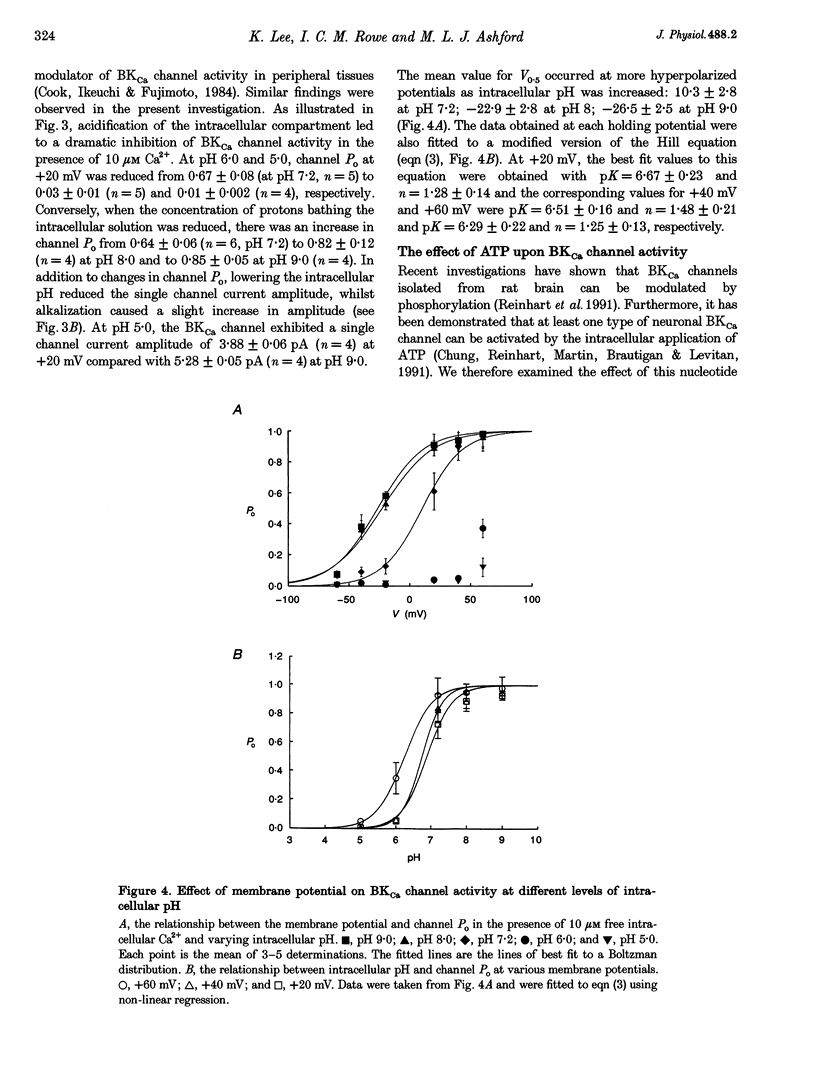
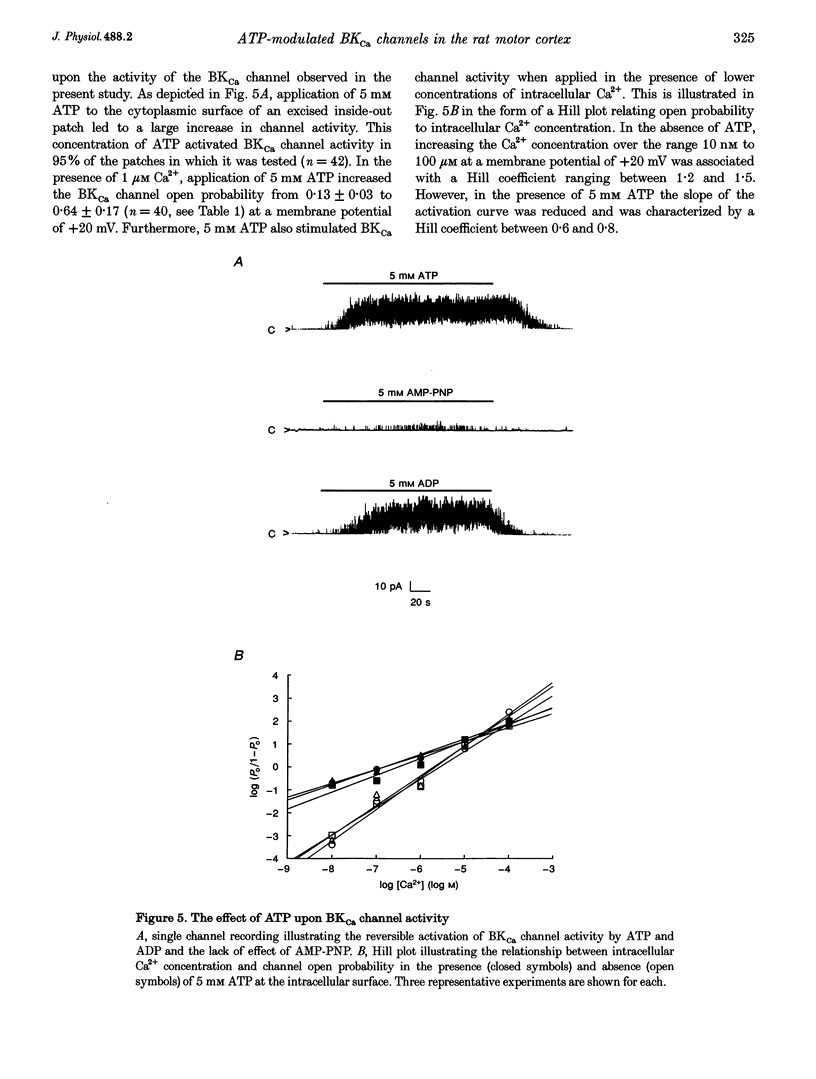
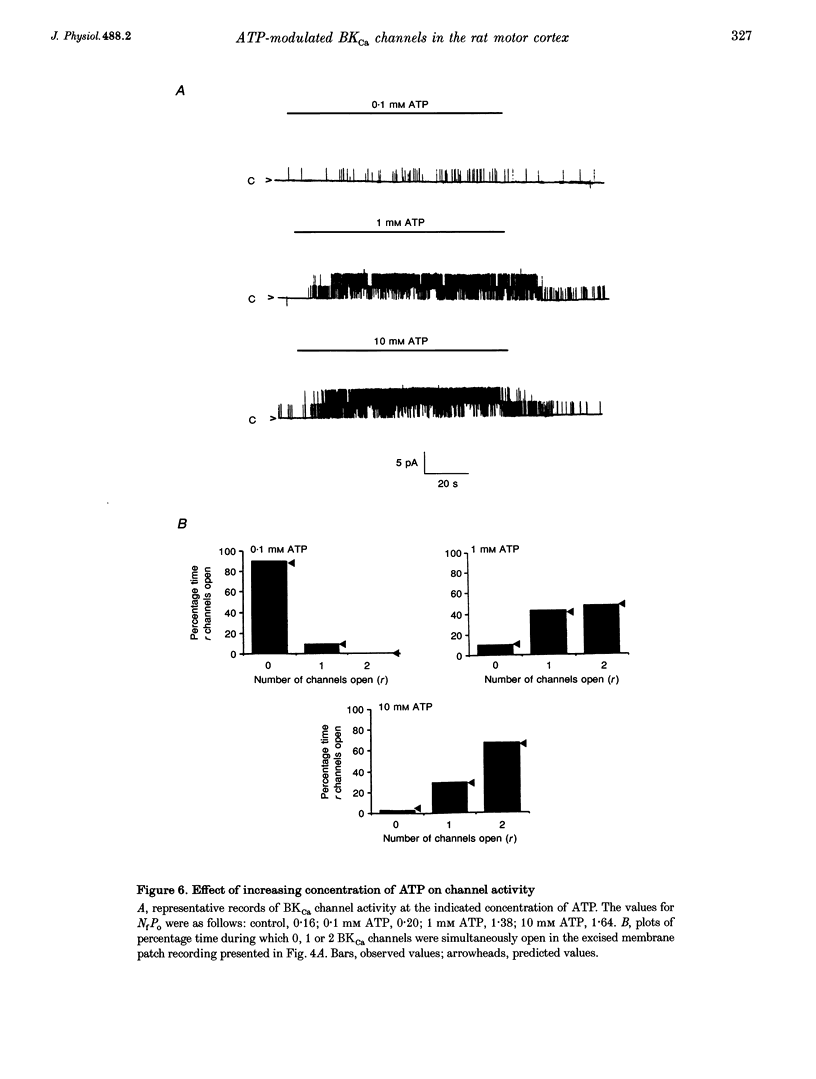
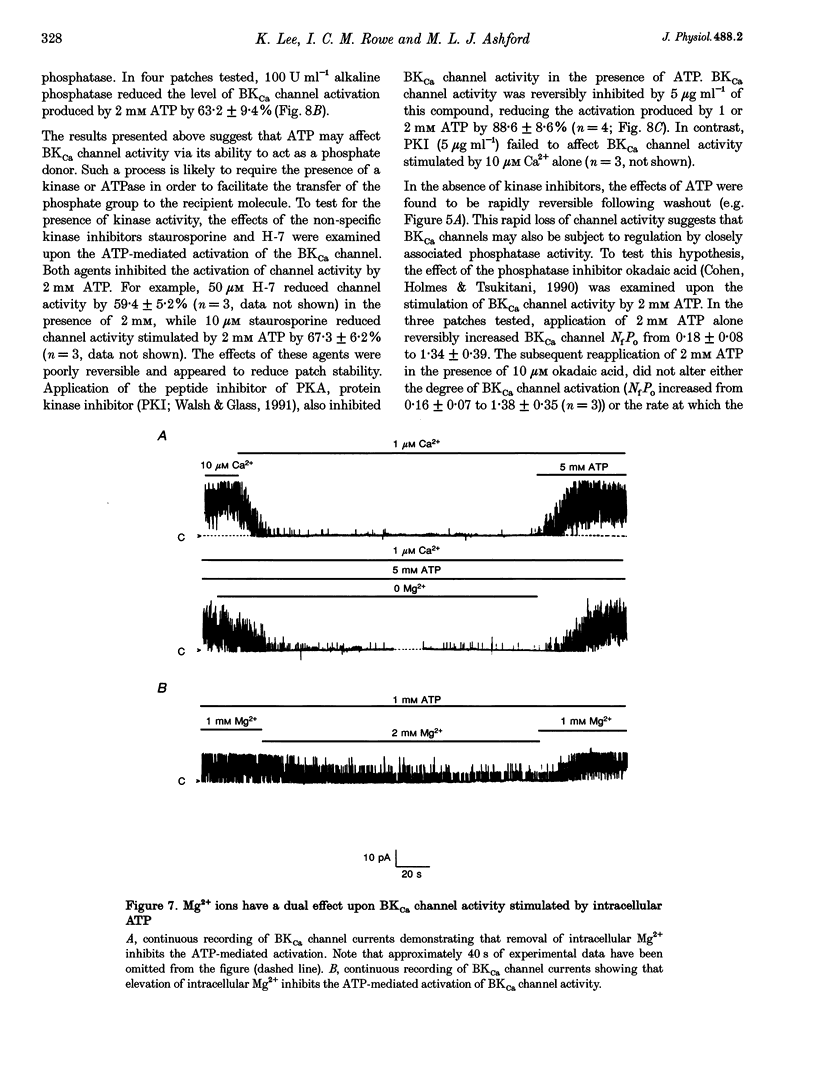
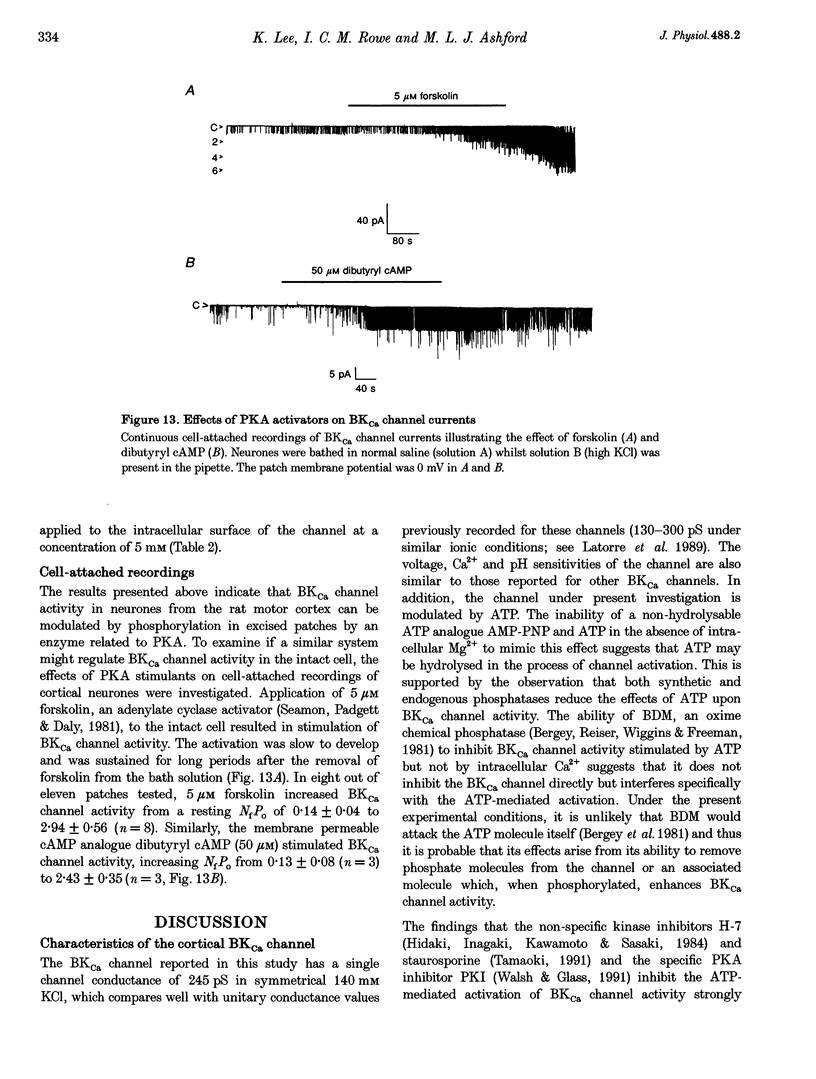
1. Single channel current recordings were used to study the characteristics of a large conductance Ca(2+)-activated K+ (BKCa) channel present in neurones acutely dissociated from the rat motor cortex. Application of ATP to the intracellular surface of excised inside-out patches produced a large, concentration-dependent increase in BKCa channel activity. 2. This ATP-mediated activation was dependent upon the presence of Mg2+ in the intracellular bathing solution and was diminished by the phosphatases 2,3-butanedione monoxime (BDM) or alkaline phosphatase and by the protein kinase inhibitors staurosporine, H-7 and PKI. 3. ADP stimulated BKCa channel activity in a Mg(2+)-dependent manner, an action also inhibited by the concomitant application of PKI or BDM. The effect of ADP was reduced by application of hexokinase and glucose or by application of the adenylate kinase inhibitor Ap5A. 4. Of other nucleotides tested, only CTP consistently activated BKCa channel activity. 5. Using the cell-attached configuration, bath application of forskolin or dibutyryl cAMP stimulated BKCa channel activity. 6. It is concluded that BKCa channel activity in the rat motor cortex is subject to modulation by the activity of a closely associated kinase. The ability of cAMP activators to stimulate BKCa channel activity in the intact cell suggests that this system may be of physiological importance.
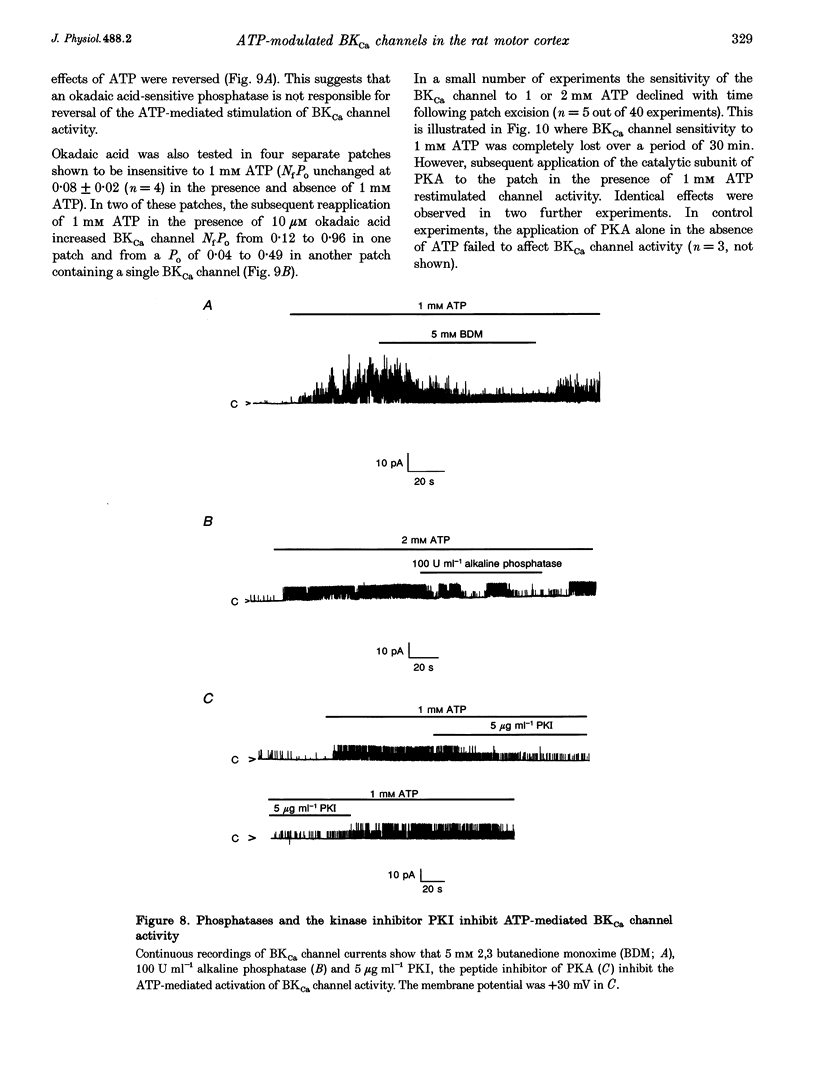
Full text
PDF


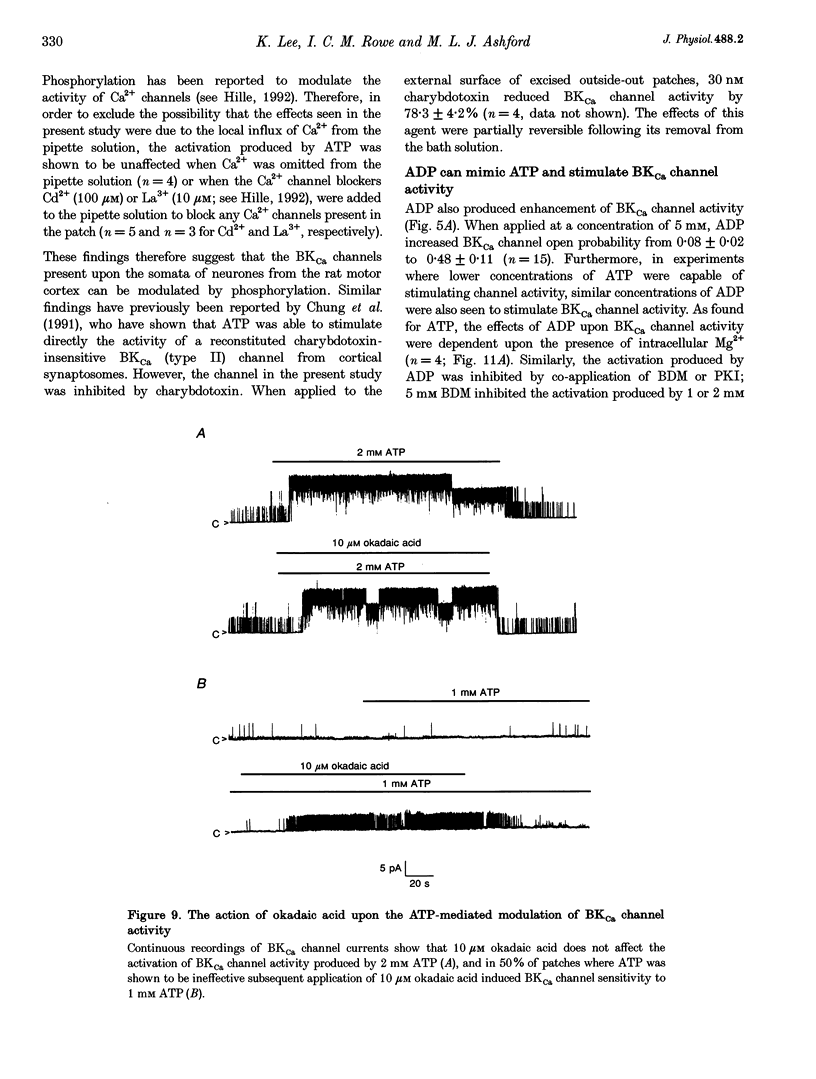
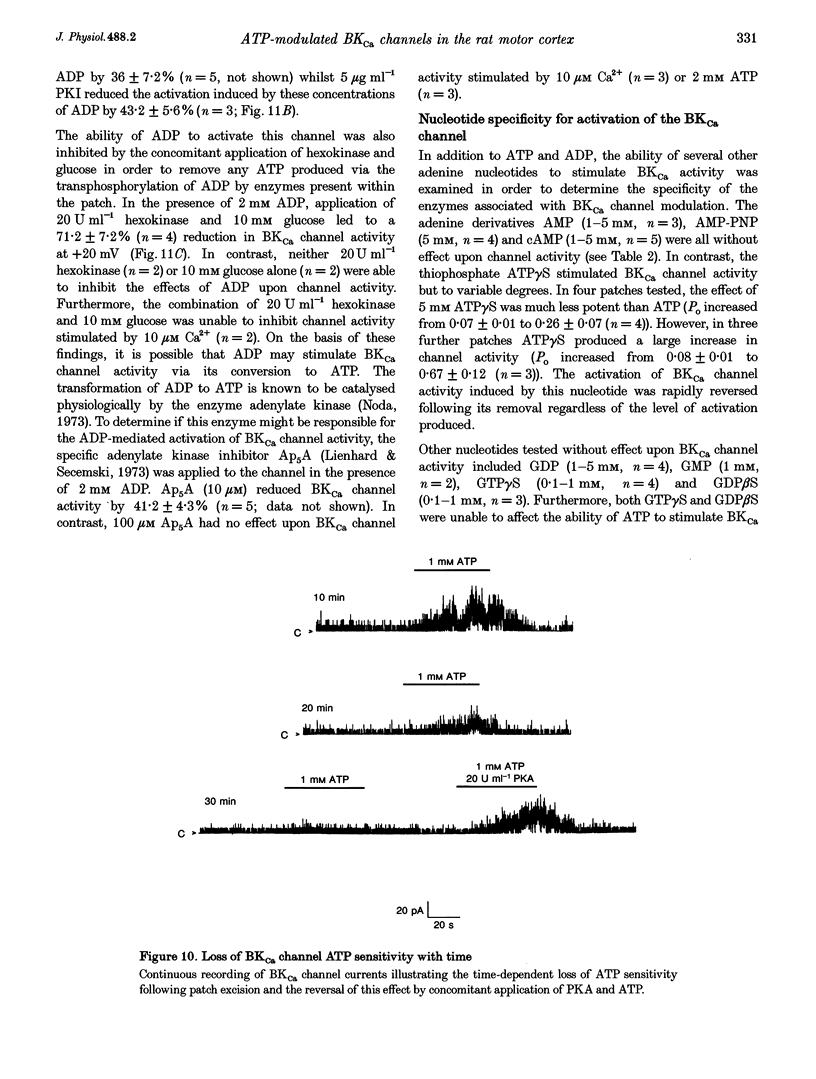
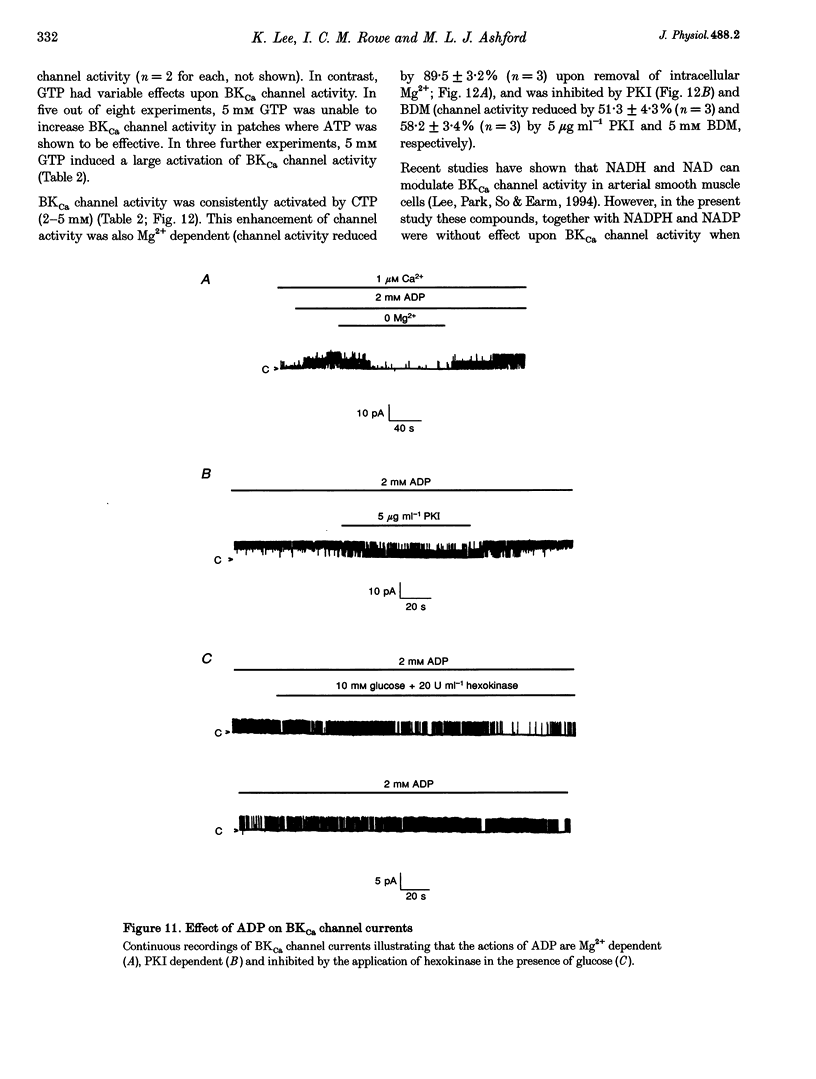
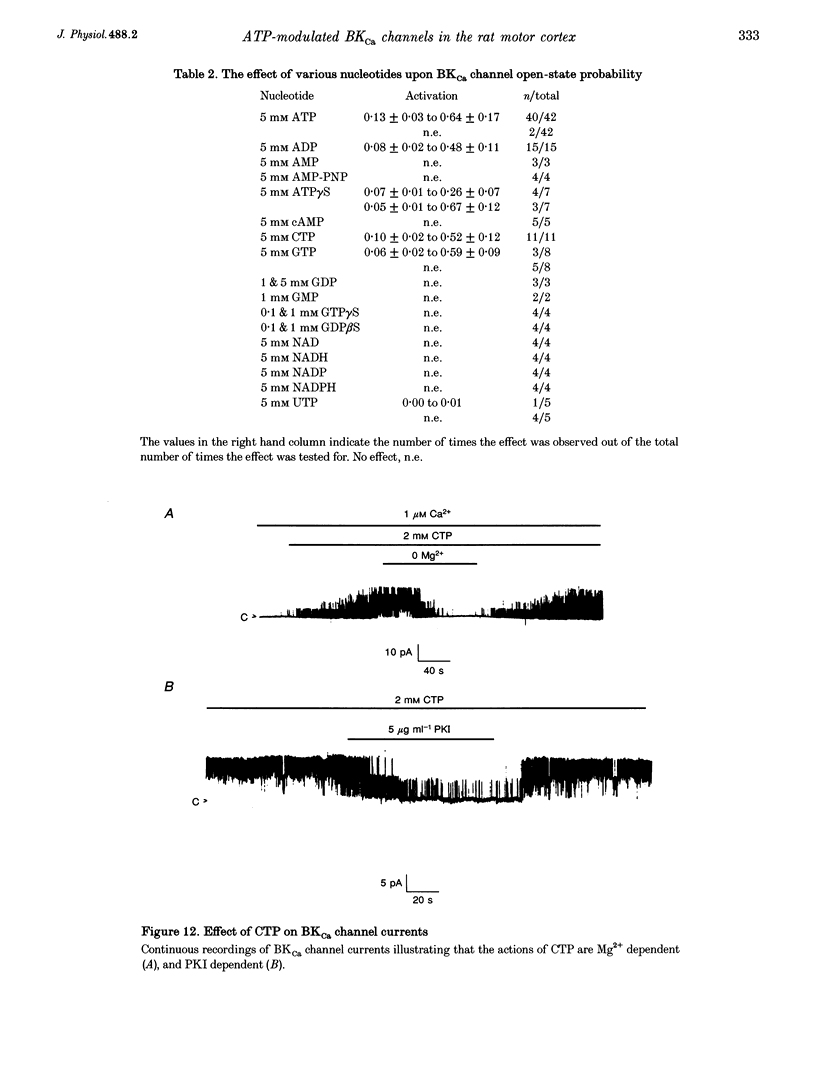



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford M. L., Boden P. R., Treherne J. M. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990 Jan;415(4):479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey J. L., Reiser J., Wiggins J. R., Freeman A. R. Oximes: 'enzymatic' slow channel antagonists in canine cardiac purkinje fibers? Eur J Pharmacol. 1981 May 8;71(2-3):307–319. doi: 10.1016/0014-2999(81)90033-9. [DOI] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Valdeolmillos M., O'Neill S. C., Eisner D. A. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol. 1990 May;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Silver I. A. Ions and energy in mammalian brain. Prog Neurobiol. 1994 May;43(1):37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Farley J., Rudy B. Multiple types of voltage-dependent Ca2+-activated K+ channels of large conductance in rat brain synaptosomal membranes. Biophys J. 1988 Jun;53(6):919–934. doi: 10.1016/S0006-3495(88)83173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lee S., Park M., So I., Earm Y. E. NADH and NAD modulates Ca(2+)-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflugers Arch. 1994 Jun;427(3-4):378–380. doi: 10.1007/BF00374548. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan T. K., Criss W. E. Three major forms of adenylate kinase from adult and fetal rat tissues. Enzyme. 1976;21(4):327–331. doi: 10.1159/000458875. [DOI] [PubMed] [Google Scholar]
- Rehm H., Pelzer S., Cochet C., Chambaz E., Tempel B. L., Trautwein W., Pelzer D., Lazdunski M. Dendrotoxin-binding brain membrane protein displays a K+ channel activity that is stimulated by both cAMP-dependent and endogenous phosphorylations. Biochemistry. 1989 Jul 25;28(15):6455–6460. doi: 10.1021/bi00441a044. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Levitan I. B. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989 Jan;2(1):1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991 Jun;11(6):1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Kanaide H., Nakamura M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am J Physiol. 1988 Oct;255(4 Pt 2):H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T. Use and specificity of staurosporine, UCN-01, and calphostin C as protein kinase inhibitors. Methods Enzymol. 1991;201:340–347. doi: 10.1016/0076-6879(91)01030-6. [DOI] [PubMed] [Google Scholar]
- Wacker W. E. The biochemistry of magnesium. Ann N Y Acad Sci. 1969 Aug 15;162(2):717–726. doi: 10.1111/j.1749-6632.1969.tb13003.x. [DOI] [PubMed] [Google Scholar]
- Wiemann S., Kinzel V., Pyerin W. Isoform C beta 2, an unusual form of the bovine catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1991 Mar 15;266(8):5140–5146. [PubMed] [Google Scholar]


