Abstract
Actinic keratosis (AK) is considered a chronic skin disease mostly caused by long-term exposure to UV radiation and other risk factors such as immunosuppression, leading to an individual susceptibility for skin cancer manifestation. The treatment of AK is laborious and costly, and the incidence of skin cancer is forecasted to double until the year 2030 in an aging society.
Risk factors in AK for malignant transformation in cutaneous squamous cell carcinoma (cSCC) are not fully understood, but studies suggest that histological features, such as atypia in the basal epidermal third and basal proliferation (PRO score) in AK play a pivotal role for development of malignancy. As the clinical appearance of AK does not correlate with the risk for malignancy, guidelines suggest treating every single AK lesion upon diagnosis. Skin imaging techniques, such as line-field confocal optical coherence tomography (LC-OCT) can help to provide an individual holistic follow-up for AK lesions by non-invasive visualization of atypia and basal proliferation. A follow-up for patients with AK may be critical for treatment success in terms of strengthening therapy adherence. When AK presents therapy refractory, cSCC manifests in nearly 30% of the cases after several years. Patients with AK suffering from field cancerization and immunosuppression are susceptible for a severe course of disease including metastasis and high mortality rates. Those vulnerable subgroups benefit from close skin cancer screening, early adequate treatment and chemoprevention, such as niacinamide or acitretin. Skin cancer prevention is substantial. Primary prevention should include chemical and physical UV-light protection and avoidance of indoor tanning. Secondary prevention is essential in high-risk populations, such as fair skin type elderly men and STORs. Tertiary prevention should comprise adequate treatment strategies to prevent therapy resistance, reoccurrence and cSCC, especially when field cancerization and immunosuppression are present.
Keywords: actinic keratosis, skin cancer, prevention, non-invasive imaging, PRO score
Introduction – Epidemiology
Actinic keratosis (AK) is characterized by the presence of atypical keratinocytes within the epidermal skin layer and is considered an in-situ neoplasia of the skin and a progenitor of cutaneous squamous cell cancer (cSCC) [1]. AK usually manifest on body areas, such as the face, scalp, head and neck area, hands, and forearms, being exposed to long-term ultraviolet (UV) radiation (UVR) over several years, usually decades. UVR and especially UV-B radiation (UVBR) causes damage to the skin and its keratinocytes at several stages of cell regulation and homeostasis, underlined by a complex interplay of local immunosuppression, skin inflammation, oxidative stress and DNA alteration. Clinically, AK is characterized by erythema and scaling. AK may appear erosive or may be accompanied by itching, but usually elderly patients do not consider these lesions as harmful, as AK progress or even regress slowly over time. The estimated prevalence of AK in the group of individuals being 60 years of age and older is 4.6–14.57% [2]. Moreover, the male population is affected at a higher rate of 3.5% compared to females (1.5%) [3]. Patients suffering from multiple AK, face a cumulative lifetime risk of 6–10% for malignant transformation into cSCC and the annual risk for malignant development for a single AK lesion ranges from 0.03–20% [4–6]. Furthermore, in up to 85% of cases of treated AK, recurrence or manifestation of new lesions can be observed after a one-year follow up, making AK a chronic and recurring disease [7]. The aging society and the high rate of recurrence may partly explain, why the treatment of AK is considered a laborious and costly task, placing an estimated financial burden on the US healthcare system of nearly $1–1.68 billion annually [8, 9]. These spendings cover the evaluation of almost 10 million patients for actinic skin damaged, annually [8]. Respectively, the costs for treatment of AK and cSCC reached an enormous amount of $4.59 billion for the year 2013 and accounted for more than 15% of health care spendings related to skin disease treatment [8]. However, treatment of cSCC remains significantly more costly per year than treatment of AK ($791 vs. $143) [10]. This burden may increase in an aging society and is supported by the fact that the incidence of cSCC has partly quadrupled in the last 30 years among the population in Germany [11]. Moreover, for the year 2030, Leiter et al. predict that, the incidence rate of NMSC will double again, with a continuous long-term trend in increasing incidence [12]. The estimated age-standardized incidence rates are 230 for males and 180–200/100.000 cases for females per annum in the year 2030 [12]. The chronic course of AK disease, including spontaneous regression, but also progression from sun damaged actinic skin, has influence on the quality of life (QoL) in several aspects, including fear about cSCC manifestation, cosmesis and clinical symptoms [13]. As AK is considered a chronic disease, QoL scores of those patients are comparable to scores of patients suffering from other chronic skin conditions, such as atopic dermatitis and psoriasis and are remarkably lower compared to patients without AK [13–15]. Interestingly, the number of AK correlates inversely with the QoL scores of patients and may cause a high sense of affliction in patients with multiple AK, field cancerization (FC) or recurring disease [13, 14, 16, 17]. Moreover, disease burden regarding lower QoL scores is significantly higher in female patients and patients younger than 60 years of age [18, 19]. For the reasons named, the complex management of AK, placing an immense financial burden on health care providers and psychological stress on diseased individuals, requires improved strategies for AK and cSCC prevention as well as adequate treatment strategies. This section will discuss the identification of possible risk factors in AK for early adequate therapy referral, as well as insights into recent approaches for therapy monitoring. Especially the quantification of objectifiable treatment response parameters may become substantial, as detection of non-responding lesions and reduction of malignant transformation in AK should remain primary goals in the handling of AK.
Pathogenesis of AK and Histological Risk Factors for Development of cSCC
The development of AK and the progression into invasive cSCC is multifactorial and is driven by a variety of endogenous and exogenous factors. Sunlight, and especially UVRB, was identified as a carcinogen for its ability to mutate TP53 [20, 21]. On gene level, TP53 functions as a tumor suppressor gene in the human genome, which controls various cell cycle mechanisms and is of importance in the regulation of cell proliferation and induction of apoptosis in mutated cells [21, 22]. Additionally, UVB irradiation of the skin initiates production of pro-inflammatory cytokines in keratinocytes, those include IL-1, IL-6, IL-8, interferon gamma (IFN-γ), granulocyte colony-stimulating factor (C-GSF), macrophage inflammatory protein (MIP-β) and tumor necrosis factor alpha (TNF-α) [23]. Together with other neuroand vasoactive mediators this inflammation causes “sunburn” within 24 hours of exposure [24]. UVR is reported to cause formation of reactive oxygen species (ROS) in the skin, such as hydroxide peroxide and other hydroxyl radicals [25]. As DNA nucleotides targeted by ROS are highly susceptible to mutagenesis, a well-characterized nucleotide mispairing found in DNA of sun damaged skin is the change of G/C pair into an A/T pair [26]. NADPH-dependent DNA repair mechanism play a pivotal role in keratinocytes to reverse free radical damage in DNA to avoid oxidative mutagenesis eventually. Typically, mutations of TP53 are predominantly found in human cancer tissue, including cSCC, where mutations of TP53 are found in approximately 50% of the cases [21, 27, 28]. When the mechanism of DNA repair is absent due to mutation, the immunosuppressive mechanism of action of UVR, and especially UVAR, which already comes into play after the first ten days of sun exposure, mediated through DNA damage in the form of cyclobutene pyrimidine dimers (CPDs), leads the sequel AK development and progression to cSCC [24, 29, 30]. TP53 mutations found in UVB-induced skin patches of mice were like mutations in cSCC, suggesting that these patch lesions are precursors of cSCC [31]. A characteristic UVBR induced mutation in the TP53 gene is the transition of cytidine to thymidine, leading to a loss of function of the TP53 gene product [32]. Beside TP53, other additional mutations in the genome are considered to increase the risk of atypia in cSCC or stimulate uncontrolled cell proliferation. Mutations in the KNSTRN oncogene are reported to drive progression towards cell atypia [33]. On protein level, KNSTRN translates into a kinetochore-associated protein responsible for mitotic chromosome segregation during anaphase in the cell cycle. Mutations in this gene, caused by substitution of the genome bases cytosine for adenine and induced by UVR, lead to cell aneuploidy and enhanced tumor genesis [32, 33]. The mutation was observed in 19% of cSCC and in 13% of AK, contrary this mutation was never found in healthy skin [32].
The resulting uncontrolled cell cycle finally leads to keratinocyte cell atypia and dysplasia, which can initially be observed in the basal layers of the epidermis [34]. Beside genomic mutations found in AK, other co-factors seem to stimulate the malignant transformation of AK. It is estimated that in up to 35% of the cases of cancer manifestation, viral infections play a co-stimulatory role in carcinogenesis of cSCC [35]. For the skin, HPV DNA is found in forms of non-melanoma skin cancer (NMSC), such as basal cell carcinoma (BCC) and cSCC, but also in AK and healthy skin. For people under immunosuppression HPV DNA is found in 80% of NMSC. Still, its causal role in the pathogenesis for NMSC is poorly understood. It is assumed that HPV skin infection acts co-stimulatory in the pathogeneses of AK, but it is not considered to be a co-carcinogen for malignant transformation into NMSC [32]. Since AK is considered an insitu SCC, the proliferation of atypic keratinocytes is limited to the epidermis, while the dermo-epidermal junction (DEJ) remains intact. From the evaluation of histological specimen of cSCC and adjacent AK tissue, Fernandez-Figueras et al. suggested two major pathways of malignant transformation from AK to cSCC [36]. The classic pathway describes the stepwise evolution of cSCC rom atypia in the lowest third of the epidermis (AK I) evolving to atypia in the middle (AK II) and finally upper third of the epidermis (AK III), according to the previously proposed atypia grading by Röwert-Huber [34, 36]. At the final stage of the disease, invasive proliferation is defined by loss of DEJ integrity and invasive infiltration of atypical keratinocytes into the dermis. Beside the classic pathway, it was found that AK I can even directly progress into cSCC, without stagewise atypia evolvement, especially when AK I grows in close contact to adnexal tissue [36]. Beside atypia, Schmitz et al. [37] identified distinct basal growth patterns in AK to be suggestive for cSCC development. Those basal growth patterns, found in histological sections, can be subsumed under the so-called PRO score, which grades the basal growth pattern in AK on a tier grading system (PRO I–III) [37]. For PRO I, the epidermal layer is flat and no protrusions in the dermis can be observed. For PRO II, the DEJ appears undulated and slight protrusion into the dermis can be appreciated. PRO III AK lesions are considered to be high proliferative, as protrusions found in those lesions reach a deep, cone-like penetration into the dermis. The DEJ remains still intact [37]. In 2019, Schmitz et al. histologically investigated the epidermis adjacent to samples of cSCC [38]. The epidermal layers were assessed for keratinocyte atypia (Röwert-Huber, AK I–III) and the basal growth pattern (PRO I–III). The majority (39.4%) of cSCC harbored PRO III in the adjacent epidermis, followed by PRO II (31.9%) and PRO I (25.7%) [38]. Also, basal proliferation of atypical keratinocytes (AK I) was found in more than 50% of the evaluated adjacent AK lesions [38]. The finding of AK I, being predominantly associated with cSCC was determined in accordance with the study of Fernandez-Figueras et al., who reported that cSCC often emerges from atypia (AK I) in the lower third of the epidermis [36, 38]. When AK I advances along adnexal structures this might even further facilitate the development of cSCC from AK I lesions [36].
The clinical Olsen classification, and both histological classifications (PRO score and Röwert-Huber) have in common to provide a possible grading to the malignant potential of AK. However, the clinical value of these classifications is debated and critically discussed in the literature. Recent studies imply that the clinical Olsen score is not sufficient to predict the potential of malignant transformation alone [11, 36, 39]. In a study conducted by Schmitz et al. in 2016, it was proven that the clinical appearance of AK, graded by the Olsen scale, did not correlate with the extent of keratinocyte atypia underneath, measured using the Röwert-Huber scale [39]. Only in 53.8% lesions, a matching clinical and histological classification was found, with the majority (83.1%) being Olsen II and AK II [39]. Another study found that the expression of the mutated TP53 gene in AK, tended to increase with a higher rate of keratinocyte dysplasia, but no significant level was reached [28]. Moreover, in AK no significant correlation was found between TP53 expression, the extent of dysplasia in the epidermis and the clinical thickness as well as thickness of the stratum corneum (SC) [28]. From these findings, Heerfordt et al.[28] supported the suggestion by Schmitz et al.[39] that the clinical appearance of AK is not a sufficient and reliable predictor of malignant transformation in AK. In contrast, Bakshi et al. were able to show that the mutational status of TP53 and its increased level of protein expression was found in clinical apparent AK, sun-exposed skin, cSCC and BCC, while significantly lower levels were found in regressive AK [40]. It was found that a progressive increase in nuclear TP53 staining was associated with a progress from actinically damaged skin to AK to cSCC eventually [40]. From these findings they concluded that TP53 may be a good biomarker of AK progression towards invasiveness [40]. The progression from AK to cSCC appears to be a complex process of interactions between the named co-carcinogenic factors. Their underlying mechanism are still insufficiently understood and thus subject to controversially discussion in the available literature. With these assumptions, Schmitz et al. suggest to use clinical and histological features, found in AK lesions, to predict the risk of malignant transformation in AK [32].
From these controversies, the latest S3-guideline for AK and SCC does not give any advice on risk factors for malignancy in AK [11]. Moreover, no predictive value for the risk of malignant transformation can be assumed by the clinical appearance of AK [11]. Still, the reported findings imply that the downward proliferation and basal atypia in AK are two major factors for discrimination of high-risk AK. Unfortunately, they cannot be assessed by clinical appearance and are not routinely evaluated in the follow-up of AK, due to invasiveness of skin biopsy [36, 39]. Hence, no long-term follow-up data of AK exists, reporting and documenting distinct ongoing changes in cellular morphology and epidermal architecture in the pathway of malignant transformation. Beside these histopathological features in AK, which may contribute to malignant transformation, Schmitz et al. [41] identified painfulness and refractory of therapy in AK as clinical warning sign for malignant transformation, as those therapy-resistant AK have an underlying histology of highgrade atypia (AK III) and high basal proliferation rates (PRO III).
Beside intrinsic risk factors for the development of AK, extrinsic factors such as intake of photosensitizing cardiovascular drugs seem to affect the likeliness of AK development. Studies report exposure to angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors and calcium channel blockers [42]. Moreover, the intake of antiplatelet agents was identified as an independent risk factor for AK development [43].
Risk Factors for Development of AK and cSCC – Immunosuppression
Solid organ transplant recipients (STORs) under immunosuppression face a significant risk of developing NMSC during their lifetime. 80% of transplant recipient develop AK during their lifetime, with up to 30% of them suffering from more than five AK lesions [44, 45]. Although, immunosuppression is associated with the likelihood of HPV colonization, immunosuppression alone, significantly correlates with the development NMSC, depending on its prescription duration [32]. While for a period of two years under immunosuppression, the reported incidence of NMSC is 5%, it significantly increases over time and reaches up to 60% in patients, being under immunosuppression for a period of at least 20 years [32]. Studies report a median of eight years after transplantation for NMSC to manifest, while transplant recipients older than 60 years of age seem to develop NMSC already after three to five years of transplantation [46, 47]. Patients under immunosuppression also face a ten times increased risk to develop a second cSCC when field cancerization (FC) is present [48, 49]. In a renal transplant cohort, 7% of recipients with AK developed cSCC, while recipients with FC developed cSCC in 15% of the cases. The authors concluded a correlation between risk of SCC development and the skin area suffering from AK, such as FC [50]. Studies found that, manifestation of BCC is predominantly observed after initiation of immunosuppression and the risk follows a linear trend, but for cSCC the risk even develops exponentially [51]. For immunosuppressed patients, the ratio of cSCC vs. BCC occurrence is 4:1 and therefore inverse proportionally to the ratio observed in immunocompetent patients [32]. According to Schmitz et al. this may partly be explained by the higher observed progression rate of up to 30% from AK to SCC in immunodeficient patients [32]. Recent follow-up data from the year 2022 for a Finnish cohort of organ-transplanted patients, who were retrospectively evaluated for the last 30 years, revealed that NMSC was found predominately in 53% of all observed cancer manifestations [52]. Moreover, NSMC was found to be the leading cause for tumor-associated mortality in kidney transplanted patients in the Australian and New Zealand based population [53]. In general, patients under immunosuppression are reported to suffer from a more aggressive course of disease, coming along with higher rates of malignant infiltration, such as perineural spreading, high risk of recurrence (up to 13,4%), metastasis (5–8%) and higher rates of mortality [49, 54–57]. Moreover, NMSC manifests multifocal and eruptive. Similarly, patients with chronic lymphatic leukemia have a 5- to 8.6-fold higher risk to suffer from cSCC, compared to immunocompetent patients [58–60]. In this patient population, cSCC occurs with significantly higher rates of poor outcome such as metastasis, recurrence, or tumor related death [61–64].
For the reasons named, STORs profit from close therapy monitoring and disease management to control and prevent development of NMSC in the first place, or to provide mechanism of control for secondary and tertiary prophylaxis. In terms of management of immunosuppressive medication in STORs, mammalian target of rapamycin (mTOR) inhibitors should be implemented as the preferred immunosuppressive medication of choice [65]. Unlike immunosuppressive drugs such as cyclosporine, azathioprin or high dose corticosteroids, mTOR inhibitors prevent multiple mechanisms involved in carcinogenesis, such as angiogenesis, cell expansion and cell survival [66–68]. Moreover, mTOR inhibitors block the HPV related induction of mTOR pathway and therefore act as antivirals, by inhibiting growth of HPV-16-immortalised keratinocytes [66–69]. Other strategies should include, early treatment of premalignant and malignant lesions, by non-invasive and invasive means, but should also comprise prophylactic approaches, such as photoprotection, reduction of immunosuppression, use of mTOR and chemoprophylaxis. Retinoids, such as acitretin, a synthetic vitamin A substitute, seem to provide a favorable approach for prophylaxis of NMSC in this population, due to their positive effects on cell cycle control [70]. These include stimulation of cellular differentiation and induction of apoptosis, beside immunomodulatory aspects, cellular proliferation, and keratinization [70]. Another favorable attribute of acitretin is the inhibition of ornithine decarboxylase, the initial rate-limiting enzyme in the polyamine biosynthetic pathway, being responsible for elevated levels of polyamines found in highly proliferating tumor cells [70, 71]. Several studies report a reduction of cSCC incidence in cohorts under chemoprophylaxis with acitretin for at least three to five years and report ‘rebound effect’ with rapid increases in cSCC incidence after discontinuation of acitretin, but beneficial long-term effects are not fully studied to this date [72–75]. Still the named favorable aspects of acitretin find recognition in a consensus-based recommendation on the prevention of cSCC from the year 2021, advocating for the beneficial use of acitretin as chemoprophylaxis in STORs, who either develop a single high-risk cutaneous cSCC after multiple low-risk cSCCs, or more than ten low-risk cSCCs per year [76].
Challenges in AK Treatment – Field Cancerization
Patients suffering from actinic FC are at the highest risk to develop multiple NMSC during their lifetime usually being associated with poor outcomes [77]. The concept of FC was first described in 1953 by Slaughter et al. [78], examining more than 700 tissue samples of oropharyngeal carcinoma. In a predominant number of the reported cases, the unsuspicious appearing surrounding tissue harbored pathologically relevant cell atypia, leading to a substantial number of second tumor manifestation in the subsequent [78]. On cellular level, FC correlates with the clonal expansion of a mutant cell clone, spreading in tumor adjacent tissue and being susceptible for malignant transformation [79]. While FC was found in several tumor types, such as head and neck, breast and cervix, skin tissue is especially vulnerable to develop cell atypia, due to a chronic UVR stimulus on sun-exposed body areas such as scalp, forearms, and dorsum of the hand [79]. Still, there is no standardized definition of FC implemented to this date. Vague definition describes FC as an anatomical area suffering from AK or skin adjacent to AK showing at least two clinical markers of chronic actinic sun exposure, such as telangiectasia, skin atrophy, pigmentation disorder or hyperkeratosis [80]. It remains unclear, whether FC can be assumed without clinical appearance of AK, or not [80]. While, AK and FC are currently not considered distinct diagnosis from each other, presence of FC is associated with development of a poor disease-related outcome compared to presence of several discrete AK [81]. The lack of understanding FC correctly and treating FC and multiple AK equally, may lead to undertreatment of FC patients and poor outcomes eventually [81]. Studies found that the risk for development of cSCC correlates with the number of AK present. Even immunocompetent patients have a 5.7-fold increased risk for cSCC manifestation, when more than 15 Aks are present on the head and neck area [82]. Willenbrink et al. [81] presume, that regarding FC, patients face an even higher risk, due to the confluent nature of AK and the wide field of premalignant atypical keratinocytes, prone to malignant transformation.
Risk factors for developing FC are similar to those of AK and cSCC and can be narrowed down to duration of UVR exposure, age, fair skin type, male sex and immunosuppression [81]. For age, a 4-fold increased risk of AK development is reported for the age group of 61 to 70 year of age. The same increased risk is reported for male sex in the German population [83, 84]. In males the prevalence of extensive sun damage (minimum of ten AK) is three times more present compared to females [85]. In terms of body site, the single strongest risk factor for development of ten AK is reported to be scalp baldness in males [85]. Willenbrink et al.[81] experienced the highest risk for the occurrence of FC in immunosuppressed patients, such as STORs. This assumption is backed by evidence reporting a prevalence of 17% of FC in STOR cohorts [81]. As patients suffering from FC may develop cSCC more likely, the development of multiple cSCC during a patient’s lifetime is clearly associated with a significant worsening of disease burden in terms of aggressive disease progress. It is reported that patients suffering from more than ten cSCC have a 3.8–4.2 times increased risk for nodal metastasis and diseases recurrence, compared to patients with a single cSCC [77].
Prevention of AK and cSCC
For the US American population, trends regarding the protective behavior of sun light exposure seem to have increased among adults, throughout 2010 to 2020 [86]. McKenzie et al. [86] reported that sun-protective behaviors significantly improved through the reported time period, not only for the use of sunscreen but also in regard to seeking shade, avoiding sun and sun burns, and by wearing physical UV-light protection, such as long-sleeved shirts and hats.
In terms of primary prevention of skin cancer, the focus lies on educational programs, risk assessment models for individuals, the use of sunscreen and legislative regulation [87]. As 80–90% of skin cancer seems to be associated with exposure to UV-radiation, sun protective behavior is promoted and appears to be essential [88, 89]. Educational programs seem to account for the most widely studied primary prevention strategy [87]. Those programs seem to be especially effective in terms of awareness and knowledge of sun protective behavior, when targeting minors in primary and secondary schools, compared to adults [90, 91]. The use of educational images is reported to be effective in terms of sun protective behavior, knowledge and for self-examination in terms of melanoma [92–94]. Other strategies, such as using reminders via text messages and email to strengthen sun protective behavior, lack evidence and studies report controversial results. While Finch et al. [95] found that electronic text reminders lead to a reduction in sunburns, the data was not unambiguous to interpret as the number of sunburns was self-reported. Other studies do not report any evidence for text message reminders [96]. Beside schools, the occupational setting appears to be a critical targeting point for the implementation of skin cancer awareness and protection, as behavior towards UV-light exposures differs tremendously between outdoor workers [97]. Education on skin cancer prevention at the workplace is reported to be effective in terms of sun protective behavior, using sunscreen to reduce the number of sunburns eventually [98, 99].
Although studies report imprecise results between the use of sunscreen and the risk of developing melanoma, Waldman et al.[100] report the beneficial use of sunscreen to reduce the manifestation of AK, SCC and less clear also for BCC. The use of indoor tanning modalities is rightly criticized to promote skin cancer development. A large meta-analysis conducted by Wehner et al. [101], evaluating more than 9000 cases of skin cancer, found the population attributable risk fraction for cSCC to be 8.2% and 3.7% for BCC in the US population. These numbers are estimated to account for more than 170 000 annual cases of NMSC related to indoor tanning in the US alone [101]. The presented numbers were published in the year 2012. Since then, only a handful of countries have implemented policies to fully ban tanning beds, such as Australia and Brazil [102]. In Austria, Belgium, France, Germany, Portugal, Spain, and the United Kingdom, only bans for minors under the age of 18 exist [102]. Data from the US shows that, while indoor tanning prevalence decreased significantly among all US adults from 2007 to 2018 (10% vs. 4%), frequent indoor tanning was still common in 2018 with nearly 25% of respondents reporting the use of indoor tanning 25 times or more per year [103].
Oral Drugs for Skin Cancer Prevention
Beside topical ointment, several systemic agents have been investigated for its use and effectiveness in the secondary prevention of AK development. Chemoprophylaxis of AK can be implemented to prevent the occurrence or reoccurrence of new AK lesions.
Niacinamide
Niacinamide is the water-soluble derivate of vitamin B3, a key-coenzyme in the generation of ADP on cellular level, by its role for the formation of NAD+ complex. Atypical keratinocytes in AK are reported to produce significant lower levels of ADP, which is physiologically required as a source for intracellular energy production in terms of providing functioning DNA strand repair mechanism [104]. This loss of mechanism is reported to reduce the effectiveness of DNA repair and promotes development of cSCC [105, 106]. Niacinamide also reduces UV radiation induced skin inflammation by significant downregulation of IL-6, IL-10, MCP-1 and TNF-α mRNA expression, in vitro [107]. Additionally, oral and topical Nicotinamide have shown to be immune protective against UVBR and UVAR [108, 109].
Multiple studies have been conducted, investing this underlying pathway in vitro. Still clinical studies additionally suggest the beneficial use of niacinamide in skin cancer prevention [106, 109]. Park et al. [104] were able to show by microarray studies on in vivo irradiated human skin, that UV-induced cellular ATP loss was reduced by substitution of niacinamide but did not affect ROS formation or keratinocyte apoptosis. Chen et al. [110] were able to show in a double-blinded, randomized, controlled trial, including 386 immunocompetent Australians with a history of least two NMSCs, that the intake of 500 mg of niacinamide twice daily, significantly lowered the incidence of NMSC by 23% versus the placebo. For cSCC, a reduction by 30% was observed [110]. Interestingly also the number of AK lesions was significantly lower by 13% after 12 months of follow-up [110]. In a recent systemic review conducted by Mainville et al. [111] five trials were identified, reporting a significant reduction for cSCC and BCC for patients with untreated AK and previous manifestation of BCC and cSCC [110, 112–115].
For AK, the current knowledge remains heterogenous. Mainville et al.[111] could not identify a beneficial use for the prevention of AK evaluating three trials [110, 112, 113]. Although, the level of evidence for AK was estimated very low because of study inconsistency and imprecision [111]. Based on these findings, recent recommendations published in the Journal of the American Academy of Dermatology in the years 2018 and 2020, emphasize the beneficial use of oral niacinamide 500 mg twice daily in patients with a field cancerization or more than one previous manifestation of SCC [116, 117]. For STORs the use of niacinamide did not provide any beneficial use over the placebo [118]. In this vulnerable group, the use of acitretin is recommended for skin cancer prevention [76], see above. As most of the studies present results on tertiary prophylaxis of skin cancer, further studies should focus on chemoprevention of AK [111]. The intake of niacinamide appears to be safe and well tolerated, but for high doses exceeding 3 g/d reversible hepatotoxicity is reported [110, 116, 119, 120].
The Role of Vitamin D in AK and its Possible Preventive use
The role of vitamin D in the development of NMSC is discussed controversially in the available literature and is not fully understood to date. Yet, vitamin D is attributed with skin cancer protective abilities. Vitamin D contributes to retain cell homeostasis, by mediating and promoting apoptosis and antiproliferative effects in melanocytes and keratinocytes in vitro [121]. Moreover, vitamin D acts protective in sun damaged skin, by reducing cyclobutene pyrimidine dimers and by inducing the formation of antioxidants such as metallothionein in vitro [122–125]. Vitamin D a true prohormone comes in two major configurations, namely cholecalciferol (D3) and ergocalciferol (D2). Both forms can be substituted by dietary intake while the larger amounts of vitamin D3 are endogenously produced by photochemical modification of 7-dehydrocholesterol in the skin upon UVBR stimulus [121].
Vitamin D production in the skin largely depends on the distribution of melanin, as melanin absorbs and scatters UVBR, leading to effective conversion of 7-dehydrocholesterol into vitamin D derivates [126]. The amount of endogenous synthesized vitamin D3 also largely depends on several independent aspects related to sun exposure, such as cumulative exposure time, amount of sun-exposed skin, age, skin phototype and body mass index [127]. While vitamin D is formed by UVBR stimulus in the skin, the dilemma in understanding the definite role of vitamin D in prevention and development of skin cancer lies in the complex interplay of UVBR as a key driver of skin cancer development, but also being the activator of vitamin D synthesis. Different hypothesis on vitamin D levels related to skin type exist. Assumptions are made that individuals with the fairest phototypes suffer from the lowest vitamin D levels due to minimum sun exposure, given their photosensitivity [128]. The hypothesis for skin pigmentation evolution proposes that progressive skin depigmentation was critical for our ancestors to ensure sufficient vitamin D production through UVBR, when migrating to areas with reduced sunlight [129].
Although fair-skinned individuals seem to produce higher levels of vitamin D, they are susceptible to skin cancer due to lower tanning ability and greater sunburn response [130, 131]. However, Bonilla et al.[126] found that fairer-skinned children with higher pigmentation score values had increased vitamin D levels, while applying more sun protective measures. They concluded that sun protection does not eradicate the positive effect on vitamin D production in less pigmented skin [126].
The challenge seems to identify the ideal balance between generating enough vitamin D while limiting skin damage caused by UVBR, but to this date no data exists identifying the optimal dose of daily vitamin D intake, to reduce skin cancer eventually. Inconsistent findings exist regarding the amount of sun exposure and vitamin D supplementation for skin cancer prevention [132]. Yet, several studies report, 25-hydroxyvitamin D (25(OH)D), the circulating form of vitamin D, being associated with skin cancer development risk and therefore may function as a biomarker to reflect long-term sun exposure and predict the risk of NMSC also in AK patients [133–138].
Current knowledge on the chemoprotective role of vitamin D remains to be discussed. Sutedja et al. evaluated a total of 18 studies on this topic, including 11 in vivo studies, with five of them being either randomized controlled trials or interventional studies [139]. Evaluating the study of Passarelli et al., the oral intake of 1000 IU/day of vitamin D alone or in combination with calcium was reported to be protective for cSCC development [139, 140]. Rosenberg et al. evaluated the efficacy of topical application of 5-fluorouracil (5-FU) in combination with calcipotriol 0.005% (low-calcemic vitamin D analog) over a course of 4 days, implemented as an immunotherapy for AK on the scalp and face skin to prevent cSCC development [141]. Over the course of three years, 5-FU + calcipotriol were effective in reducing the incidence of cSCC significantly, compared to only 5-FU, while for BCC no difference was reported [141]. Rosenberg et al. also found that the treated skin harbored significantly more tissue-resident memory T-cells compared to the control (5-FU) [141]. In analogy, Cunningham et al. demonstrated that application of 5-FU combined with calcipotriol led to a robust and sustainable CD4+ T-cell response against atypical premalignant keratinocytes in AK, induced by upregulation of thymic stromal lymphopoietin cytokine in keratinocytes by calcipotriol, which is also reported for treatment of psoriatic lesions [142–144]. A similar positive effect was found for the oral pretreatment of AK using vitamin D3 10.000 IU daily for 5 or 14 days, followed by blue light photodynamic therapy (30 minutes; 20 J/cm2) [145]. In the control group with no vitamin D3 supplementation, individuals with 25(OH)D deficiency (< 31 ng/dL) had a clearance rate of 40.9% ± 42%, while in patients with normal 25(OH)D levels a clearance was found in 62.6% ± 14.2% of the cases. For high-dose vitamin D3 supplementation a significantly improved lesion clearance was found in 72.5% ± 13.6% cases [145].
Discussion – Management and Monitoring of Actinic Keratosis and Prevention of cSCC
Further research is needed to fully understand the effect of AK treatment on cSCC risk and outcomes of cSCC. A longitudinal cohort study conducted by Madani et al. [146] evaluated the risk of cSCC development for more than 200.000 AK patients vs. a control without AK for a period of ten years (2009–2020). After ten years, the cumulative incidence of cSCC reached 17.1% for AK patients vs. 5.7% for the control, with the number of AK being associated with the incidence of cSCC [146]. Interestingly, individuals being diagnosed with AK under 49 years of age, were nearly 7 times more likely to be diagnosed with cSCC than those without AK [146]. Therefore, early detection of AK may be critical, but the role of screenings for NMSC is not well understood to date.
While for the detection of melanoma, schemes such as the “ABCDE” rule exist and validation data in terms of sensitivity and specificity is available, the detection rate of NMSC in skin cancer screenings is not well documented [147, 148]. Often cancer registries even lack reliable epidemiologic data, because NMSC is common and usually curable and is therefore not monitored precisely [147]. A holistic review conducted by Henrikson et al. [149] found that routine clinician skin examination in terms of skin cancer screening, is not associated with higher detection rates of NMSC, skin cancer precursor lesions and melanoma compared to lesion-directed examination. Moreover, the included studies did not report NMSC mortality by stage at detection [149].
In terms of harms related to skin cancer screenings, little evidence exists for negative effects on psychological harms or cosmetic concerns. In a German study population 7% of taken shave biopsies were rated with poor cosmetic outcome at six months of follow-up and after a period of eight months after skin cancer examination, the patient’s wellbeing in terms of anxiety disorders an depression did not differ significantly from the normal range [149]. The insufficient evidence for screening reinforces the need for primary prevention of NMSC and monitoring of AK appears to be substantial in the follow-up of AK.
Especially, patients suffering from FC and/ or immunosuppression are vulnerable populations benefiting from close skin cancer screening and implementation of aggressive and early adequate therapy [81, 116]. For these patients, acitretin chemoprophylaxis should be implemented [76]. But also, when AK presents therapy refractory and additional treatment is required the risk for cSCC development increases dramatically up to 33.5% for a 4-year risk assessment [150]. Although, when AK is treated sufficiently, the risk of cSCC occurrence within the field of treatment is 2.2 to 5.8% over a course of four years, depending on the topical ointment applied [150]. These numbers imply that patients may benefit from a close follow-up for close AK lesion monitoring. This assumption may even be validated as studies suggest that the appliance of topical therapy often lacks adequate implementation by patients, as they are often not well informed about the specific topical intervention regime [151]. For a cross-sectional cohort of 113 patients, Koch et al .[151] found a concerning non-adherence rate of 46.9% to the implemented topical AK treatment. Only 30.9% of the patients used the administered therapy in accordance with the product characteristics [151]. Patients, who did not adhere with the medical product guidelines were significantly less informed about the product and adjusted application timeframe and therapy frequency independently [151]. Recent literature suggests that some patient groups suffering from AK are not well informed about the condition of AK. Elderly patients (over 77 years of age) and those suffering from more than seven lesions were identified at high risk for not seeking treatment due to intrinsic and extrinsic motivation deficits [152]. But also, patients who never had AK related treatment and those suffering from only one to three AK lesions are more likely to expect a one-time treatment, indicating that they may not yet be aware that AK is considered a chronic condition, which usually requires multiple treatment modalities and lifelong surveillance [152].
Regularly patient visits may be a useful opportunity to inform patients about AK and to reinforce the patients’ therapy adherence as one of the main goals in the patient’s motivation for AK treatment is the prevention of malignant transformation [152]. Moreover, patients are strengthened in their will to treat AK, when it is recommended by the physician [152].
However, monitoring of FC and AK only by measuring lesion counts can be cumbersome and studies have shown that this approach is imprecise and impracticable, due to the sometimes-difficult identification of subclinical AK and confluent transition of AK [153]. Tools such as the actinic keratosis field assessment scale (AK-FAS) or the actinic keratosis area and severity index (AKASI) have in common to characterize and objectivize the extent of AK or FC. The AKASI evaluates the percentage of the head area affected by AK and graded by the severities of distribution, erythema and thickness and higher scores are associated with the incidence of SCC according to Schmitz et al. [154]. Another tool for the evaluation of sun damaged skin regarding the extent of AK and FC located at the face and scalp area is the AK-FAS scale [155]. AK-FAS takes total affected skin area, hyperkeratosis and aspects of sun damage into account, but was also evaluated from standardized clinical photographs so far and was not tested in the clinical setting [155]. Willenbrink et al.[81] concluded that both grading systems (AKASI and AK-FAS) are partly impractical in the clinical setting, because the assessment is time-consuming and laborious. Another disadvantage of both scoring systems may be the neglection of in situ cSCC and invasive cSCC within the sun damaged area [81]. Therefore Willenbrink et al.[81] assumed that those assessment tools are prone to fail for risk stratification towards SCC progression, as both tools were not validated in large prospective study cohorts.
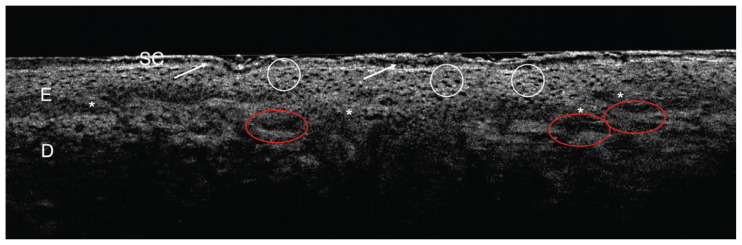
When AK is present on the skin, non-invasive imaging techniques may provide a substantial benefit for risk assessment and monitoring of AK. Using skin imaging techniques allows non-invasive assessment of independent risk factors in AK such as PRO score and atypia score. Since the introduction of line-field confocal optical coherence tomography (LC-OCT) to the field of dermatology, numerous studies investigated its use in clinical dermatology to visualize benign and malignant skin lesions. LC-OCT images were found to strongly correlate with conventional histopathological images [156]. Further, Ruini et al. found that non-invasive real-time evaluation of the dermo-epidermal junction (DEJ) and subsequent PRO score quantification is possible, using LC-OCT [157, 158]. The non-invasive diagnosis of AK, using LC-OCT, can be made in analogy to AK features in histological sections with a focus on keratinocyte morphology and epidermis architecture (Figure 1) [1]. By the definition of AK, being an intraepidermal neoplasia, the integrity of the DEJ must be contained throughout the whole suspicious lesion under surveillance. The diagnosis of AK can be made by visualization of hyper- and parakeratosis, as well as epidermal thickening and the notion of atypical, basal and suprabasal keratinocytes of heterogenous size. Also, basal proliferation may be present. In the papillary dermis dilated vessels can be appreciated. In contrast, for the diagnosis of sSCC, the most critical feature which must be noted during lesion imaging is the lack of DEJ integrity, this morphological feature defines the invasiveness of this tumor in accordance with histology. Other features such as ulceration and keratin plugs may underline the diagnosis of sSCC [158–160].
Figure 1.
Diagnosis of AK using LC-OCT.
AK lesion on the forehead of a patient. Stratum corneum (SC) presents hyper-/parakeratosis (white arrows), while the epidermis harbors keratinocytes which are heterogenous in size (white circle). The epidermis shows beginning basal proliferation (white asterisk), so PRO II can be assumed. In the papillary dermis dilated vessels are present. Based on the named features the clinician can be guided in making the diagnosis of AK using LC-OCT. (LC-OCT, deepLive™, DAMAE Medical, Paris, France; image size: 1.2 × 0.5 mm2, lateral and axial resolution: 1.1 × 1.3 μm).
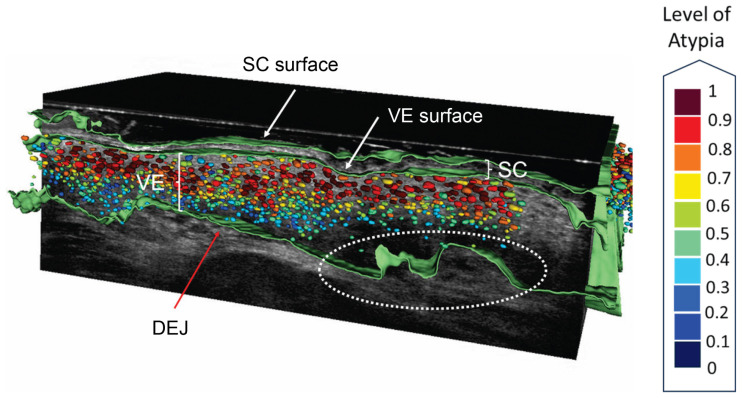
Beside using non-invasive imaging for identifying AK, AI integration tools are able to characterize AK by objectifiable features, such as PRO score and atypia to provide a grading to AK [Figure 2) [161–163]. These developments may resemble a further step to provide an effective follow-up for AK, which should be non-invasive and should be able to detect and monitor risk factors in AK, such as high levels of cell atypia and basal proliferation, in an effortless and time-sparing manner, for secondary prevention of cSCC. Moreover, the notion of atypia in histopathological sections can be very subjective with poor inter-rater agreement reported in the literature [163]. When comparing the automated atypia scoring algorithm to the experts’ consensus, the algorithm outperformed for the entire dataset [163]. Hence, this automated atypia grading tool may provide an objective and time saving alternative to conventional atypia scoring when it comes to non-invasive AK lesion monitoring. Moreover, for clinicians, the simple detection of atypia using LC-OCT may help to distinguish AK from other benign skin lesions (Figure 1). Previous studies found that the AI-automated PRO score, assessed by the implemented grading algorithm, correlates well with the experts’ consensus [161]. Also, 2D imaging allows visualization of the PRO score in accordance with histology [157]. Still, 2D vertical section imaging of AK only allows a very limited glimpse under the lesions’ surface under the assumption that single AK lesions comprise heterogenous patterns of basal proliferation. This fact resembles the possible additional value of automated 3D visualization allowing a much broader lesion imaging and therefore describe the DEJ undulation not just by a single vertical section, so that the computed DEJ undulation index allows a broader understanding for basal growth patterns in AK.
Figure 2.
AI-generated evaluation of AK features in LC-OCT.
AK lesion on the forehead of a patient. White arrows show the surface of stratum corneum (SC) and the viable epidermis (VE) detected by the skin segmentation algorithm. The red arrow shows the detected intact DEJ. Epidermal protrusions are found and indicated by the white circle. Keratinocytes colored in red show high atypia within the epidermis. The following parameters were detected by the implemented algorithms: SC thickness: 24.2 μm, VE thickness: 108.7 μm, DEJ undulation: 33%, KN atypia: 0.61. From this AK lesion, prevalence of atypia in 2/3 of the epidermal layer and PRO II can be assumed (SC= stratum corneum, VE= viable epidermis, KN= keratinocyte nuclei, DEJ= dermo-epidermal junction; LC-OCT, deepLive™, DAMAE Medical, Paris, France).
For a cohort with 24 AK lesions under treatment with tirbanibulin 1%, Thamm et al.[164] were able to provide a holistic follow-up for AK over 90 days, using LC-OCT (reference). By non-invasive imaging it was possible to show that keratinocyte atypia decreased significantly over all cases after the treatment during the follow-up. Interestingly, for a single lesion a slight worsening in dermoscopic strawberry pattern was clinically noticed during the follow-up. In LC-OCT imaging the persistence of atypia was observed accordingly at the end of the follow-up and an inadequate therapy response was therefore assumed. By the means of non-invasive imaging this lesion was identified as therapy non-responder, while clinically therapy associated inflammation and lesion clearing was assumed over the follow-up to some extent. These findings highlight the additional valuable input of non-invasive imaging as it allows to underline the clinical assumption of therapy refractory by objective parameters such as presence of atypia and therefore should be used as an automated tool for therapy monitoring of AK, as suggested by Fishman et al. [163].
The treatment and monitoring of AK remains a laborious task. The primary goal needs to be prevention or at least the early detection of cSCC. A follow-up for AK may be helpful to strengthen patient adherence to therapy and LC-OCT may add value to a non-invasive follow-up for AK by visualization of epidermal recovery processes and the evaluation of its objective parameters such as SC/epidermal thickness, DEJ undulation (PRO score) and keratinocyte atypia. This allows a more comprehensive follow-up of AK rather than considering clinical lesion aspects only. Moreover, lesions non-responding to the implemented therapy can be identified and can subsequently be referred to an adequate treatment regime early on.
Short Summary
AK are considered a chronic skin disease and reflect long-term exposure to UV radiation, coming with an individual susceptibility for skin cancer manifestation.
Risk factors in AK for development of cSCC are not fully understood, but studies suggest that atypia and basal proliferation in AK play a pivotal role for malignant transformation.
Skin imaging can help to facilitate individual risk assessment of AK lesions by non-invasive visualization of atypia and basal proliferation.
Guidelines suggest treating every single AK lesion independently from severity of clinical appearance. Patients with AK may benefit from a close follow-up to strengthen therapy adherence.
Patients with AK and field cancerization and/ or immunosuppression are highly vulnerable subgroups who benefit from close skin cancer screening, early adequate treatment, and chemoprevention.
Skin cancer prevention is substantial. Primary prevention should include chemical and physical UV-light protection and avoidance of indoor tanning. Secondary prevention is essential in high-risk populations, such as fair skin type elderly men and STORs. Tertiary prevention should comprise adequate treatment strategies to prevent therapy resistance, reoccurrence and cSCC development, especially when field cancerization and immunosuppression are present.
Footnotes
Funding: None.
Competing Interests: Julia Welzel: Consulting fees from Almirall, Janssen, Lecture fees from Almirall, Novartis, Janssen, Leo, Boehringer Ingelheim, BMS, Travel Grant from Janssen, President of the German Dermatological Society.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Thamm JR, Welzel J, Schuh S. Diagnosis and therapy of actinic keratosis. J Dtsch Dermatol Ges. 2024 doi: 10.1111/ddg.15288. [DOI] [PubMed] [Google Scholar]
- 2.Yaldiz M. Prevalence of actinic keratosis in patients attending the dermatology outpatient clinic. Medicine (Baltimore) 2019;98(28):e16465. doi: 10.1097/MD.0000000000016465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer I, Reusch M, Siebert J, Spehr C, Augustin M. Health care characteristics of basal cell carcinoma in Germany: the role of insurance status and socio-demographic factors. J Dtsch Dermatol Ges. 2014;12(9):803–11. doi: 10.1111/ddg.12415. [DOI] [PubMed] [Google Scholar]
- 4.Foote JA, Harris RB, Giuliano AR, et al. Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults. Int J Cancer. 2001;95(1):7–11. doi: 10.1002/1097-0215(20010120)95:1<7::aid-ijc1001>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1(8589):795–7. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 6.Quaedvlieg PJ, Tirsi E, Thissen MR, Krekels GA. Actinic keratosis: how to differentiate the good from the bad ones? Eur J Dermatol. 2006;16(4):335–9. [PubMed] [Google Scholar]
- 7.Steeb T, Wessely A, Petzold A, et al. Long-term recurrence rates of actinic keratosis: A systematic review and pooled analysis of randomized controlled trials. J Am Acad Dermatol. 2022;86(5):1116–9. doi: 10.1016/j.jaad.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Lim HW, Collins SAB, Resneck JS, Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76(5):958–72 e2. doi: 10.1016/j.jaad.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 9.Kirby JS, Gregory T, Liu G, Leslie DL, Miller JJ. Variation in the Cost of Managing Actinic Keratosis. JAMA Dermatol. 2017;153(4):264–9. doi: 10.1001/jamadermatol.2016.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Housman TS, Feldman SR, Williford PM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48(3):425–9. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 11.Heppt MV, Leiter U, Steeb T, et al. S3 guideline for actinic keratosis and cutaneous squamous cell carcinoma - short version, part 1: diagnosis, interventions for actinic keratoses, care structures and quality-of-care indicators. J Dtsch Dermatol Ges. 2020;18(3):275–94. doi: 10.1111/ddg.14048. [DOI] [PubMed] [Google Scholar]
- 12.Leiter U, Keim U, Eigentler T, Katalinic A, Holleczek B, Martus P, Garbe C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J Invest Dermatol. 2017;137(9):1860–7. doi: 10.1016/j.jid.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock MA, Lee KC, Chren MM, Marcolivio K, Group VT. Quality of life in the actinic neoplasia syndrome: The VA Topical Tretinoin Chemoprevention (VATTC) Trial. J Am Acad Dermatol. 2009;61(2):207–15. doi: 10.1016/j.jaad.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tennvall GR, Norlin JM, Malmberg I, Erlendsson AM, Haedersdal M. Health related quality of life in patients with actinic keratosis--an observational study of patients treated in dermatology specialist care in Denmark. Health Qual Life Outcomes. 2015;13:111. doi: 10.1186/s12955-015-0295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller IM, Vinding G, Zarchi K, Esmann S, Murrell DF, Jemec GB. Differences in Disease-specific Quality of Life in Patients with Actinic Keratosis in Australia and Denmark. Acta Dermatovenerol Croat. 2016;24(1):25–8. [PubMed] [Google Scholar]
- 16.Lee K, Weinstock M. Prospective quality of life impact of actinic keratoses: observations from the veterans affairs topical tretinoin chemoprevention trial. Acta Derm Venereol. 2011;91(1):101–2. doi: 10.2340/00015555-0972. [DOI] [PubMed] [Google Scholar]
- 17.Vis K, Waalboer-Spuij R, Snels D, Hollestein LM. Validity and Reliability of the Dutch Adaptation of the Actinic Keratosis Quality of Life Questionnaire (AKQoL) Dermatology. 2018;234(1–2):60–5. doi: 10.1159/000489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philipp-Dormston WG, Muller K, Novak B, Stromer K, Termeer C, Hammann U, et al. Patient-reported health outcomes in patients with non-melanoma skin cancer and actinic keratosis: results from a large-scale observational study analysing effects of diagnoses and disease progression. J Eur Acad Dermatol Venereol. 2018;32(7):1138–46. doi: 10.1111/jdv.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo I, Serra-Guillen C. Quality of Life, Behaviour and Attitudes towards Actinic Keratosis in Spain: The PIQA Study. Actas Dermosifiliogr (Engl Ed) 2018;109(4):331–9. doi: 10.1016/j.ad.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt J, Seidler A, Diepgen TL, Bauer A. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br J Dermatol. 2011;164(2):291–307. doi: 10.1111/j.1365-2133.2010.10118.x. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372(6508):773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler A, Jonason A, Simon J, Leffell D, Brash DE. Tumor suppressor gene mutations and photocarcinogenesis. Photochem Photobiol. 1996;63(4):432–5. doi: 10.1111/j.1751-1097.1996.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshizumi M, Nakamura T, Kato M, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32(11):1405–11. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Yarosh DB. DNA repair, immunosuppression, and skin cancer. Cutis. 2004;74(5 Suppl):10–3. [PubMed] [Google Scholar]
- 25.Meyskens FL, Jr, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14(3):148–54. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 26.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101(14):4954–9. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Queille S, Luron L, Spatz A, et al. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis. 2007;28(3):724–31. doi: 10.1093/carcin/bgl191. [DOI] [PubMed] [Google Scholar]
- 28.Heerfordt IM, Nissen CV, Poulsen T, Philipsen PA, Wulf HC. Thickness of Actinic Keratosis Does Not Predict Dysplasia Severity or P53 Expression. Sci Rep. 2016;6:33952. doi: 10.1038/srep33952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchel JM, Barnetson RS, Halliday GM. Cyclobutane pyrimidine dimer formation is a molecular trigger for solar-simulated ultraviolet radiation-induced suppression of memory immunity in humans. Photochem Photobiol Sci. 2005;4(8):577–82. doi: 10.1039/b504068j. [DOI] [PubMed] [Google Scholar]
- 30.Halliday GM, Byrne SN, Kuchel JM, Poon TS, Barnetson RS. The suppression of immunity by ultraviolet radiation: UVA, nitric oxide and DNA damage. Photochem Photobiol Sci. 2004;3(8):736–40. doi: 10.1039/b313199h. [DOI] [PubMed] [Google Scholar]
- 31.Kramata P, Lu YP, Lou YR, Singh RN, Kwon SM, Conney AH. Patches of mutant p53-immunoreactive epidermal cells induced by chronic UVB Irradiation harbor the same p53 mutations as squamous cell carcinomas in the skin of hairless SKH-1 mice. Cancer Res. 2005;65(9):3577–85. doi: 10.1158/0008-5472.CAN-04-4537. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz L, Oster-Schmidt C, Stockfleth E. Nonmelanoma skin cancer - from actinic keratosis to cutaneous squamous cell carcinoma. J Dtsch Dermatol Ges. 2018;16(8):1002–13. doi: 10.1111/ddg.13614. [DOI] [PubMed] [Google Scholar]
- 33.Lee CS, Bhaduri A, Mah A, et al. Recurrent point mutations in the kinetochore gene KNSTRN in cutaneous squamous cell carcinoma. Nat Genet. 2014;46(10):1060–2. doi: 10.1038/ng.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowert-Huber J, Patel MJ, Forschner T, et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol. 2007;156(Suppl 3):8–12. doi: 10.1111/j.1365-2133.2007.07860.x. [DOI] [PubMed] [Google Scholar]
- 35.Ajila V, Shetty H, Babu S, Shetty V, Hegde S. Human Papilloma Virus Associated Squamous Cell Carcinoma of the Head and Neck. J Sex Transm Dis. 2015;2015:791024. doi: 10.1155/2015/791024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Figueras MT, Carrato C, Saenz X, et al. Actinic keratosis with atypical basal cells (AK I) is the most common lesion associated with invasive squamous cell carcinoma of the skin. J Eur Acad Dermatol Venereol. 2015;29(5):991–7. doi: 10.1111/jdv.12848. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz L, Gambichler T, Gupta G, et al. Actinic keratoses show variable histological basal growth patterns - a proposed classification adjustment. J Eur Acad Dermatol Venereol. 2018;32(5):745–51. doi: 10.1111/jdv.14512. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz L, Gambichler T, Kost C, et al. Cutaneous squamous cell carcinomas are associated with basal proliferating actinic keratoses. Br J Dermatol. 2019;180(4):916–21. doi: 10.1111/bjd.16536. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz L, Kahl P, Majores M, Bierhoff E, Stockfleth E, Dirschka T. Actinic keratosis: correlation between clinical and histological classification systems. J Eur Acad Dermatol Venereol. 2016;30(8):1303–7. doi: 10.1111/jdv.13626. [DOI] [PubMed] [Google Scholar]
- 40.Bakshi A, Shafi R, Nelson J, et al. The clinical course of actinic keratosis correlates with underlying molecular mechanisms. Br J Dermatol. 2020;182(4):995–1002. doi: 10.1111/bjd.18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz L, Brehmer A, Falkenberg C, et al. Treatment-resistant actinic keratoses are characterized by distinct clinical and histological features. Ital J Dermatol Venerol. 2021;156(2):213–9. doi: 10.23736/S2784-8671.21.06892-9. [DOI] [PubMed] [Google Scholar]
- 42.Warszawik-Hendzel O, Olszewska M, Rakowska A, Sikora M, Hendzel P, Rudnicka L. Cardiovascular Drug Use and Risk of Actinic Keratosis: A Case-Control Study. Dermatol Ther (Heidelb) 2020;10(4):735–43. doi: 10.1007/s13555-020-00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sechi A, di Altobrando A, Cerciello E, Maietti E, Patrizi A, Savoia F. Drug Intake and Actinic Keratosis: A Case-Control Study. Dermatol Pract Concept. 2021;11(2):e2021031. doi: 10.5826/dpc.1102a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iannacone MR, Sinnya S, Pandeya N, et al. Prevalence of Skin Cancer and Related Skin Tumors in High-Risk Kidney and Liver Transplant Recipients in Queensland, Australia. J Invest Dermatol. 2016;136(7):1382–6. doi: 10.1016/j.jid.2016.02.804. [DOI] [PubMed] [Google Scholar]
- 45.Jiyad Z, O’Rourke P, Soyer HP, Green AC. Actinic keratosis-related signs predictive of squamous cell carcinoma in renal transplant recipients: a nested case-control study. Br J Dermatol. 2017;176(4):965–70. doi: 10.1111/bjd.15019. [DOI] [PubMed] [Google Scholar]
- 46.Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143(3):513–9. [PubMed] [Google Scholar]
- 47.Euvrard S, Kanitakis J, Pouteil-Noble C, Dureau G, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 Pt 1):222–9. doi: 10.1016/0190-9622(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 48.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136(12):1524–30. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 49.Harwood CA, Mesher D, McGregor JM, et al. A surveillance model for skin cancer in organ transplant recipients: a 22-year prospective study in an ethnically diverse population. Am J Transplant. 2013;13(1):119–29. doi: 10.1111/j.1600-6143.2012.04292.x. [DOI] [PubMed] [Google Scholar]
- 50.Wallingford SC, Russell SA, Vail A, Proby CM, Lear JT, Green AC. Actinic keratoses, actinic field change and associations with squamous cell carcinoma in renal transplant recipients in Manchester, UK. Acta Derm Venereol. 2015;95(7):830–4. doi: 10.2340/00015555-2098. [DOI] [PubMed] [Google Scholar]
- 51.Ferrandiz C, Fuente MJ, Ribera M, Bielsa I, Fernandez MT, Lauzurica R, Roca J. Epidermal dysplasia and neoplasia in kidney transplant recipients. J Am Acad Dermatol. 1995;33(4):590–6. doi: 10.1016/0190-9622(95)91276-2. [DOI] [PubMed] [Google Scholar]
- 52.Friman TK, Jaamaa-Holmberg S, Aberg F, et al. Cancer risk and mortality after solid organ transplantation: A population-based 30-year cohort study in Finland. Int J Cancer. 2022;150(11):1779–91. doi: 10.1002/ijc.33934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosales BM, De La Mata N, Vajdic CM, Kelly PJ, Wyburn K, Webster AC. Cancer mortality in kidney transplant recipients: An Australian and New Zealand population-based cohort study, 1980–2013. Int J Cancer. 2020;146(10):2703–11. doi: 10.1002/ijc.32585. [DOI] [PubMed] [Google Scholar]
- 54.Garrett GL, Lowenstein SE, Singer JP, He SY, Arron ST. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J Am Acad Dermatol. 2016;75(1):106–12. doi: 10.1016/j.jaad.2016.02.1155. [DOI] [PubMed] [Google Scholar]
- 55.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 56.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1–17. doi: 10.1067/mjd.2002.125579. quiz 8–20. [DOI] [PubMed] [Google Scholar]
- 57.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016;152(4):419–28. doi: 10.1001/jamadermatol.2015.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene MH, Hoover RN, Fraumeni JF., Jr Subsequent cancer in patients with chronic lymphocytic leukemia—a possible immunologic mechanism. J Natl Cancer Inst. 1978;61(2):337–40. [PubMed] [Google Scholar]
- 59.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;310(6993):1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levi F, Randimbison L, Te VC, La Vecchia C. Non-Hodgkin’s lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74(11):1847–50. doi: 10.1038/bjc.1996.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105(7):1076–81. doi: 10.1038/bjc.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Velez NF, Karia PS, Vartanov AR, Davids MS, Brown JR, Schmults CD. Association of advanced leukemic stage and skin cancer tumor stage with poor skin cancer outcomes in patients with chronic lymphocytic leukemia. JAMA Dermatol. 2014;150(3):280–7. doi: 10.1001/jamadermatol.2013.6249. [DOI] [PubMed] [Google Scholar]
- 63.Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol. 2015;72(2):302–9. doi: 10.1016/j.jaad.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehrany K, Weenig RH, Lee KK, Pittelkow MR, Otley CC. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 2005;53(6):1067–71. doi: 10.1016/j.jaad.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 65.Perez HC, Benavides X, Perez JS, et al. Basic aspects of the pathogenesis and prevention of non-melanoma skin cancer in solid organ transplant recipients: a review. Int J Dermatol. 2017;56(4):370–8. doi: 10.1111/ijd.13409. [DOI] [PubMed] [Google Scholar]
- 66.Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367(4):329–39. doi: 10.1056/NEJMoa1204166. [DOI] [PubMed] [Google Scholar]
- 67.Salgo R, Gossmann J, Schofer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10(6):1385–93. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 68.Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17(2):581–9. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 69.Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61(3):289–97. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 70.Lens M, Medenica L. Systemic retinoids in chemoprevention of non-melanoma skin cancer. Expert Opin Pharmacother. 2008;9(8):1363–74. doi: 10.1517/14656566.9.8.1363. [DOI] [PubMed] [Google Scholar]
- 71.Somani RR, Rai PR, Kandpile PS. Ornithine Decarboxylase Inhibition: A Strategy to Combat Various Diseases. Mini Rev Med Chem. 2018;18(12):1008–21. doi: 10.2174/1389557517666170927130526. [DOI] [PubMed] [Google Scholar]
- 72.Allnutt KJ, Vogrin S, Li J, et al. A long-term cohort study of acitretin for prevention of keratinocyte carcinoma in solid organ transplant recipients. Australas J Dermatol. 2022;63(2):e121–e6. doi: 10.1111/ajd.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebocontrolled study. J Clin Oncol. 1995;13(8):1933–8. doi: 10.1200/JCO.1995.13.8.1933. [DOI] [PubMed] [Google Scholar]
- 74.George R, Weightman W, Russ GR, Bannister KM, Mathew TH. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43(4):269–73. doi: 10.1046/j.1440-0960.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 75.Harwood CA, Leedham-Green M, Leigh IM, Proby CM. Low-dose retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients: a 16-year retrospective study. Arch Dermatol. 2005;141(4):456–64. doi: 10.1001/archderm.141.4.456. [DOI] [PubMed] [Google Scholar]
- 76.Massey PR, Schmults CD, Li SJ, et al. Consensus-Based Recommendations on the Prevention of Squamous Cell Carcinoma in Solid Organ Transplant Recipients: A Delphi Consensus Statement. JAMA Dermatol. 2021;157(10):1219–26. doi: 10.1001/jamadermatol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levine DE, Karia PS, Schmults CD. Outcomes of Patients With Multiple Cutaneous Squamous Cell Carcinomas: A 10-Year Single-Institution Cohort Study. JAMA Dermatol. 2015;151(11):1220–5. doi: 10.1001/jamadermatol.2015.1702. [DOI] [PubMed] [Google Scholar]
- 78.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 79.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63(8):1727–30. [PubMed] [Google Scholar]
- 80.Figueras Nart I, Cerio R, Dirschka T, et al. Defining the actinic keratosis field: a literature review and discussion. J Eur Acad Dermatol Venereol. 2018;32(4):544–63. doi: 10.1111/jdv.14652. [DOI] [PubMed] [Google Scholar]
- 81.Willenbrink TJ, Ruiz ES, Cornejo CM, Schmults CD, Arron ST, Jambusaria-Pahlajani A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83(3):709–17. doi: 10.1016/j.jaad.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 82.Xiong MY, Rizzo AE, Cohen TS, et al. Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial. J Invest Dermatol. 2013;133(6):1521–32. doi: 10.1038/jid.2013.35. [DOI] [PubMed] [Google Scholar]
- 83.Schaefer I, Augustin M, Spehr C, Reusch M, Kornek T. Prevalence and risk factors of actinic keratoses in Germany–analysis of multisource data. J Eur Acad Dermatol Venereol. 2014;28(3):309–13. doi: 10.1111/jdv.12102. [DOI] [PubMed] [Google Scholar]
- 84.Hensen P, Muller ML, Haschemi R, et al. Predisposing factors of actinic keratosis in a North-West German population. Eur J Dermatol. 2009;19(4):345–54. doi: 10.1684/ejd.2009.0706. [DOI] [PubMed] [Google Scholar]
- 85.Flohil SC, van der Leest RJ, Dowlatshahi EA, Hofman A, de Vries E, Nijsten T. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol. 2013;133(8):1971–8. doi: 10.1038/jid.2013.134. [DOI] [PubMed] [Google Scholar]
- 86.McKenzie C, Nahm WJ, Kearney CA, Zampella JG. Sunprotective behaviors and sunburn among US adults. Arch Dermatol Res. 2023;315(6):1665–74. doi: 10.1007/s00403-023-02547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alonso-Belmonte C, Montero-Vilchez T, Arias-Santiago S, Buendia-Eisman A. Current State of Skin Cancer Prevention: A Systematic Review. Actas Dermosifiliogr. 2022;113(8):781–91. doi: 10.1016/j.ad.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Koh HK, Geller AC, Miller DR, Grossbart TA, Lew RA. Prevention and early detection strategies for melanoma and skin cancer. Current status. Arch Dermatol. 1996;132(4):436–43. [PubMed] [Google Scholar]
- 89.Parkin DM, Mesher D, Sasieni P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(Suppl 2(Suppl 2)):S66–9. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reyes-Marcelino G, Wang R, Gultekin S, et al. School-based interventions to improve sun-safe knowledge, attitudes and behaviors in childhood and adolescence: A systematic review. Prev Med. 2021;146:106459. doi: 10.1016/j.ypmed.2021.106459. [DOI] [PubMed] [Google Scholar]
- 91.Thoonen K, Osch LV, Vries H, Jongen S, Schneider F. Are Environmental Interventions Targeting Skin Cancer Prevention among Children and Adolescents Effective? A Systematic Review. Int J Environ Res Public Health. 2020;17(2) doi: 10.3390/ijerph17020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McWhirter JE, Hoffman-Goetz L. Visual images for skin cancer prevention: a systematic review of qualitative studies. J Cancer Educ. 2012;27(2):202–16. doi: 10.1007/s13187-012-0355-y. [DOI] [PubMed] [Google Scholar]
- 93.McWhirter JE, Hoffman-Goetz L. Visual images for patient skin self-examination and melanoma detection: a systematic review of published studies. J Am Acad Dermatol. 2013;69(1):47–55. doi: 10.1016/j.jaad.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 94.McWhirter JE, Hoffman-Goetz L. Systematic review of population-based studies on the impact of images on UV attitudes and behaviours. Health Promot Int. 2015;30(2):397–410. doi: 10.1093/heapro/dat031. [DOI] [PubMed] [Google Scholar]
- 95.Finch L, Janda M, Loescher LJ, Hacker E. Can skin cancer prevention be improved through mobile technology interventions? A systematic review. Prev Med. 2016;90:121–32. doi: 10.1016/j.ypmed.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 96.Chambergo-Michilot D, Tellez WA, Becerra-Chauca N, Zafra-Tanaka JH, Taype-Rondan A. Text message reminders for improving sun protection habits: A systematic review. PLoS One. 2020;15(5):e0233220. doi: 10.1371/journal.pone.0233220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ziehfreund S, Schuster B, Zink A. Primary prevention of keratinocyte carcinoma among outdoor workers, the general population and medical professionals: a systematic review updated for 2019. J Eur Acad Dermatol Venereol. 2019;33(8):1477–95. doi: 10.1111/jdv.15525. [DOI] [PubMed] [Google Scholar]
- 98.Reinau D, Weiss M, Meier CR, Diepgen TL, Surber C. Outdoor workers’ sun-related knowledge, attitudes and protective behaviours: a systematic review of cross-sectional and interventional studies. Br J Dermatol. 2013;168(5):928–40. doi: 10.1111/bjd.12160. [DOI] [PubMed] [Google Scholar]
- 99.Horsham C, Auster J, Sendall MC, et al. Interventions to decrease skin cancer risk in outdoor workers: update to a 2007 systematic review. BMC Res Notes. 2014;7:10. doi: 10.1186/1756-0500-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waldman RA, Grant-Kels JM. The role of sunscreen in the prevention of cutaneous melanoma and nonmelanoma skin cancer. J Am Acad Dermatol. 2019;80(2):574–6 e1. doi: 10.1016/j.jaad.2018.06.069. [DOI] [PubMed] [Google Scholar]
- 101.Wehner MR, Shive ML, Chren MM, Han J, Qureshi AA, Linos E. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. doi: 10.1136/bmj.e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eskander A, Marqueen KE, Edwards HA, et al. To ban or not to ban tanning bed use for minors: A cost-effectiveness analysis from multiple US perspectives for invasive melanoma. Cancer. 2021;127(13):2333–41. doi: 10.1002/cncr.33499. [DOI] [PubMed] [Google Scholar]
- 103.Bowers JM, Geller AC, Schofield E, Li Y, Hay JL. Indoor Tanning Trends Among US Adults, 2007–2018. Am J Public Health. 2020;110(6):823–8. doi: 10.2105/AJPH.2020.305605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park J, Halliday GM, Surjana D, Damian DL. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem Photobiol. 2010;86(4):942–8. doi: 10.1111/j.1751-1097.2010.00746.x. [DOI] [PubMed] [Google Scholar]
- 105.Surjana D, Halliday GM, Damian DL. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis. 2013;34(5):1144–9. doi: 10.1093/carcin/bgt017. [DOI] [PubMed] [Google Scholar]
- 106.Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol. 2008;128(2):447–54. doi: 10.1038/sj.jid.5701058. [DOI] [PubMed] [Google Scholar]
- 107.Monfrecola G, Gaudiello F, Cirillo T, Fabbrocini G, Balato A, Lembo S. Nicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor-alpha gene expression in HaCaT keratinocytes after ultraviolet B irradiation. Clin Exp Dermatol. 2013;38(2):185–8. doi: 10.1111/ced.12018. [DOI] [PubMed] [Google Scholar]
- 108.Sivapirabu G, Yiasemides E, Halliday GM, Park J, Damian DL. Topical nicotinamide modulates cellular energy metabolism and provides broad-spectrum protection against ultraviolet radiation-induced immunosuppression in humans. Br J Dermatol. 2009;161(6):1357–64. doi: 10.1111/j.1365-2133.2009.09244.x. [DOI] [PubMed] [Google Scholar]
- 109.Yiasemides E, Sivapirabu G, Halliday GM, Park J, Damian DL. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30(1):101–5. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- 110.Chen AC, Martin AJ, Choy B, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med. 2015;373(17):1618–26. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 111.Mainville L, Smilga AS, Fortin PR. Effect of Nicotinamide in Skin Cancer and Actinic Keratoses Chemoprophylaxis, and Adverse Effects Related to Nicotinamide: A Systematic Review and Meta-Analysis. J Cutan Med Surg. 2022;26(3):297–308. doi: 10.1177/12034754221078201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moloney F, Vestergaard M, Radojkovic B, Damian D. Randomized, double-blinded, placebo controlled study to assess the effect of topical 1% nicotinamide on actinic keratoses. Br J Dermatol. 2010;162(5):1138–9. doi: 10.1111/j.1365-2133.2010.09659.x. [DOI] [PubMed] [Google Scholar]
- 113.Surjana D, Halliday GM, Martin AJ, Moloney FJ, Damian DL. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132(5):1497–500. doi: 10.1038/jid.2011.459. [DOI] [PubMed] [Google Scholar]
- 114.Drago F, Ciccarese G, Cogorno L, Calvi C, Marsano LA, Parodi A. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27(4):382–5. doi: 10.1684/ejd.2017.3025. [DOI] [PubMed] [Google Scholar]
- 115.Chen AC, Martin AJ, Dalziell RA, et al. A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients. Br J Dermatol. 2016;175(5):1073–5. doi: 10.1111/bjd.14662. [DOI] [PubMed] [Google Scholar]
- 116.Cornejo CM, Jambusaria-Pahlajani A, Willenbrink TJ, Schmults CD, Arron ST, Ruiz ES. Field cancerization: Treatment. J Am Acad Dermatol. 2020;83(3):719–30. doi: 10.1016/j.jaad.2020.03.127. [DOI] [PubMed] [Google Scholar]
- 117.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78(2):249–61. doi: 10.1016/j.jaad.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 118.Chung EYM, Palmer SC, Strippoli GFM. Interventions to Prevent Nonmelanoma Skin Cancers in Recipients of a Solid Organ Transplant: Systematic Review of Randomized Controlled Trials. Transplantation. 2019;103(6):1206–15. doi: 10.1097/TP.0000000000002641. [DOI] [PubMed] [Google Scholar]
- 119.Knip M, Douek IF, Moore WP, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43(11):1337–45. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 120.Gale EA, Bingley PJ, Emmett CL, Collier T European Nicotinamide Diabetes Intervention Trial G. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–31. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 121.Martin-Gorgojo A, Gilaberte Y, Nagore E. Vitamin D and Skin Cancer: An Epidemiological, Patient-Centered Update and Review. Nutrients. 2021;13(12) doi: 10.3390/nu13124292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part II. J Am Acad Dermatol. 2012;67(5):817 e1–11. doi: 10.1016/j.jaad.2012.07.022. quiz 27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dixon KM, Deo SS, Wong G, et al. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97(1–2):137–43. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Hanada K, Sawamura D, Nakano H, Hashimoto I. Possible role of 1,25-dihydroxyvitamin D3-induced metallothionein in photoprotection against UVB injury in mouse skin and cultured rat keratinocytes. J Dermatol Sci. 1995;9(3):203–8. doi: 10.1016/0923-1811(94)00378-r. [DOI] [PubMed] [Google Scholar]
- 125.Lee J, Youn JI. The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci. 1998;18(1):11–8. doi: 10.1016/s0923-1811(98)00015-2. [DOI] [PubMed] [Google Scholar]
- 126.Bonilla C, Ness AR, Wills AK, Lawlor DA, Lewis SJ, Davey Smith G. Skin pigmentation, sun exposure and vitamin D levels in children of the Avon Longitudinal Study of Parents and Children. BMC Public Health. 2014;14:597. doi: 10.1186/1471-2458-14-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Giorgi V, Gori A, Grazzini M, et al. Epidemiology of melanoma: is it still epidemic? What is the role of the sun, sunbeds, Vit D, betablocks, and others? Dermatol Ther. 2012;25(5):392–6. doi: 10.1111/j.1529-8019.2012.01483.x. [DOI] [PubMed] [Google Scholar]
- 128.Bataille V. Sun exposure, sunbeds and sunscreens and melanoma. What are the controversies? Curr Oncol Rep. 2013;15(6):526–32. doi: 10.1007/s11912-013-0342-4. [DOI] [PubMed] [Google Scholar]
- 129.Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34(4):707–29. doi: 10.1111/pcmr.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wagner JK, Parra EJ, HLN, Jovel C, Shriver MD. Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry. Pigment Cell Res. 2002;15(5):385–90. doi: 10.1034/j.1600-0749.2002.02046.x. [DOI] [PubMed] [Google Scholar]
- 131.Sturm RA. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 2002;12(5):405–16. doi: 10.1097/00008390-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 132.Burns EM, Elmets CA, Yusuf N. Vitamin D and skin cancer. Photochem Photobiol. 2015;91(1):201–9. doi: 10.1111/php.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liang G, Nan H, Qureshi AA, Han J. Pre-diagnostic plasma 25-hydroxyvitamin D levels and risk of non-melanoma skin cancer in women. PLoS One. 2012;7(4):e35211. doi: 10.1371/journal.pone.0035211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van der Pols JC, Russell A, Bauer U, Neale RE, Kimlin MG, Green AC. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133(3):637–41. doi: 10.1038/jid.2012.346. [DOI] [PubMed] [Google Scholar]
- 135.Nemazannikova N, Antonas K, Dass CR. Role of vitamin D metabolism in cutaneous tumour formation and progression. J Pharm Pharmacol. 2013;65(1):2–10. doi: 10.1111/j.2042-7158.2012.01527.x. [DOI] [PubMed] [Google Scholar]
- 136.Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133(3):629–36. doi: 10.1038/jid.2012.395. [DOI] [PubMed] [Google Scholar]
- 137.Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21(3):387–91. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cerman AA, Karabay EA, Altunay IK, Cesur SK. Vitamin D levels in actinic keratosis: a preliminary study. An Bras Dermatol. 2018;93(4):535–8. doi: 10.1590/abd1806-4841.20186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sutedja EK, Arianto TR, Lesmana R, Suwarsa O, Setiabudiawan B. The Chemoprotective Role of Vitamin D in Skin Cancer: A Systematic Review. Cancer Manag Res. 2022;14:3551–65. doi: 10.2147/CMAR.S389591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Passarelli MN, Karagas MR, Mott LA, Rees JR, Barry EL, Baron JA. Risk of keratinocyte carcinomas with vitamin D and calcium supplementation: a secondary analysis of a randomized clinical trial. Am J Clin Nutr. 2020;112(6):1532–9. doi: 10.1093/ajcn/nqaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4(6) doi: 10.1172/jci.insight.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127(1):106–16. doi: 10.1172/JCI89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103(31):11736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sato-Deguchi E, Imafuku S, Chou B, Ishii K, Hiromatsu K, Nakayama J. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167(1):77–84. doi: 10.1111/j.1365-2133.2012.10917.x. [DOI] [PubMed] [Google Scholar]
- 145.Bullock TA, Negrey J, Hu B, Warren CB, Hasan T, Maytin EV. Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: An interventional cohort-controlled trial. J Am Acad Dermatol. 2022;87(1):80–6. doi: 10.1016/j.jaad.2022.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Madani S, Marwaha S, Dusendang JR, et al. Ten-Year Follow-up of Persons With Sun-Damaged Skin Associated With Subsequent Development of Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2021;157(5):559–65. doi: 10.1001/jamadermatol.2021.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Force USPST, Mangione CM, Barry MJ, et al. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2023;329(15):1290–5. doi: 10.1001/jama.2023.4342. [DOI] [PubMed] [Google Scholar]
- 148.Garbe C, Augustin M, Augustin J, et al. Evaluation of skin cancer screening in Germany - participation, tumor detection and interval tumors based on SHI data. J Dtsch Dermatol Ges. 2023;21(Suppl 5):3–11. doi: 10.1111/ddg.15170. [DOI] [PubMed] [Google Scholar]
- 149.Henrikson NB, Ivlev I, Blasi PR, et al. Screening for Skin Cancer: An Evidence Update for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); Apr, 2023. [PubMed] [Google Scholar]
- 150.Ahmady S, Jansen MHE, Nelemans PJ, et al. Risk of Invasive Cutaneous Squamous Cell Carcinoma After Different Treatments for Actinic Keratosis: A Secondary Analysis of a Randomized Clinical Trial. JAMA Dermatol. 2022;158(6):634–40. doi: 10.1001/jamadermatol.2022.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Koch EAT, Steeb T, Bender-Sabelkampf S, et al. Poor Adherence to Self-Applied Topical Drug Treatment Is a Common Source of Low Lesion Clearance in Patients with Actinic Keratosis-A Cross-Sectional Study. J Clin Med. 2023;12(11) doi: 10.3390/jcm12113813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Steeb T, Wessely A, von Bubnoff D, et al. Treatment Motivations and Expectations in Patients with Actinic Keratosis: A German-Wide Multicenter, Cross-Sectional Trial. J Clin Med. 2020;9(5) doi: 10.3390/jcm9051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weinstock MA, Bingham SF, Cole GW, et al. Reliability of counting actinic keratoses before and after brief consensus discussion: the VA topical tretinoin chemoprevention (VATTC) trial. Arch Dermatol. 2001;137(8):1055–8. [PubMed] [Google Scholar]
- 154.Schmitz L, Gambichler T, Gupta G, Stucker M, Dirschka T. Actinic keratosis area and severity index (AKASI) is associated with the incidence of squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2018;32(5):752–6. doi: 10.1111/jdv.14682. [DOI] [PubMed] [Google Scholar]
- 155.Dreno B, Cerio R, Dirschka T, et al. A Novel Actinic Keratosis Field Assessment Scale for Grading Actinic Keratosis Disease Severity. Acta Derm Venereol. 2017;97(9):1108–13. doi: 10.2340/00015555-2710. [DOI] [PubMed] [Google Scholar]
- 156.Dubois A, Levecq O, Azimani H, et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J Biomed Opt. 2018;23(10):1–9. doi: 10.1117/1.JBO.23.10.106007. [DOI] [PubMed] [Google Scholar]
- 157.Ruini C, Schuh S, Gust C, et al. In-Vivo LC-OCT Evaluation of the Downward Proliferation Pattern of Keratinocytes in Actinic Keratosis in Comparison with Histology: First Impressions from a Pilot Study. Cancers (Basel) 2021;13(12) doi: 10.3390/cancers13122856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ruini C, Schuh S, Gust C, et al. Line-field confocal optical coherence tomography for the in vivo real-time diagnosis of different stages of keratinocyte skin cancer: a preliminary study. J Eur Acad Dermatol Venereol. 2021;35(12):2388–97. doi: 10.1111/jdv.17603. [DOI] [PubMed] [Google Scholar]
- 159.Cinotti E, Bertello M, Cartocci A, et al. Comparison of reflectance confocal microscopy and line-field optical coherence tomography for the identification of keratinocyte skin tumours. Skin Res Technol. 2023;29(1):e13215. doi: 10.1111/srt.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lenoir C, Cinotti E, Tognetti L, et al. Line-field confocal optical coherence tomography of actinic keratosis: a case series. J Eur Acad Dermatol Venereol. 2021;35(12):e900–e2. doi: 10.1111/jdv.17548. [DOI] [PubMed] [Google Scholar]
- 161.Daxenberger F, Deussing M, Eijkenboom Q, et al. Innovation in Actinic Keratosis Assessment: Artificial Intelligence-Based Approach to LC-OCT PRO Score Evaluation. Cancers (Basel) 2023;15(18) doi: 10.3390/cancers15184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thamm JR, Daxenberger F, Viel T, et al. Artificial intelligence-based PRO score assessment in actinic keratoses from LC-OCT imaging using Convolutional Neural Networks. J Dtsch Dermatol Ges. 2023;21(11):1359–66. doi: 10.1111/ddg.15194. [DOI] [PubMed] [Google Scholar]
- 163.Fischman S, Pérez-Anker J, Tognetti L, et al. Non-invasive scoring of cellular atypia in keratinocyte cancers in 3D LC-OCT images using Deep Learning. Sci Rep. 2022;12(1):481. doi: 10.1038/s41598-021-04395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thamm J, Welzel J, Schuh S. AI-based and LC-OCT-guided follow-up of actinic keratoses under treatment with Tirbanibulin 1% doi: 10.1111/jdv.20059. Submitted to JEADV and currently under review. [DOI] [PubMed] [Google Scholar]