Abstract
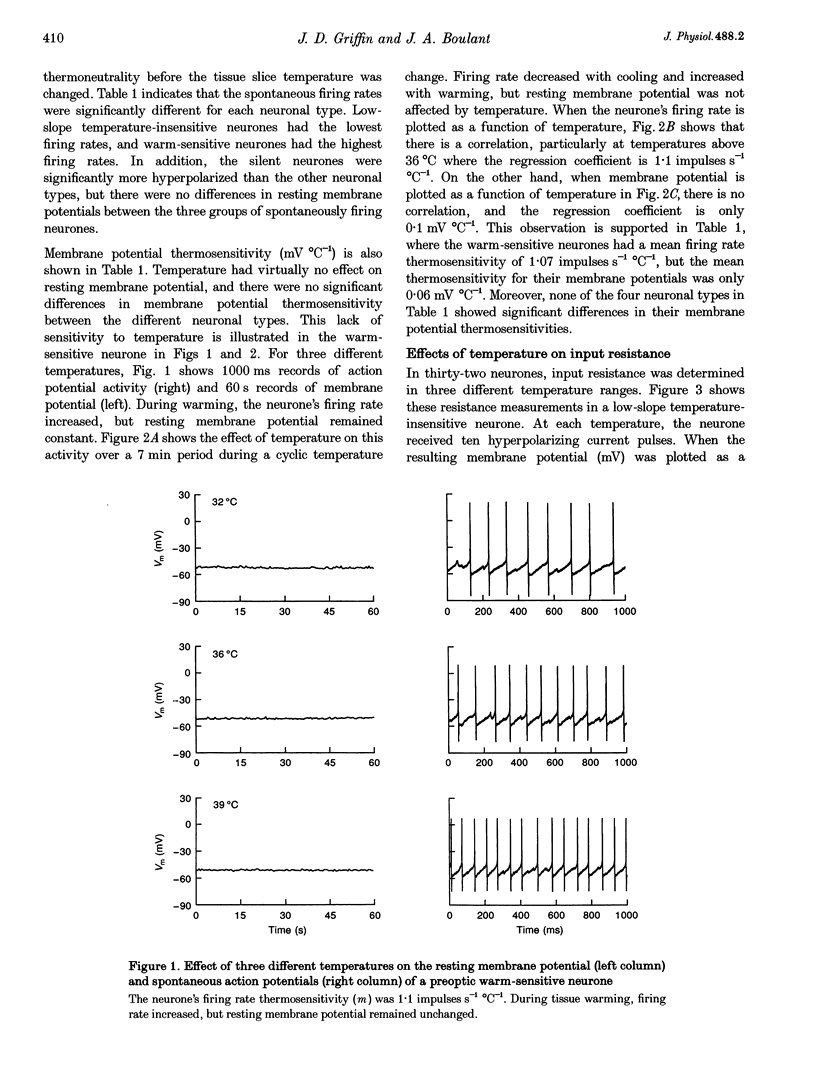
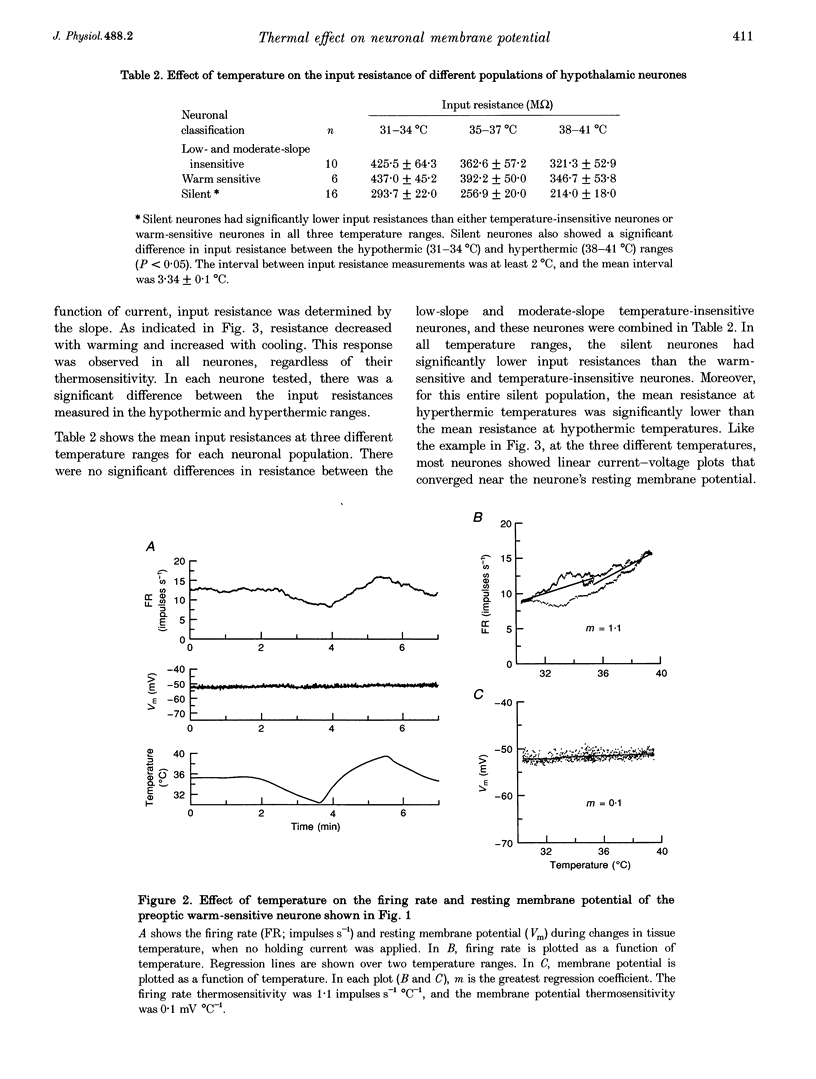
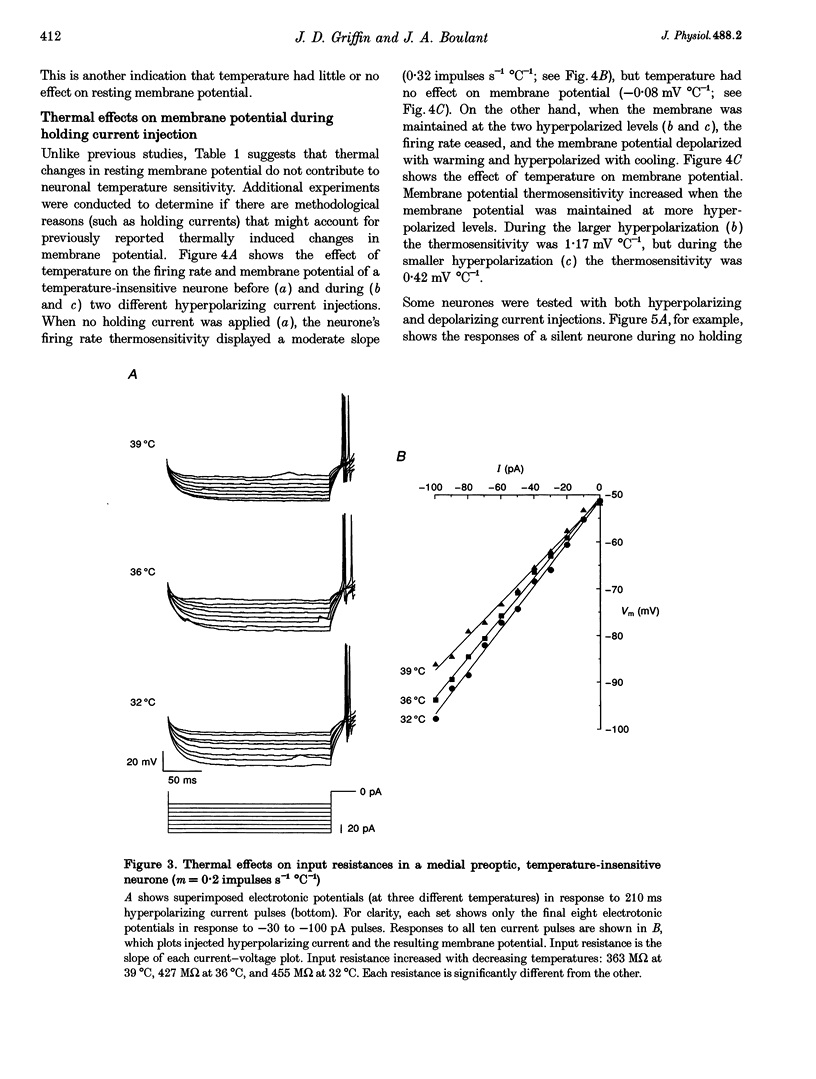
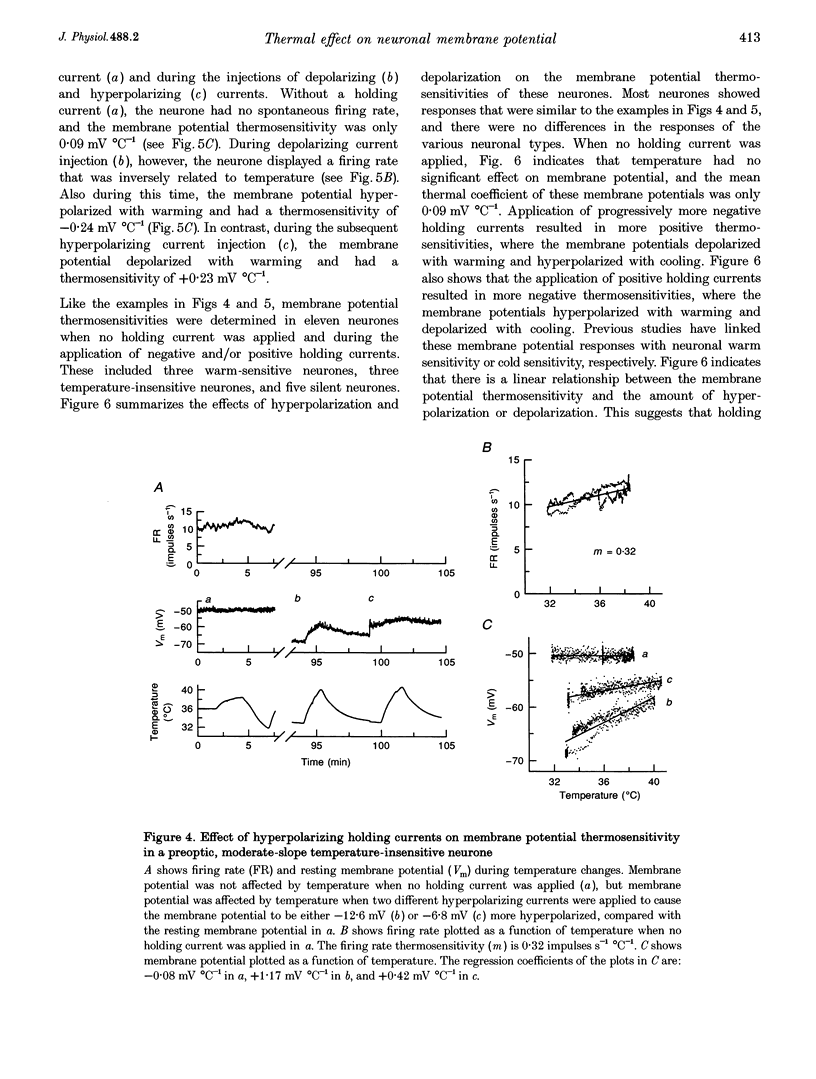
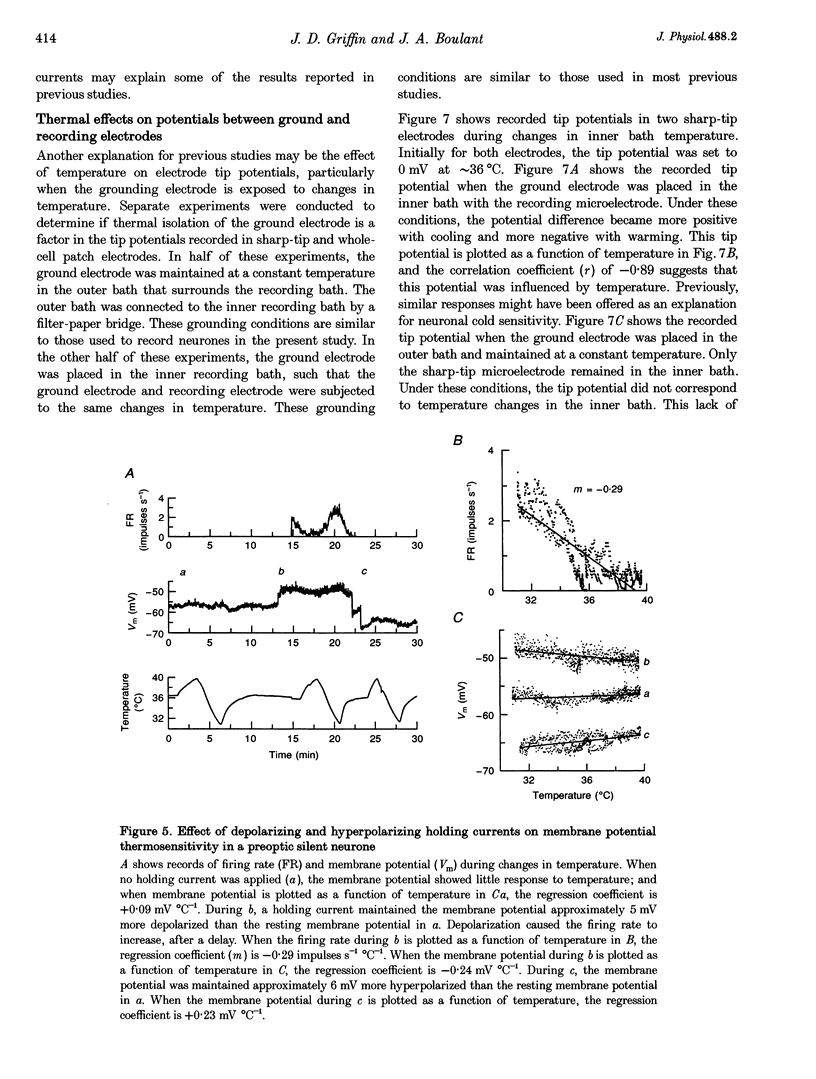
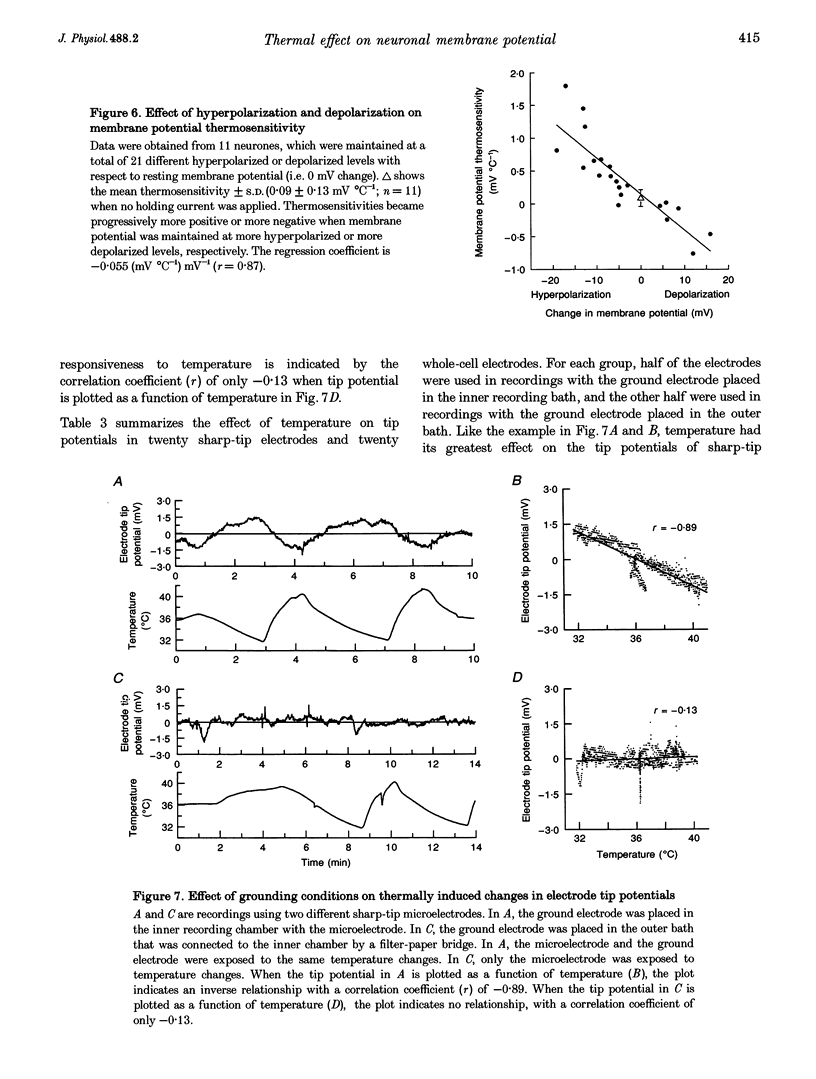
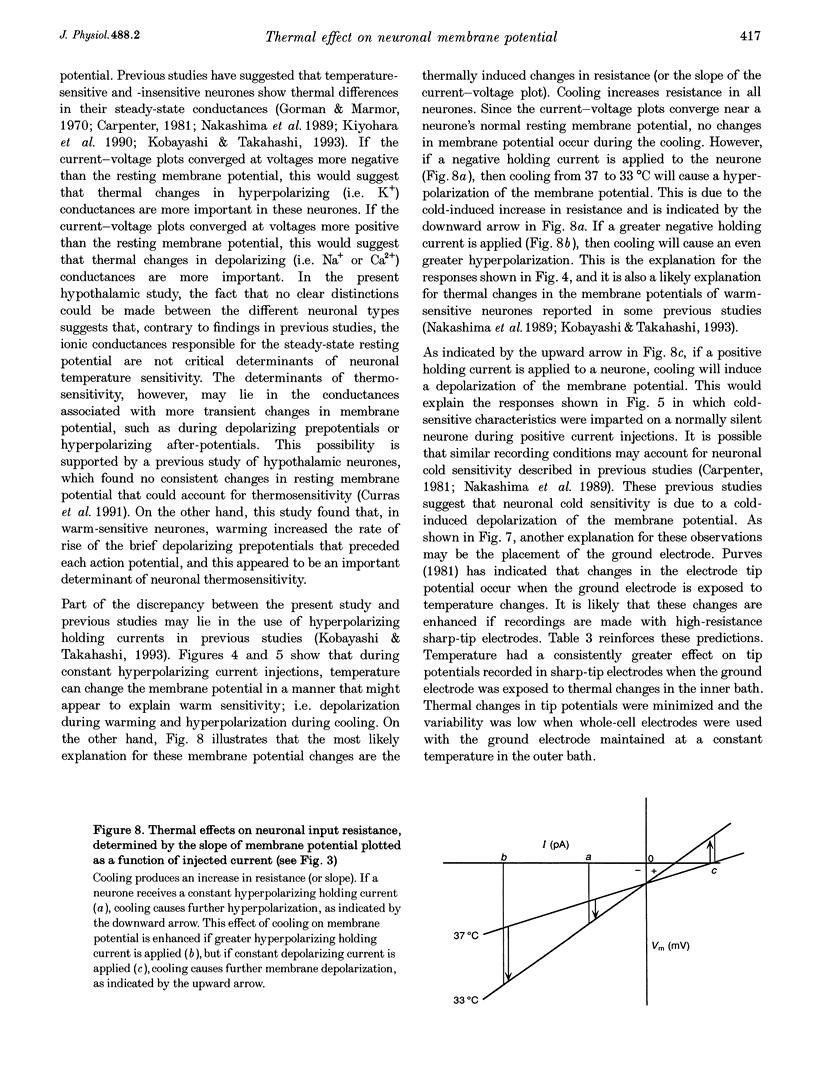
1. Whole-cell recordings were conducted in rat hypothalamic tissue slices to test the hypothesis that thermal changes in membrane potential contribute to neuronal thermosensitivity. Intracellular recordings of membrane potential and input resistance were made in eighty-two neurones, including twenty-four silent neurones and fifty-eight spontaneously firing neurones (22 warm-sensitive neurones and 36 temperature-insensitive neurones). Fifty-seven of the neurones were recorded in the preoptic and anterior hypothalamus. 2. Warm-sensitive neurones increased their firing rates during increases in temperature (1.07 +/- 0.06 impulses s-1 degree C-1), but their resting membrane potentials were not affected by temperature (0.06 +/- 0.06 mV degree C-1). Similarly, temperature did not affect the membrane potentials of temperature-insensitive neurones or silent neurones. 3. Silent neurones had significantly lower input resistances (256.9 +/- 20.0 M omega), compared with temperature-insensitive (362.6 +/- 57.2 M omega) and warm-sensitive neurones (392.2 +/- 50.0 M omega). Temperature had the same effect on all three types of neurones, such that resistance increased during cooling and decreased during warming. 4. If hyperpolarizing or depolarizing holding currents were applied to neurones, temperature caused changes in the membrane potentials. This spurious effect can be explained by thermally induced changes in the input resistance. 5. Measurements of electrode tip potentials indicated that artificial changes in membrane potential may also be recorded if grounding electrodes are not isolated from the changes in temperature. 6. These results suggest that physiological changes in resting membrane potentials do not determine neuronal warm sensitivity, and thermal changes in input resistance do not determine the primary differences between warm-sensitive and temperature-insensitive hypothalamic neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry P. H., Lynch J. W. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991 Apr;121(2):101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Boulant J. A., Dean J. B. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Carpenter D. O. Ionic and metabolic bases of neuronal thermosensitivity. Fed Proc. 1981 Dec;40(14):2808–2813. [PubMed] [Google Scholar]
- Curras M. C., Kelso S. R., Boulant J. A. Intracellular analysis of inherent and synaptic activity in hypothalamic thermosensitive neurones in the rat. J Physiol. 1991;440:257–271. doi: 10.1113/jphysiol.1991.sp018707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J. B., Boulant J. A. A diencephalic slice preparation and chamber for studying neuronal thermosensitivity. J Neurosci Methods. 1988 Apr;23(3):225–232. doi: 10.1016/0165-0270(88)90006-4. [DOI] [PubMed] [Google Scholar]
- Derambure P. S., Boulant J. A. Circadian thermosensitive characteristics of suprachiasmatic neurons in vitro. Am J Physiol. 1994 Jun;266(6 Pt 2):R1876–R1884. doi: 10.1152/ajpregu.1994.266.6.R1876. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Temperature dependence of the sodium-potassium permeability ratio of a molluscan neurone. J Physiol. 1970 Nov;210(4):919–931. doi: 10.1113/jphysiol.1970.sp009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso S. R., Nelson D. O., Silva N. L., Boulant J. A. A slice chamber for intracellular and extracellular recording during continuous perfusion. Brain Res Bull. 1983 Jun;10(6):853–857. doi: 10.1016/0361-9230(83)90219-8. [DOI] [PubMed] [Google Scholar]
- Kiyohara T., Hirata M., Hori T., Akaike N. Hypothalamic warm-sensitive neurons possess a tetrodotoxin-sensitive sodium channel with a high Q10. Neurosci Res. 1990 Apr;8(1):48–53. doi: 10.1016/0168-0102(90)90056-k. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Takahashi T. Whole-cell properties of temperature-sensitive neurons in rat hypothalamic slices. Proc Biol Sci. 1993 Feb 22;251(1331):89–94. doi: 10.1098/rspb.1993.0013. [DOI] [PubMed] [Google Scholar]
- Konnerth A. Patch-clamping in slices of mammalian CNS. Trends Neurosci. 1990 Aug;13(8):321–323. doi: 10.1016/0166-2236(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Neher E. The use of the patch clamp technique to study second messenger-mediated cellular events. Neuroscience. 1988 Sep;26(3):727–734. doi: 10.1016/0306-4522(88)90094-2. [DOI] [PubMed] [Google Scholar]
- Pierau F. R., Klee M. R., Klussmann F. W. Effect of temperature on postsynaptic potentials of cat spinal motoneurones. Brain Res. 1976 Sep 10;114(1):21–34. doi: 10.1016/0006-8993(76)91004-0. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Masukawa L. M., Prince D. A. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. 1985 Mar;5(3):817–824. doi: 10.1523/JNEUROSCI.05-03-00817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


