Abstract
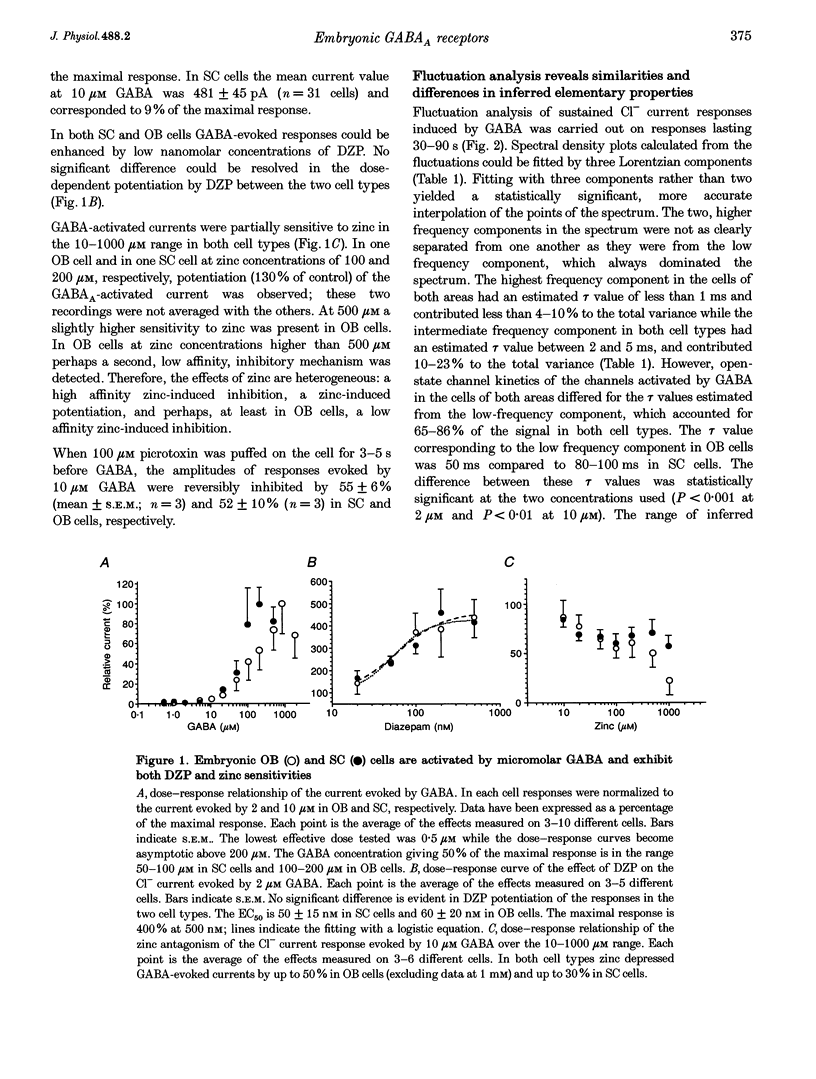
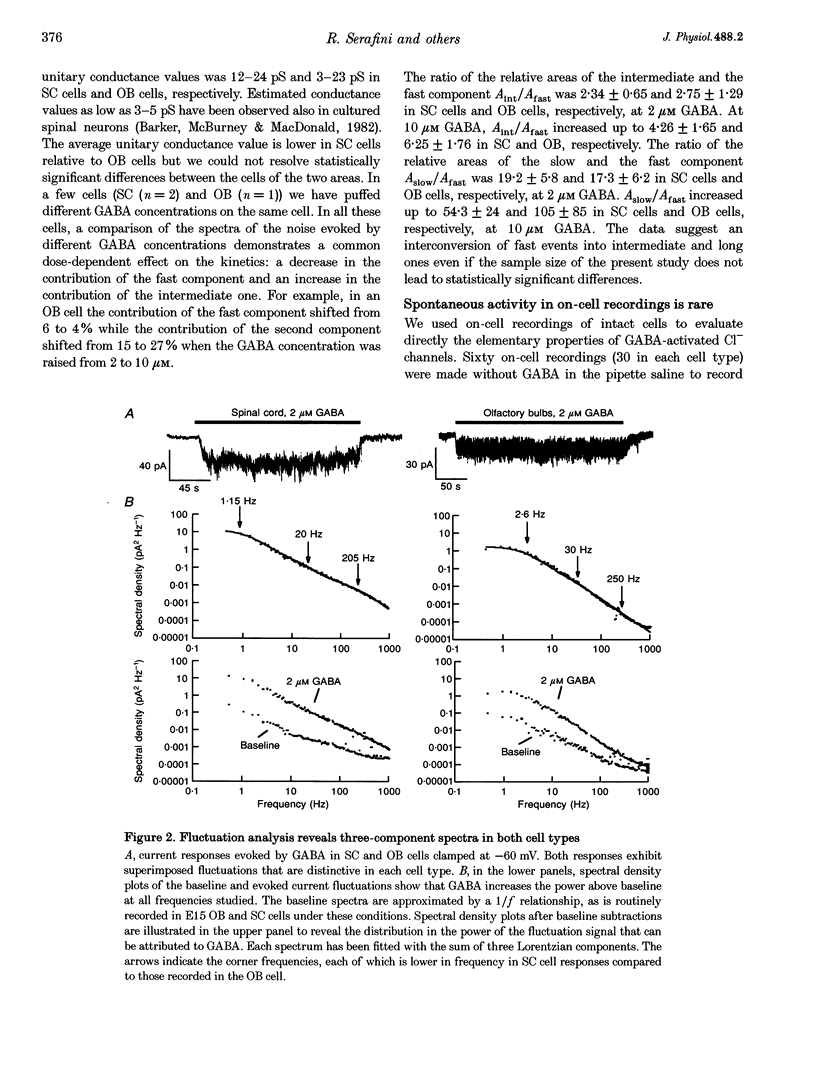
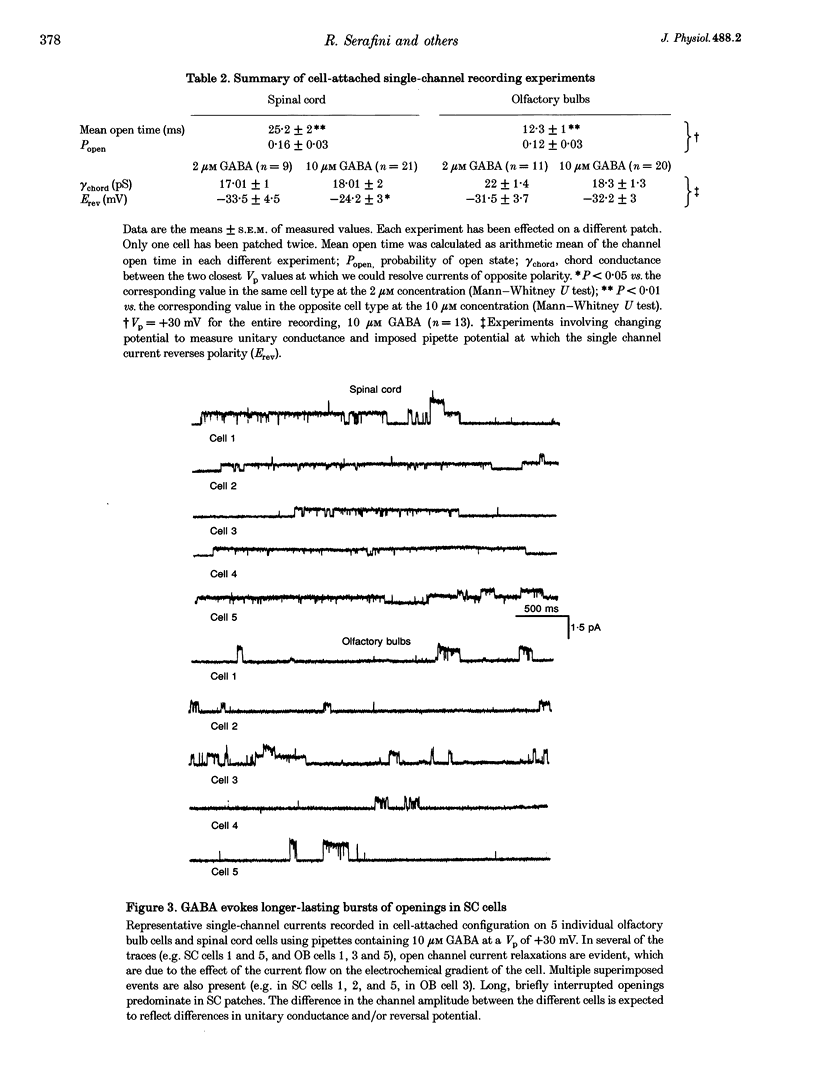
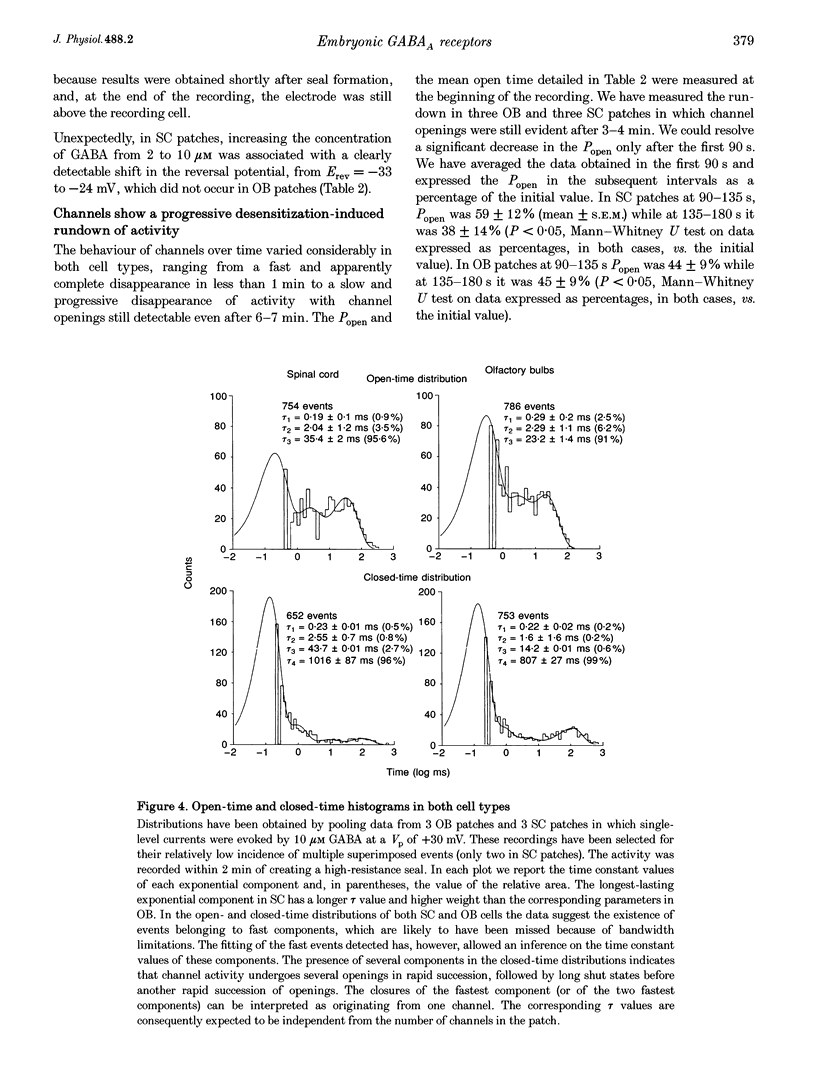
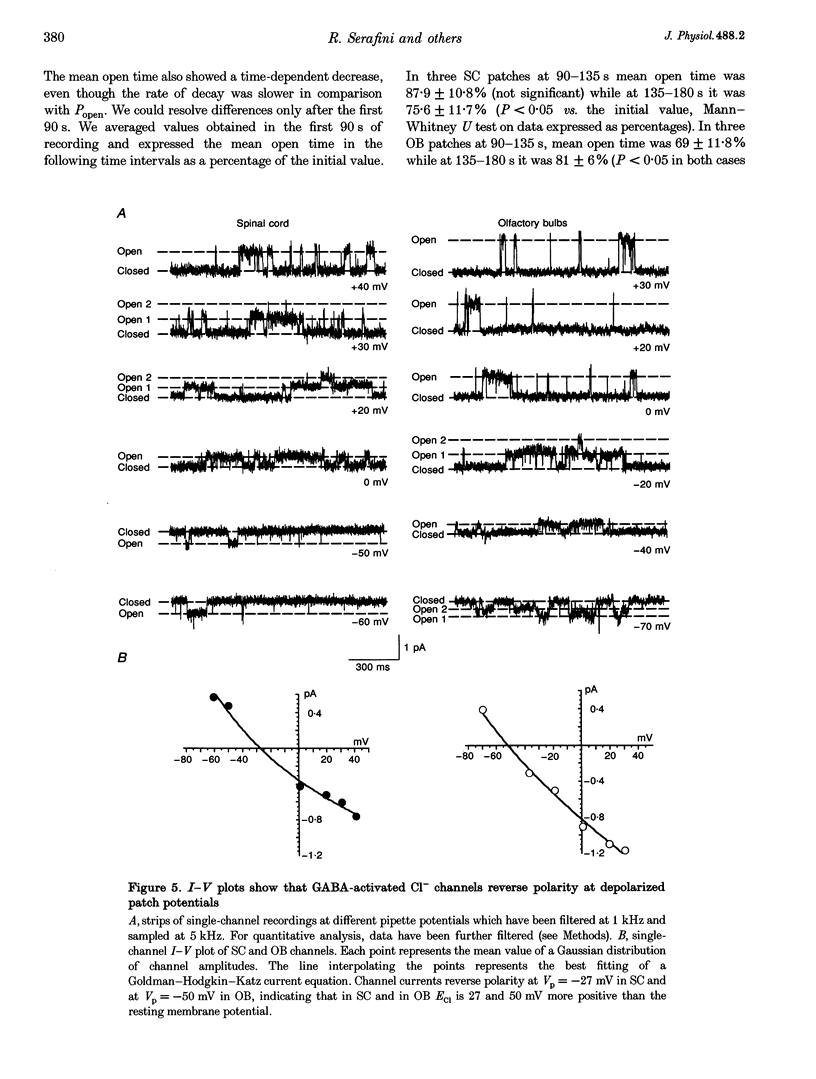
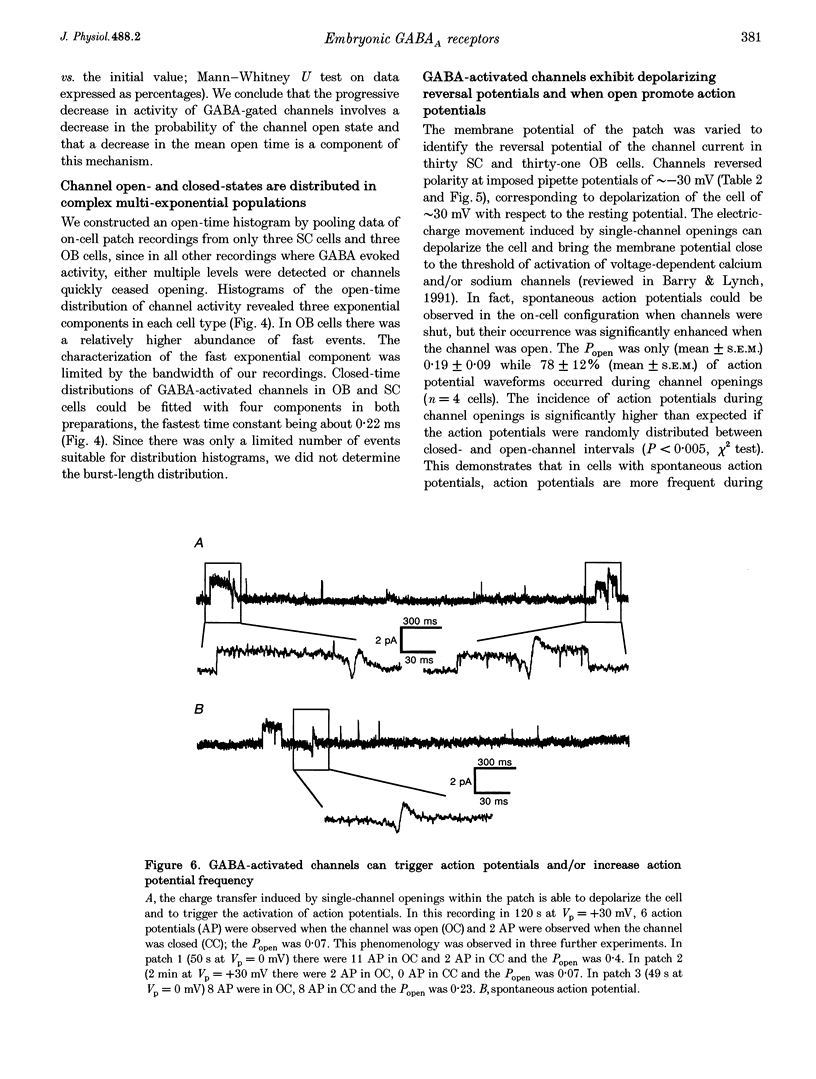
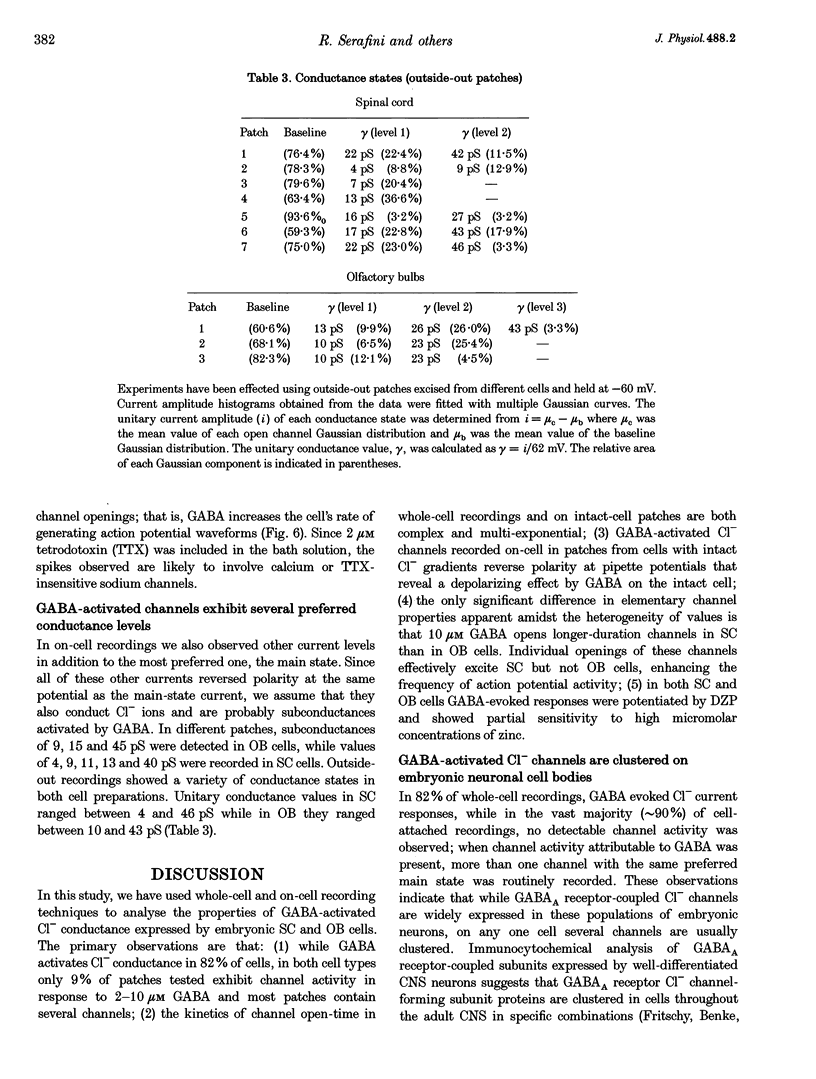
1. We have compared the electrical properties of the Cl- channels activated by GABA in cells acutely dissociated from embryonic (E) spinal cord (SC) and olfactory bulb (OB) regions at E15 using different configurations of the patch-recording technique. By in situ analysis these cells express GABAA receptor mRNAs encoding a common set of subunits (alpha 2, beta 2, and beta 3). SC cells also express alpha 3, alpha 5 and gamma 2s transcripts. 2. Whole-cell recordings revealed current responses to GABA (0.5 microM to 1 mM) in 242 out of 294 cells. In both SC and OB cells, currents evoked by 2 microM GABA could be potentiated by diazepam (DZP) in a dose-dependent manner with an EC50 of approximately 50 nM in both SC and OB. The maximal effect was approximately 300%. Both SC and OB cells exhibited GABA-activated currents that were only partially sensitive to zinc even at high micromolar concentrations. The effect of DZP and the relatively modest sensitivity to zinc suggest the presence of gamma subunits in both preparations. 3. Spectral analysis of current responses in twenty-six cells showed that power spectra could be fitted by three exponential components (tau 1-3) in the cells of both areas. The tau of the longest-lasting component (tau 3) was significantly different in the cells of the two areas: approximately 50 ms in OB and 80-100 ms in SC. No statistically significant differences in the average inferred unitary conductance between the two cell types could be resolved. 4. Single-channel properties were examined directly using the cell-attached configuration. GABA-activated channels could be recorded in only 89 out of well-sealed 984 patches and most of them exhibited multiple channel activity. The mean open time in the response to 10 microM GABA was significantly shorter in OB cells (12 ms) compared to SC cells (25 ms) while the average conductance values were not significantly different between the two cell types. 5. On average, Cl- channels reversed polarity when the on-cell patch pipette potential was approximately -30 mV. Thus, in these embryonic neurons, micromolar GABA activates Cl- channels, which, when open, effectively depolarize cells by approximately 30 mV. 6. Cl- channels activated by GABA are open longer in embryonic SC cells than in OB cells. This statistically significant difference in native GABAA receptor Cl- channel properties correlates with, and may be related to differences in subunit mRNA expression.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelotti T. P., Macdonald R. L. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993 Apr;13(4):1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. H., Lynch J. W. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991 Apr;121(2):101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Steinbach J. H. Excision of membrane patches reduces the mean open time of nicotinic acetylcholine receptors. Pflugers Arch. 1990 Jun;416(4):385–392. doi: 10.1007/BF00370744. [DOI] [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Fritschy J. M., Benke D., Mertens S., Oertel W. H., Bachi T., Möhler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales T. G., Tyndale R. F. Few cell lines with GABAA mRNAs have functional receptors. J Neurosci. 1994 Sep;14(9):5429–5436. doi: 10.1523/JNEUROSCI.14-09-05429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Estimating the number of channels in patch recordings. Biophys J. 1991 Aug;60(2):433–439. doi: 10.1016/S0006-3495(91)82069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y. Developmental changes of transmitter gated channels. Jpn J Physiol. 1993;43(6):727–743. doi: 10.2170/jjphysiol.43.727. [DOI] [PubMed] [Google Scholar]
- Kilić G., Moran O., Cherubini E. Currents activated by GABA and their modulation by Zn2+ in cerebellar granule cells in culture. Eur J Neurosci. 1993 Jan 1;5(1):65–72. doi: 10.1111/j.1460-9568.1993.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Wisden W., Seeburg P. H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992 Nov;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H. J., Prince D. A. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991 Feb;65(2):247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Ma W., Saunders P. A., Somogyi R., Poulter M. O., Barker J. L. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1993 Dec 15;338(3):337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Wang Y. H. Effect of agonist concentration on the lifetime of GABA-activated membrane channels in spinal cord neurons. Synapse. 1988;2(6):627–632. doi: 10.1002/syn.890020608. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Cull-Candy S. G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992 Feb;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Yuzaki M. Patch-clamp studies of chloride channels activated by gamma-aminobutyric acid in cultured hippocampal neurones of the rat. Neurosci Res. 1984 Oct;1(5):275–293. doi: 10.1016/0168-0102(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Poulter M. O., Barker J. L., O'Carroll A. M., Lolait S. J., Mahan L. C. Co-existent expression of GABAA receptor beta 2, beta 3 and gamma 2 subunit messenger RNAs during embryogenesis and early postnatal development of the rat central nervous system. Neuroscience. 1993 Apr;53(4):1019–1033. doi: 10.1016/0306-4522(93)90486-y. [DOI] [PubMed] [Google Scholar]
- Poulter M. O., Barker J. L., O'Carroll A. M., Lolait S. J., Mahan L. C. Differential and transient expression of GABAA receptor alpha-subunit mRNAs in the developing rat CNS. J Neurosci. 1992 Aug;12(8):2888–2900. doi: 10.1523/JNEUROSCI.12-08-02888.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Ramanan S. V., Fan S. F., Brink P. R. Model invariant method for extracting single-channel mean open and closed times from heterogeneous multichannel records. J Neurosci Methods. 1992 Apr;42(1-2):91–103. doi: 10.1016/0165-0270(92)90139-5. [DOI] [PubMed] [Google Scholar]
- Reichling D. B., Kyrozis A., Wang J., MacDermott A. B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994 May 1;476(3):411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N. C., Macdonald R. L. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994 Nov;14(11 Pt 2):7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: properties of GABA-activated Cl- ion conductance. J Neurophysiol. 1984 Mar;51(3):500–515. doi: 10.1152/jn.1984.51.3.500. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Moss S. J., Xie X., Huganir R. L. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991 Aug;103(4):1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Wu W. L., Ziskind-Conhaim L., Sweet M. A. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J Neurosci. 1992 Oct;12(10):3935–3945. doi: 10.1523/JNEUROSCI.12-10-03935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zorumski C. F. Trifluoperazine blocks GABA-gated chloride currents in cultured chick spinal cord neurons. J Neurophysiol. 1989 Feb;61(2):363–373. doi: 10.1152/jn.1989.61.2.363. [DOI] [PubMed] [Google Scholar]
- Yasui S., Ishizuka S., Akaike N. GABA activates different types of chloride-conducting receptor-ionophore complexes in a dose-dependent manner. Brain Res. 1985 Sep 30;344(1):176–180. doi: 10.1016/0006-8993(85)91206-5. [DOI] [PubMed] [Google Scholar]
- Zhang S. J., Jackson M. B. GABA-activated chloride channels in secretory nerve endings. Science. 1993 Jan 22;259(5094):531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]



