Abstract
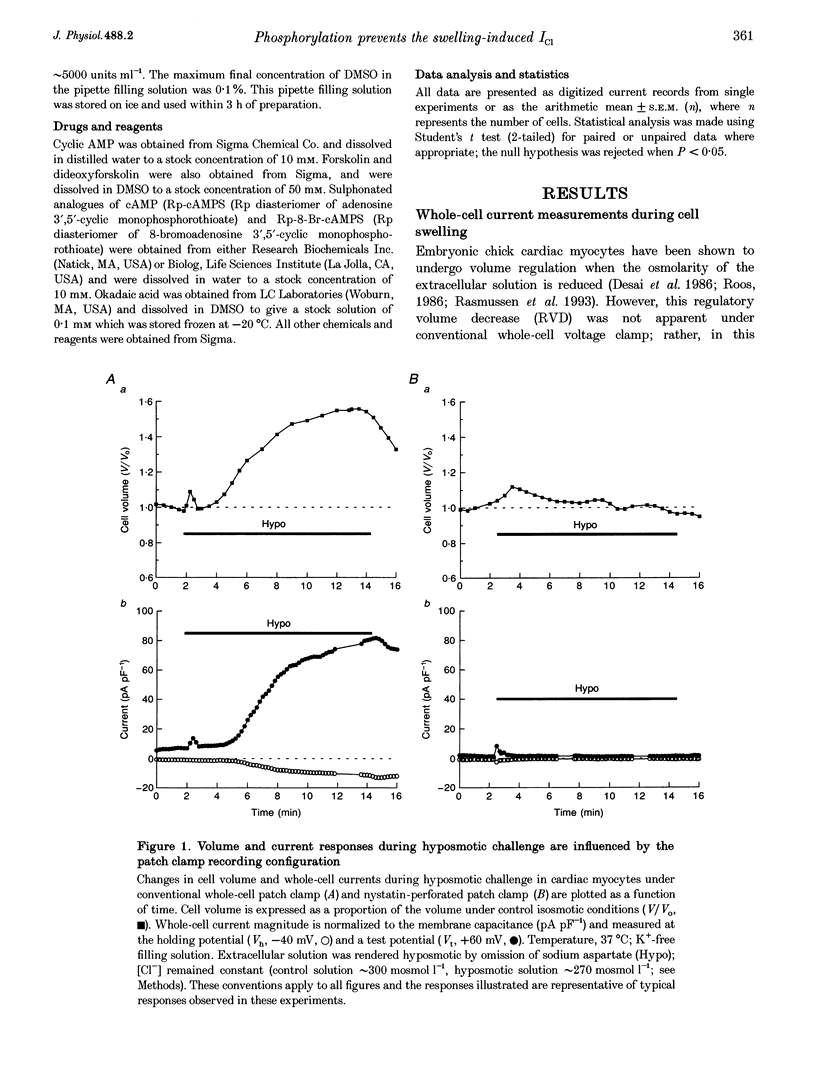
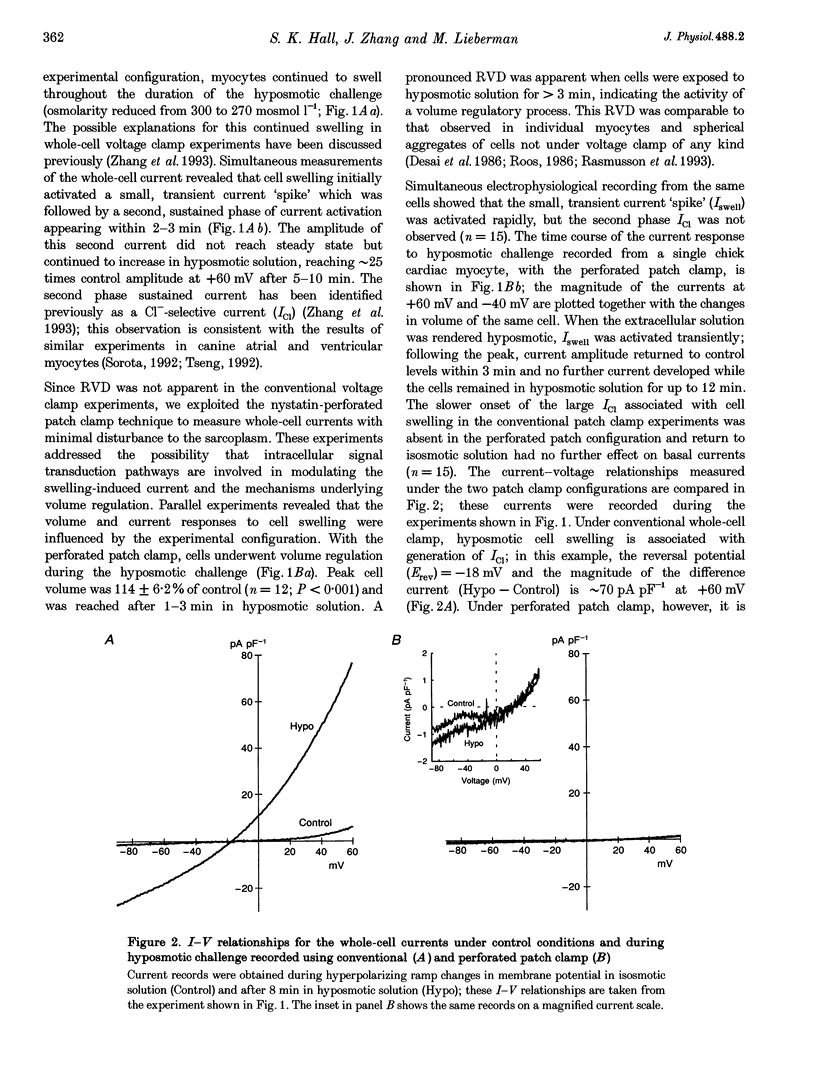
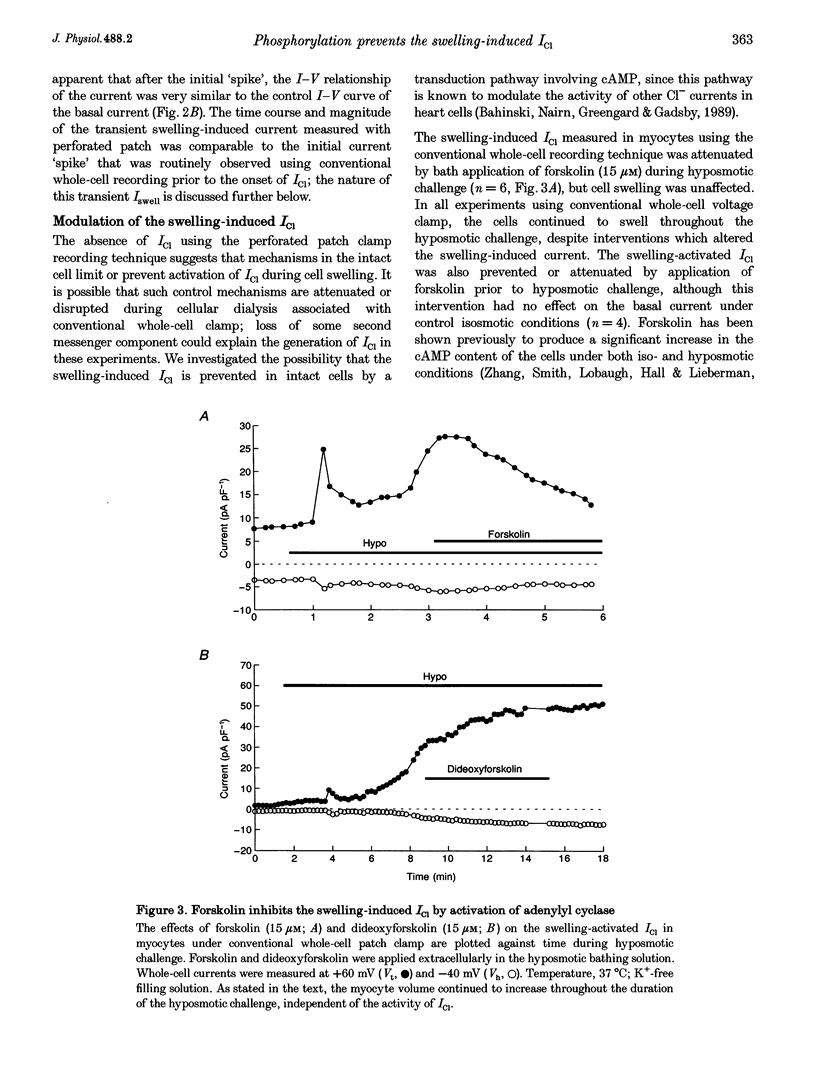
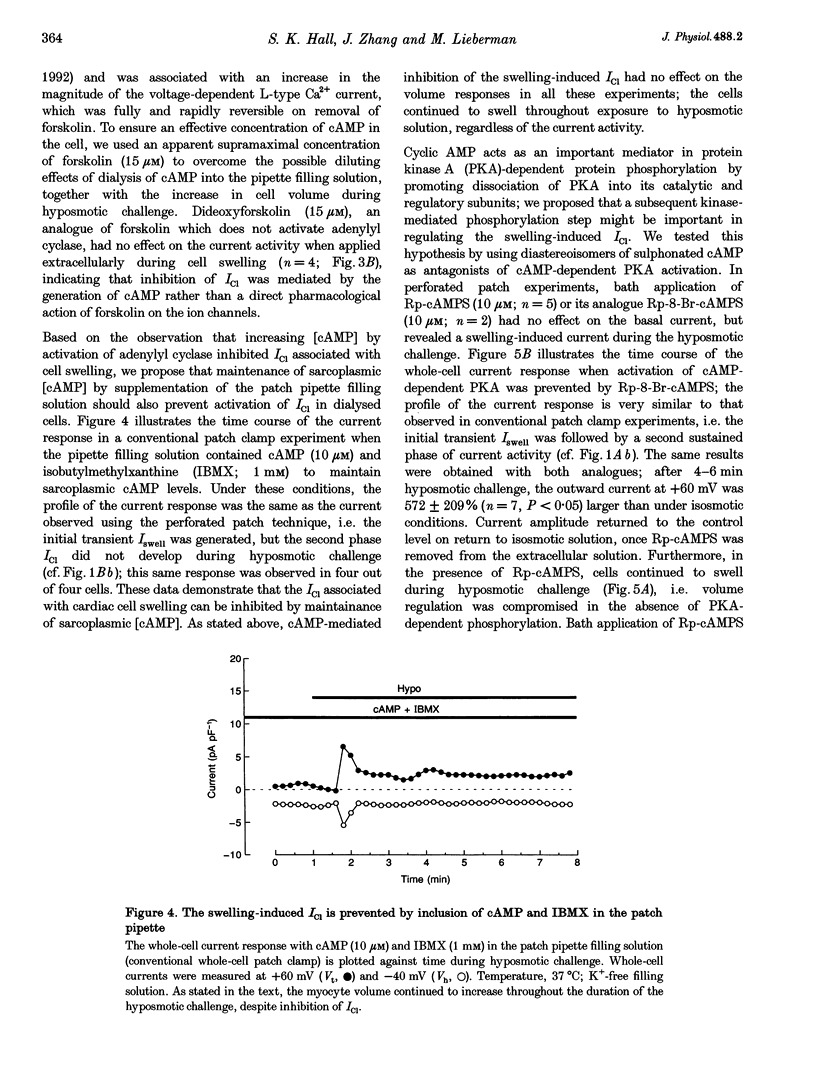
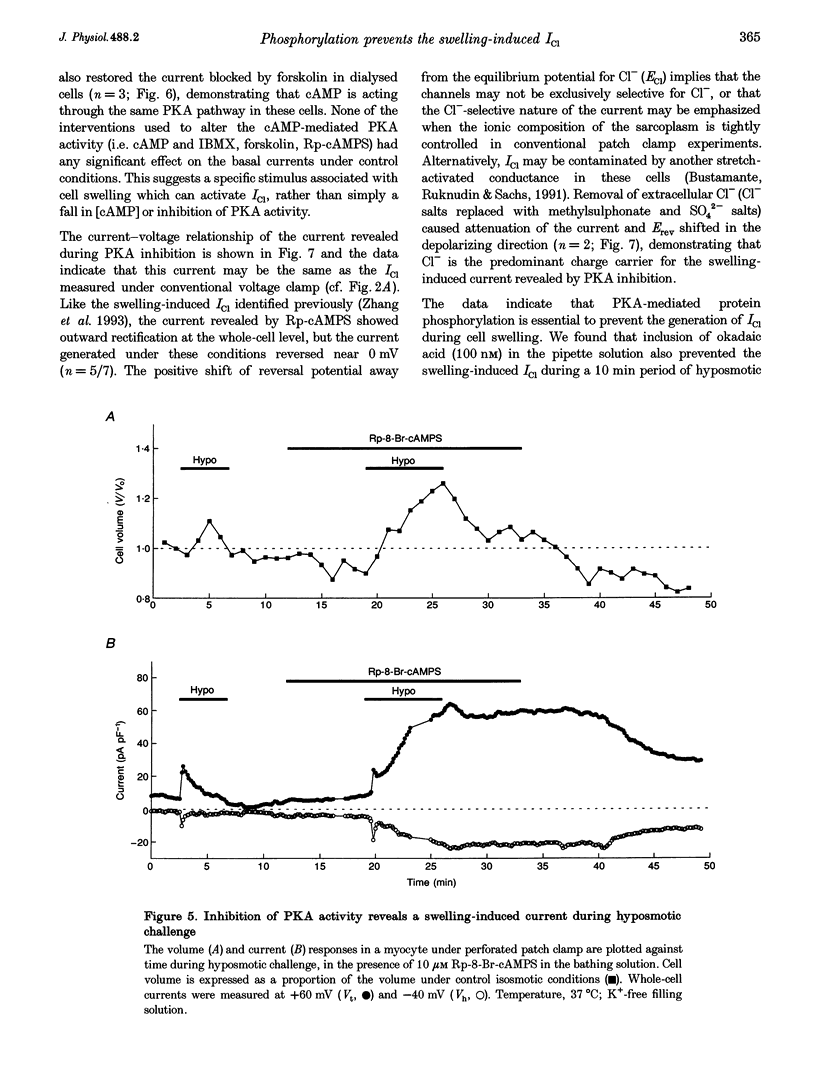
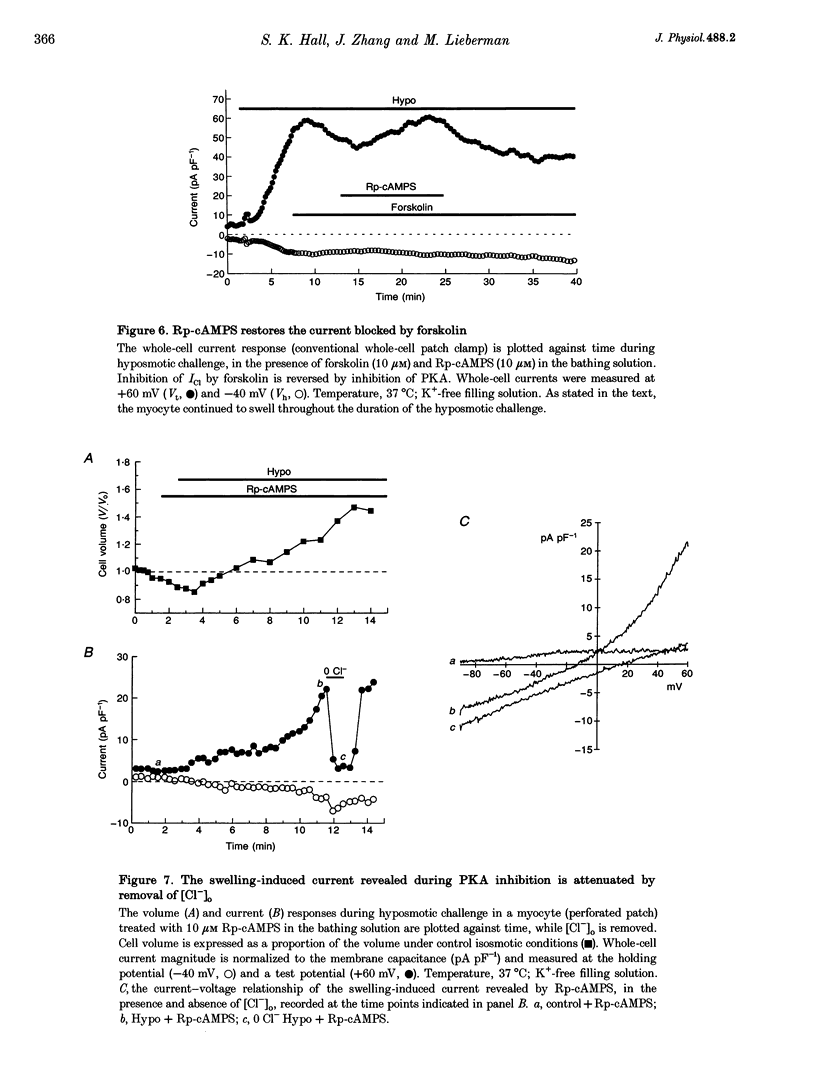
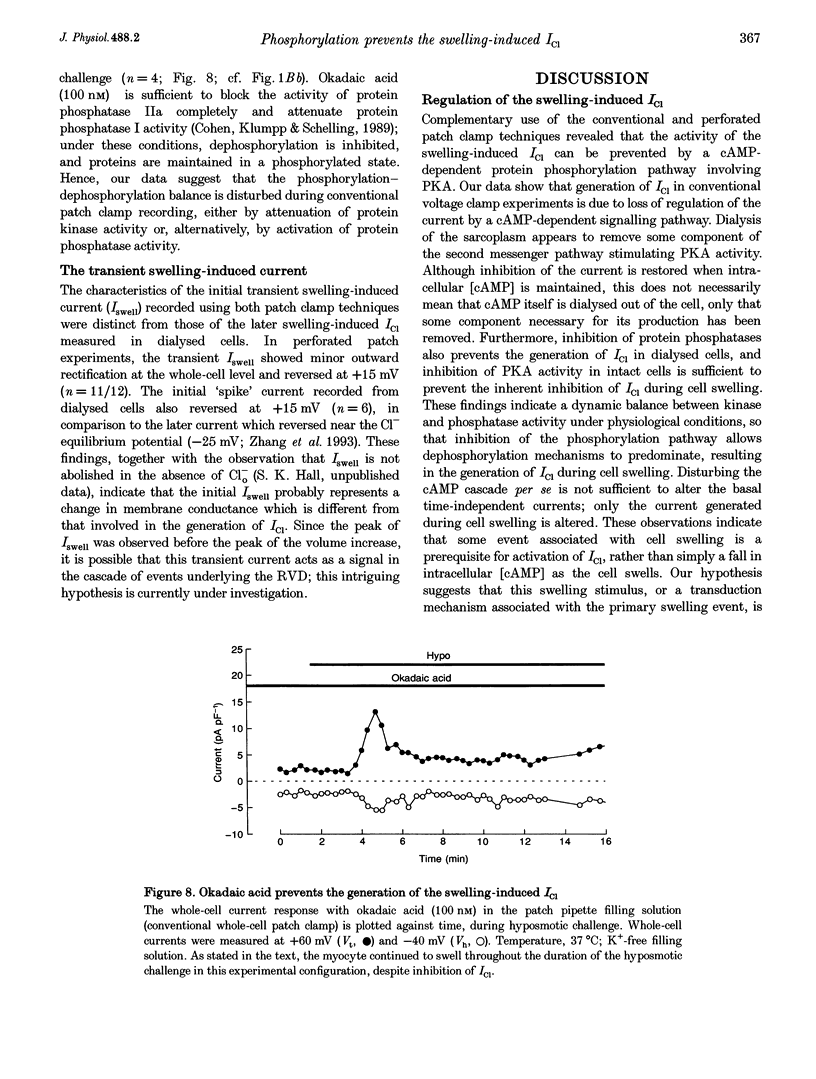
1. Changes in myocardial cell volume and whole-cell currents were measured simultaneously during hyposmotically induced cell swelling. In the conventional patch clamp configuration, hyposmotic challenge caused myocytes to swell continuously and was associated with the development of a sustained, swelling-induced chloride conductance (ICl). In contrast, perforated patch-clamped myocytes demonstrated regulatory volume decreases (RVD) during hyposmotic challenge, and ICl was not generated. 2. The swelling-induced ICl in conventionally patch-clamped myocytes was inhibited by application of forskolin (15 microM) and was prevented when the pipette filling solution contained cAMP (10 microM) and isobutylmethylxanthine (IBMX, 1 mM). ICl could also be prevented by inhibition of protein phosphatase activity, using okadaic acid (100 nM). Conversely, a swelling-induced current could be generated in myocytes under perforated patch clamp by inhibition of protein kinase A, using the antagonist Rp-cAMPS (10 microM). These data demonstrate that cAMP-dependent protein phosphorylation is both necessary and sufficient to prevent development of ICl during cell swelling. 3. Unlike other chloride currents described previously in heart muscle, generation of the novel swelling-induced ICl requires dephosphorylation of a cAMP-dependent protein phosphorylation site; hence it can be prevented by stimulation of cAMP-dependent protein phosphorylation or by inhibition of protein phosphatase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O., Ruknudin A., Sachs F. Stretch-activated channels in heart cells: relevance to cardiac hypertrophy. J Cardiovasc Pharmacol. 1991;17 (Suppl 2):S110–S113. doi: 10.1097/00005344-199117002-00024. [DOI] [PubMed] [Google Scholar]
- Cohen P., Klumpp S., Schelling D. L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989 Jul 3;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Masuda H., Shoda M., Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol. 1992 Oct;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Harvey R. D. Chloride conductance pathways in heart. Am J Physiol. 1991 Sep;261(3 Pt 1):C399–C412. doi: 10.1152/ajpcell.1991.261.3.C399. [DOI] [PubMed] [Google Scholar]
- Jacob R., Lieberman M., Liu S. Electrogenic sodium-calcium exchange in cultured embryonic chick heart cells. J Physiol. 1987 Jun;387:567–588. doi: 10.1113/jphysiol.1987.sp016589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Bolden A., Horn R. Control of action potentials and Ca2+ influx by the Ca(2+)-dependent chloride current in mouse pituitary cells. J Physiol. 1991 Aug;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Stimers J. R., Lieberman M. A novel Cl- conductance in cultured chick cardiac myocytes: role of intracellular Ca2+ and cAMP. J Membr Biol. 1994 Jul;141(1):59–68. doi: 10.1007/BF00232874. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992 Apr;70(4):851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Rasmusson R. L., Davis D. G., Lieberman M. Amino acid loss during volume regulatory decrease in cultured chick heart cells. Am J Physiol. 1993 Jan;264(1 Pt 1):C136–C145. doi: 10.1152/ajpcell.1993.264.1.C136. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Chung S., Martin B. L., Brautigan D. L., Levitan I. B. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991 Jun;11(6):1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorota S. Swelling-induced chloride-sensitive current in canine atrial cells revealed by whole-cell patch-clamp method. Circ Res. 1992 Apr;70(4):679–687. doi: 10.1161/01.res.70.4.679. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992 Apr;262(4 Pt 1):C1056–C1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- Vandenberg J. I., Yoshida A., Kirk K., Powell T. Swelling-activated and isoprenaline-activated chloride currents in guinea pig cardiac myocytes have distinct electrophysiology and pharmacology. J Gen Physiol. 1994 Dec;104(6):997–1017. doi: 10.1085/jgp.104.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B. Activation of a heart chloride current during stimulation of protein kinase C. Mol Pharmacol. 1991 Sep;40(3):342–346. [PubMed] [Google Scholar]
- White R. E., Schonbrunn A., Armstrong D. L. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991 Jun 13;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Zhang J., Rasmusson R. L., Hall S. K., Lieberman M. A chloride current associated with swelling of cultured chick heart cells. J Physiol. 1993 Dec;472:801–820. doi: 10.1113/jphysiol.1993.sp019974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Properties of the calcium-activated chloride current in heart. J Gen Physiol. 1992 Mar;99(3):391–414. doi: 10.1085/jgp.99.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]



