Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a prevalent respiratory condition and a leading cause of mortality, with acute exacerbations (AECOPD) significantly complicating its management and prognosis. Despite the development of various prognostic prediction models for patients with AECOPD, their performance and clinical applicability remain unclear, necessitating a systematic review to evaluate these models and provide guidance for their future improvement and clinical use.
Method
PubMed, Web of Science, CINAHL, Scopus, EMBASE, and Medline were searched for studies published from their inception until February 5, 2024. Data extraction and evaluation were conducted using the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS). The Prediction model Risk Of Bias Assessment Tool (PROBAST) was employed to assess the risk of bias and applicability of the models.
Results
After deduplication and screening 5942 retrieved articles, 46 studies comprising 53 models were included. Of these, 17 (37.0%) studies developed from studies conducted in China. All models were based on cohort studies. Mortality was the predicted outcome in 27 (50.9%) models. Logistic regression was used in 41 (77.4%) models, while machine learning methods were employed in 9 (17.0%) models. The median (minimum, maximum) sample size for model development was 672 (106, 150,035). The median (minimum, maximum) number of predictors per model was 5 (2, 42). Frequently used predictors included age (n = 28), dyspnea severity scores (n = 12), and PaCO2 (n = 11). The pooled AUC was 0.80 for mortality prediction models and 0.84 for hospitalization-related outcomes. 52 models have a high overall risk of bias, and all models were judged to have low concern regarding applicability. Major sources of bias included insufficient sample sizes (83.0%), reliance on univariate analysis for predictor selection (73.6%), inappropriate internal and external validation methods (54.7%), inappropriate inclusion and exclusion criteria for study subjects (50.9%) and so on. The only model with low bias was the PEARL score.
Conclusion
Current prognostic risk prediction models for patients with AECOPD generally exhibit high bias. Future efforts should standardize model development and validation methods, and develop widely usable clinical models.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03033-4.
Keywords: Chronic obstructive pulmonary disease, Acute exacerbation, Prediction model, Prognostic risk, Systematic review
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory condition defined by chronic cough, difficulty breathing, and airflow limitation [1]. In 2019, the global prevalence of COPD among individuals aged 30 to 79 was 10.3%, accounting for approximately 391 million people [2]. COPD ranks as the third leading cause of mortality among non-communicable diseases (NCDs) both globally and in China [3]. The management of COPD is significantly challenged by acute exacerbations (AECOPD). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), AECOPD is characterized by a worsening of respiratory symptoms surpassing usual day-to-day variations and leads to changes in medication [1]. The prognosis of AECOPD can include a decline in health-related quality of life, substantial medical costs, and various complications [4]. Additionally, patients with AECOPD are more likely to die from respiratory diseases compared to those without acute exacerbations [5].
Therefore, it is crucial to identify prognostic risk factors for patients with AECOPD and to develop simple, effective, and clinically valuable prediction models. These models can better estimate the risk of future deterioration in patients with exacerbations, providing reliable references for clinical care, early intervention, and personalized treatment, which are essential for improving patient survival rates and quality of life.
Owing to the heterogeneity of COPD and exacerbations, as well as the poor recognition and self-reporting of these conditions [6], early diagnosis and prognosis of patients with AECOPD have remained challenging in clinical practice and a focal point of research. Although various prognostic models for patients with AECOPD have been developed, which vary widely in type, performance, and applicability, the predictive performance and practical validity of these models remain unclear.
This study systematically reviews prognostic prediction models for patients with AECOPD and evaluates their potential to predict the risk of adverse outcomes. This review discusses the current state of research and explores future directions, providing a reference for the development, validation, and improvement of risk prediction models for patients with AECOPD. Furthermore, this study aims to provide a basis for their application in clinical practice.
Methods
Literature search
This study searched six databases: PubMed, Web of Science, CINAHL, Scopus, EMBASE, and Medline. The search period spanned from the establishment of each database to February 5, 2024. The keywords related to the study population and research methods included: (“acute exacerbation of chronic obstructive pulmonary disease” OR “AECOPD” OR “acute exacerbation of COPD” OR “exacerbation of COPD” OR “COPD exacerbation”) AND (“risk assessment” OR “risk prediction” OR “risk index” OR “risk score” OR “predict” OR “prognostic factors” OR “risk calculation” OR “prediction” OR “machine learning” OR “artificial intelligence” OR “algorithm” OR “deep learning” OR “regression”). Based on the filter functions provided by the databases, we selected English literature and journal articles. The search was conducted independently by two researchers. The results from each database were merged and deduplicated using reference management software to obtain the final total number of studies that required further screening.
Screening criteria
The inclusion and exclusion criteria for the target prognostic models in this study were defined based on the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS) [7].
The inclusion criteria were as follows: (1) the primary objective of the study is to develop or improve prognostic models for patients with AECOPD; (2) the prognostic model should assess the prognosis of patients with AECOPD to aid in early identification of adverse outcomes and inform closer monitoring and treatment decisions; (3) the study should involve the development of prognostic models using independent data or the updating of existing models; (4) the target population of the prognostic models should be adult patients diagnosed with AECOPD or those primarily diagnosed with respiratory-related diseases and secondarily with AECOPD; (5) the outcomes of the prognostic models should be any clinical outcomes that may occur in patients with AECOPD; (6) the prognostic timeframe should measure predictors and outcomes at specific points during the clinical course following AECOPD diagnosis; (7) the models should generally be used for early monitoring of patients with AECOPD; and (8) the models should have the capability to predict individual risks, with at least one metric evaluating the model's performance.
The exclusion criteria were as follows: (1) studies primarily aimed at establishing diagnostic models for AECOPD, predictive models for AECOPD complications, or prognostic models for COPD exacerbations; (2) studies solely conducting external validation or comparison of existing models; (3) cross-sectional studies where predictors and outcomes are measured simultaneously; (4) studies developing models using only one biomarker or one independent prognostic factor; (5) methodological studies and studies purely screening for risk factors; and (6) studies that are non-English, reviews, letters, conference abstracts, duplicate publications, or expert opinions.
Based on the inclusion and exclusion criteria, an initial manual screening of the deduplicated results was conducted by reviewing the titles and abstracts. Articles for which the inclusion could not be determined due to insufficient information were provisionally included. A second screening of the selected articles was conducted through full-text reading. Ultimately, a total of 46 studies were included in the systematic review. The initial screening was performed by a single researcher, while the second screening was independently conducted by two researchers. Any issues or discrepancies encountered during the process were resolved through discussion.
Data collection
The data collection process utilized standardized CHARMS forms for extraction [8]. The information collected included the study title, first author, publication year and journal, country, data source, participants, outcomes to be predicted, predictors, sample size, methods for handling missing data, modeling methods, model performance, model validation, and basic information about the final model. One researcher extracted the data, while another reviewed it. Any discrepancies were resolved through discussion. Items not found in the full text, supplementary materials, or referenced previous studies were marked as "not reported" or "unclear". The extracted data were then summarized and analyzed to provide basic study information and model characteristics.
For statistical analysis, each predictive model developed for different outcomes or characteristics of the AECOPD population was counted separately, excluding those without performance evaluation. For studies using multiple modeling methods for the same population and clinical outcome, only the best method's results were included. If the same model was validated for other outcomes, only initial modeling information was included.
Quality assessment
The Prediction model Risk Of Bias Assessment Tool (PROBAST) is used to evaluate the risk of bias in predictive model studies and the applicability of the model when used in the intended target population and clinical settings [9]. Bias is defined as systematic errors that occur in research, leading to distortion or flaws in results, which hinder the internal validity of the study. Applicability refers to the extent to which the included participants or settings, predictive factors, and outcome definitions align with the specific research question posed in the review. This tool assesses the risk of bias from four aspects: participants, predictors, outcomes, and analysis, while also evaluating the applicability of the first three aspects. The answer for each domain is categorized as low, high, or unclear. If at least one domain is assessed as having a high risk of bias or a high concern for applicability, then the overall assessment is high risk or of high concern regarding applicability. If at least one domain is rated as unclear, and there are no high risk or high applicability concerns, then the overall assessment is unclear. The assessment is conducted independently by two researchers, and any discrepancies are resolved through discussion with a third researcher.
Meta-analysis
To evaluate the performance of prediction models for AECOPD patients, a random-effects meta-analysis was conducted. As this study only included the development of cohort models, a meta-analysis was conducted on prediction models with mortality and hospitalization-related outcomes. The area under the receiver operating characteristic curve (AUC) and its 95% confidence interval were extracted from each study. Studies without reported AUCs were excluded. Heterogeneity was assessed using the I2 statistic, with high values indicating substantial heterogeneity. Publication bias was assessed using funnel plots and Egger's test.
Results
Literature screening
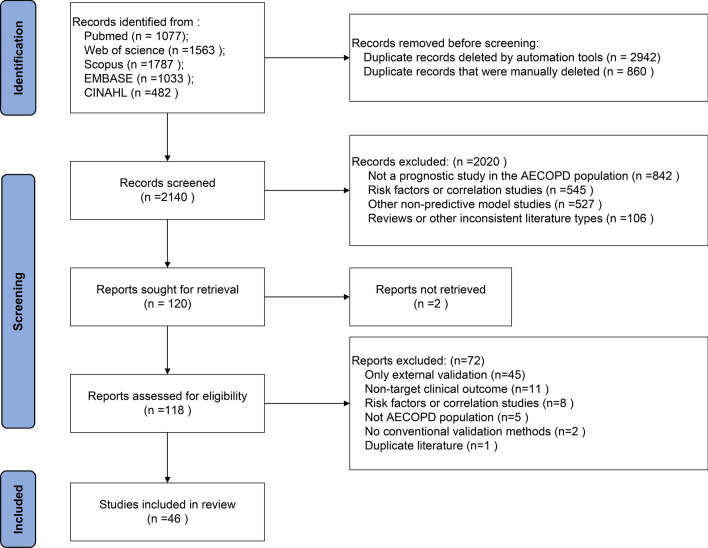
A total of 5942 articles were retrieved from various databases. After merging, 3802 duplicates were removed, resulting in 2140 unique articles. During the screening process, 2094 articles were excluded, leading to the inclusion of 46 articles in the final review (Fig. 1).
Fig. 1.
Literature screening flow diagram
Basic information of the studies
Among the 46 studies, 19 (41.3%) used a prospective study design, while 27 (58.7%) used a retrospective design, with 6 studies using aggregated data. China had the highest number of studies (n = 17; 37.0%), followed by Spain (n = 7; 15.2%), the United States (n = 6; 13.0%), and the United Kingdom (n = 5; 10.9%). Other countries represented include India, France, Canada, Thailand, Israel, Norway, and Greece (Table 1). Regarding the study setting, the majority of studies (n = 28; 60.9%) indicated that screening was conducted in hospitals, while a portion of the studies (n = 19; 41.3%) specified the departments, including 7 in emergency departments (ED), 6 in intensive care units (ICU), 3 in general respiratory wards, and 1 in respiratory and critical care medicine departments, with 3 studies investigating both internal medicine wards and ICU/ED. (See Supplement 1 for details).
Table 1.
Basic information of the studies
| Number | Author | Year | Study reigon | Study design | Enrolment period | Study setting | Model name |
|---|---|---|---|---|---|---|---|
| 1 | Ying P. Tabak [34] | 2009 | the United States | Aggregated data | 2004.1.1–2006.12.31 | Hospitals | BAP65 |
| 2 | Ying P. Tabak [35] | 2013 | the United States | Aggregated data | 2005.1.1–2007.12.31 | Hospitals | – |
| 3 | Peter K. Lindenauer [36] | 2013 | the United States | Aggregated data | 2008 | Hospitals | – |
| 4 | Cassandra M. Batzlaff [37] | 2014 | the United States | Retrospective cohort | 1995.4–2009.11 | ICU | – |
| 5 | Matthew Bonomo [38] | 2022 | the United States | Retrospective cohort | 2008.11–2018.12 | Hospital | – |
| 6 | Shi Chen [39] | 2023 | the United States | Aggregated data | 2002–2019 | ICU | – |
| 7 | M.J. Wildman [40] | 2009 | the UK | Prospective cohort | 2002.3–2003.9 | ICU and Respiratory High Dependency Units (RHDU) | – |
| 8 | Alex C. Asiimwe [41] | 2011 | the UK | Retrospective cohort | Unclear | Hospital | – |
| 9 | John Stee r[42] | 2012 | the UK | Prospective cohort | 2008.12–2010.6 | Hospitals | The DECAF Score |
| 10 | C Echevarria [31] | 2017 | the UK | Prospective cohort | 2008.12–2010.6 | Hospitals | The PEARL score |
| 11 | Tom Hartley [43] | 2021 | the UK | Retrospective cohort | 2008.11–2013.5 | Hospitals | The NIVO score |
| 12 | Prachya Mekanimitdee [44] | 2021 | Thailand | Retrospective cohort | 2015.10–2017.9 | Tertiary care center | The MAGENTA model |
| 13 | Susana García-Gutiérrez [45] | 2014 | Spain | Prospective cohort | 2008.6–2010.9 | EDs | ABC scores |
| 14 | José M Quintana [46] | 2014 | Spain | Prospective cohort | 2008.6–2010.9 | EDs | – |
| 15 | J. M. Quintana [47] | 2014 | Spain | Prospective cohort | 2008.6–2010.9 | ED | – |
| 16 | Pedro Almagro [48] | 2014 | Spain | Prospective cohort | 2009.10–2010.10 | EDs and internal medicine services | – |
| 17 | Cristóbal Esteban [49] | 2015 | Spain | Prospective cohort | 2008.6–2010.9 | ED | CART model |
| 18 | Juan Luis García-Rivero [50] | 2017 | Spain | Prospective cohort | 2013.1–2013.3 | Hospital | – |
| 19 | C esar Alameda [51] | 2021 | Spain | Prospective cohort | 2013.12–2014.11 | Health centres (HC) | – |
| 20 | Ying Wang [52] | 2014 | Norway | Retrospective cohort | 2006.3–2008.12 | Hospital | – |
| 21 | Yukiyo Sakamoto [53] | 2017 | Japan | Aggregated data | 2010.7.1–2013.3.31 | Hospitals | – |
| 22 | Akihiro Shiroshita [54] | 2022 | Japan | Retrospective cohort | 2008.4–2020.7 | Hospitals | – |
| 23 | Jiang-Chen Peng [55] | 2022 | Israel | Aggregated data | 2001–2012 | ICU | – |
| 24 | A. Mohan [56] | 2007 | India | Prospective cohort | 2004.2–2006.3 | Medical wards and ICU | – |
| 25 | Karthikeyan Ramaraju [57] | 2016 | India | Retrospective cohort | 2012.8–2013.7 | Hospital | – |
| 26 | Filia Diamantea [58] | 2014 | Greece | Prospective cohort | 2010.1–2012.6 | Respiratory medicine departments | AECOPD-F score |
| 27 | N. Roche [56] | 2008 | France | Prospective cohort | 2003.11–2004.2 | Emergency departments | – |
| 28 | Nicolas Roche [59] | 2014 | France | Retrospective cohort | 2006.10–2007.6 | Respiratory medicine department | 2008 score-new |
| 29 | Liping Fan [60] | 2014 | China | Prospective cohort | 2012.10–2014.5 | Respiratory ICU | – |
| 30 | Dong Liu [61] | 2015 | China | Prospective cohort | 2013.11–2014.2 | Respiratory medical ward or ICU | – |
| 31 | Qi‐fang Shi [62] | 2018 | China | Prospective cohort | 2016.1–2017.12 | ICU | Re-AE INDEX |
| 32 | Wei-ping Hu [63] | 2019 | China | Retrospective cohort | 2005–2007 | Hospitals | – |
| 33 | Mi Zhou [64] | 2019 | China | Retrospective cohort | 2011.2–2018.6 | Department of Respiratory and Critical Care Medicine | – |
| 34 | Yi Chen [65] | 2020 | China | Retrospective cohort | 2017.1–2018.12 | Hospital | – |
| 35 | Xing Yu [66] | 2020 | China | Retrospective cohort | 2015.1–2017.12 | Hospital | – |
| 36 | Junfeng Peng [67] | 2020 | China | Retrospective cohort | 2011–2018 | Hospital | – |
| 37 | Wei Bi [68] | 2020 | China | Prospective cohort | 2018.1–2018.12 | Department of Emergency Internal Medicine | – |
| 38 | Fen Dong [69] | 2021 | China | Retrospective cohort | 2015.1.1–2019.12.31 | Hospital | – |
| 39 | Lan Chen [70] | 2021 | China | Retrospective cohort | 2018.1–2020.9 | ED | – |
| 40 | Lili Chen and Shiping Chen [71] | 2021 | China | Prospective cohort | 2016.1–2019.12 | Hospital | – |
| 41 | Lifen Yang [72] | 2022 | China | Retrospective cohort | No information | Hospital | – |
| 42 | Dawei Chen [73] | 2023 | China | Retrospective cohort | 2014.1–2017.1 | Hospital | AAAAN Score |
| 43 | Lin Yu [74] | 2023 | China | Retrospective cohort | 2012.9–2021.8 | Hospital | – |
| 44 | Shiyi He [75] | 2023 | China | Prospective cohort | 2020.1–2022.6 | Hospital | – |
| 45 | Li-Na Yan [76] | 2024 | China | Retrospective cohort | 2020.6–2023.6 | Hospital | – |
| 46 | Reza Fakhraei [77] | 2023 | Canada | Retrospective cohort | 2012–2018 | General Internal Medicine ward | – |
Basic information of the models
Among the 46 studies, two studies developed models for two different clinical outcomes each, and one study developed six types of models, resulting in a total of 53 models. Subsequent analyses will use the models, rather than the studies, as the independent units for information summarization and statistical analysis. (See Supplement 2 for details).
Outcomes
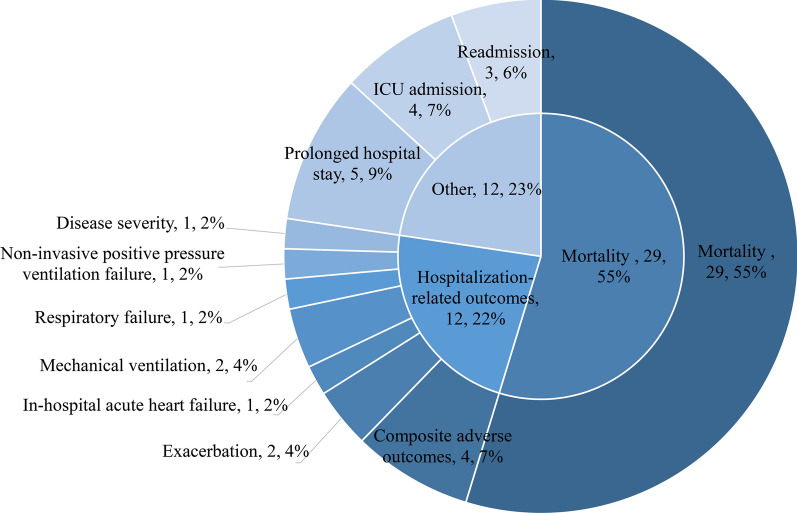
Out of the 53 models, 51 were newly developed models, while the remaining 2 models were modified models based on the DECAF score, with outcomes of mortality and ICU admission, respectively. The most common outcome was mortality (n = 29; 54.7%). Hospitalization-related outcomes were the second most common (n = 12; 22.6%), including prolonged hospital stay (n = 5; 9.4%), ICU admission (n = 4; 7.5%), and readmission (n = 3; 5.7%). Other outcomes included composite adverse outcomes, exacerbation, in-hospital acute heart failure, mechanical ventilation, respiratory failure, non-invasive positive pressure ventilation failure, post-discharge survival, and disease severity. While the remaining 2 models were modified models based on the DECAF score, with outcomes of mortality and ICU admission, respectively (Fig. 2).
Fig. 2.
Frequency Distribution of Different Outcome Types
Modeling methods
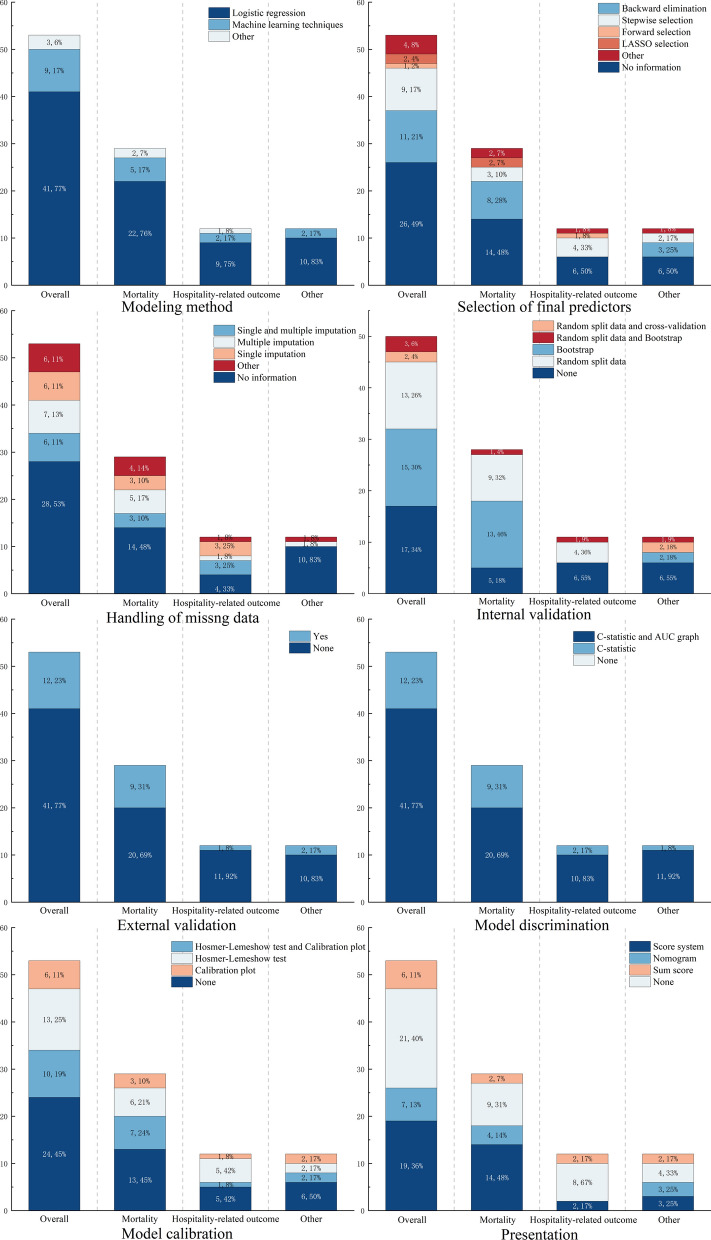
The majority of models (n = 38; 71.7%) used univariate analysis to screen predictors before including them in the model, while only a smaller portion (n = 15; 28.3%) selected predictors based on prior knowledge. For modeling methods, most models (n = 41; 77.4%) used logistic regression, while fewer (n = 9; 17.0%) applied machine learning methods. Additionally, one study created ROC curves for variables with statistical significance in univariate analysis and developed a scoring system based on variables with high predictive ability. Two modified models were updated directly based on prior knowledge and subsequently validated. In detail, the logistic regression models included 10 with backward regression, 9 with stepwise regression, 2 with LASSO regression, 1 with forward regression, and 19 with no specified method. Machine learning methods included decision tree-based algorithms such as CART (n = 3), Random Forest (n = 2), C5.0 (n = 1), RPART (n = 1), and the gradient boosting algorithm XGBoost (n = 2).
Handling of missing data
A portion of the models (n = 27; 50.9%) did not report any methods for handling missing data. Four models (7.5%) used complete case analysis. Six models (11.3%) used both single imputation and multiple imputation methods, while seven models (13.2%) used only multiple imputation, and six models (11.3%) used only single imputation. Two models analyzed missing values by placing them in separate columns. One model explicitly stated that it did not handle missing data.
Sample size and predictive factors
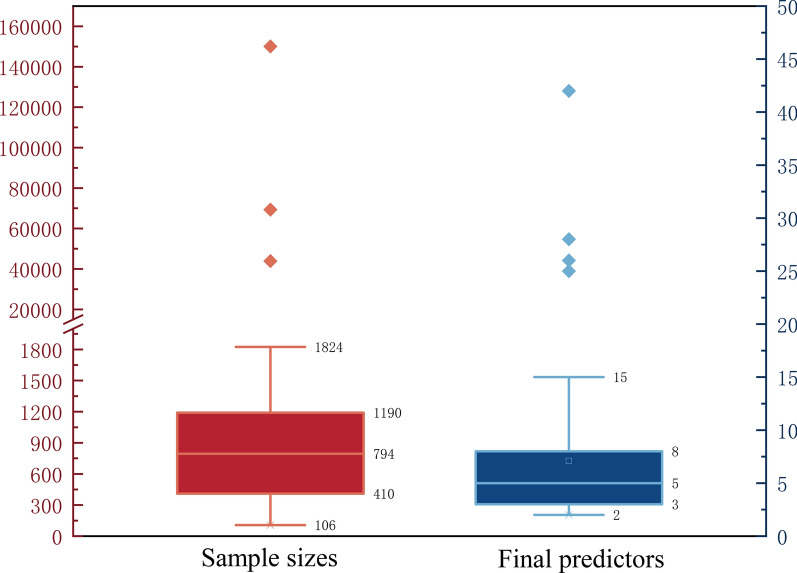
The median (minimum, maximum) sample size for model development was 672 (106, 150,035). The median (minimum, maximum) number of candidate predictors was 21 (5, 82). However, only six models had an EPV (events per variable) or EPP (events per predictor) value greater than 10, calculated based on sample size, the number of outcome events, and the number of candidate predictors before selection.
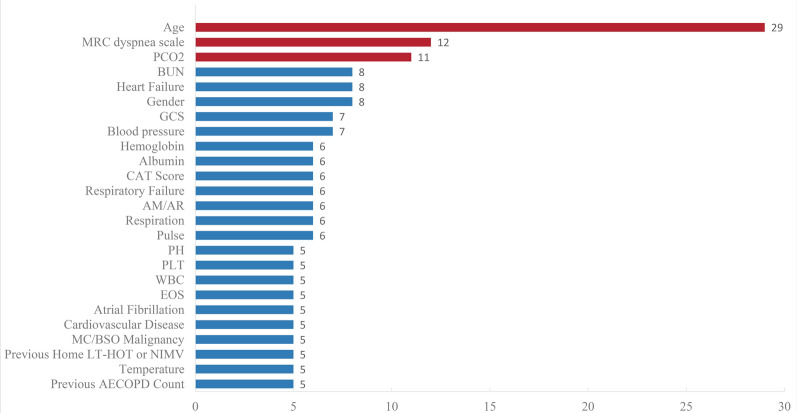
The median (minimum, maximum) number of final predictors in the models was 5 (2, 42) (Fig. 3). Predictive factors used more than ten times included age (n = 28; 52.8%), the MRC (Medical Research Council) scale for assessing dyspnea (n = 12; 22.6%), and PaCO2 (n = 11; 20.8%) (Fig. 4).
Fig. 3.
Sample size and number of predictors
Fig. 4.
Predictive factors in 53 models. MRC Medical Research Council, PCO2 Partial Pressure of Carbon Dioxide, BUN blood urea nitrogen, GCS Glasgow Coma Scale, CAT Score COPD Assessment Test Score, AM/AR Use inspiratory assistance muscle/accessory breathing, PH Potential of hydrogen; PLT Platelet count; WBC White blood cell, EOS Eosinophil count, MC/BSO Metastatic cancer/malignant tumors of the blood or solid organs, LT-HOT Long-term home oxygen therapy, NIMV Non-invasive mechanical ventilation
Internal and external validation
Three (5.7%) models did not undergo internal or external validation, and another 3 (5.7%) models underwent both internal and external validation (Fig. 5). Among the models that performed internal validation (n = 36; 67.9%), the most commonly used methods were random splitting (n = 18) and the bootstrap resampling method (n = 15). Two models used populations from the same study site but different time periods as their internal validation.
Fig. 5.
Basic information of the models
Only 12(22.6%) models conducted external validation, employing different time periods, different study sites, or entirely independent datasets.
Model performance index and presentation
For calibration, the most frequently used assessment methods were the Hosmer–Lemeshow test (n = 20; 37.7%) and calibration plots (n = 12; 22.6%). Other methods included calibration slope (n = 5; 9.4%) and intercept (n = 2; 3.8%). Twenty-five models (47.2%) did not report any calibration assessment.
For discrimination, all models used the C-statistic, with 41 (77.4%) models also employing ROC curves.
Regarding the presentation of the models, excluding the 10 machine learning models, 11 (20.8%) models did not provide a presentation format. 19 (35.8%) models developed scoring systems, 7 (13.2%) models used nomograms, and 6 (11.3%) models constructed regression-based equations. Among these, one model developed an online calculator based on the regression equation.
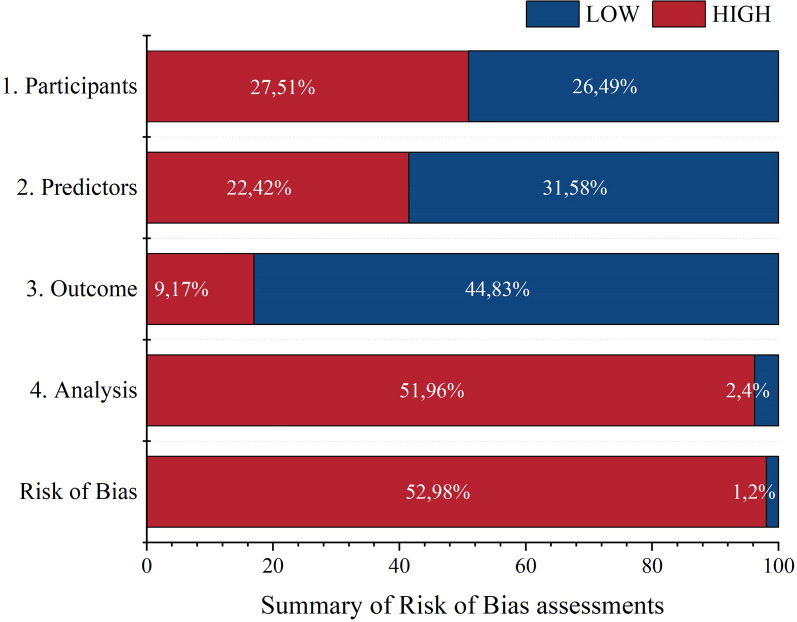
Risk of bias assessment
Among the 53 models, 52 were judged to have a high risk of bias (ROB) (Fig. 6), all models were judged to have low overall applicability. Specifically, 27 (50.9%) models were rated as high ROB in the domain of participant selection, 22 (41.5%) models in the domain of predictor assessment, 9 (17.0%) models in the domain of outcome assessment, and 50 (94.2%) models in the domain of analysis. The primary sources of bias included insufficient sample sizes (n = 44; 83.0%), the use of univariate analysis to select predictors (n = 39; 73.6%), inappropriate internal and external validation methods (n = 29; 54.7%), inappropriate inclusion and exclusion criteria for study subjects (n = 27; 50.9%), inadequate methods for assessing model performance (n = 22; 41.5%), and inconsistent definitions and measurement methods for predictors across all study subjects (n = 21; 39.6%). The only model evaluated as having a low risk of bias (ROB) was the PEARL score. (See Supplement 3 and Supplement 4 for details).
Fig. 6.
Distribution of risk of bias assessment based on four domains
Meta analysis
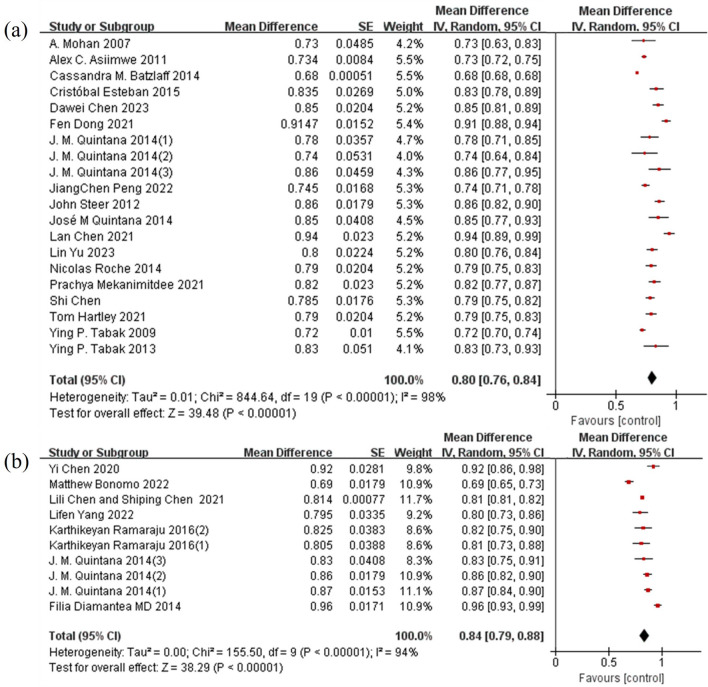
For prediction models with mortality as the outcome, the pooled AUC was 0.80 (95% CI: 0.76–0.84). For models with hospitalization-related outcomes, the pooled AUC was 0.84 (95% CI: 0.79–0.88), indicating good predictive accuracy (Fig. 7). However, heterogeneity analysis showed I2 statistics of 98% and 94%, respectively, indicating high heterogeneity among the studies (P < 0.0001). This high heterogeneity could be attributed to differences in study design, patient characteristics, types of prediction models, and follow-up periods. Funnel plots and Egger's test were used to assess publication bias, with results showing no significant publication bias.
Fig. 7.
Meta-analysis of prediction models with mortality or hospitalization as outcomes. a Mortality; b Hospitalization
Discussion
Based on the results of this review, the research on AECOPD prognostic risk prediction has garnered widespread attention globally, particularly in China. In developed countries, most studies are concentrated in the UK, the US, and Spain, following trends similar to those observed in COPD prognostic risk prediction models[10]. However, in contrast, our study found that China had the highest number of model studies, with 14 (82.4%) published in 2019 or later. In recent years, the prevalence of COPD in China has exceeded the global average and continues to rise[11]. Following the inclusion of COPD action plans in the "Healthy China Initiative (2019–2030)", there has been a surge in scientific research and policy actions, reflecting an emphasis on chronic disease management at both national and societal levels. An example of this is the early screening and comprehensive intervention project for high-risk COPD populations initiated in 2021 [12].
Researchers have explored the prognostic outcomes of patients with AECOPD in various clinical settings. Compared to general wards, the number of patients with AECOPD is higher, and their conditions are more critical in emergency departments and ICUs [13]. Prognostic models are more applicable in these acute care settings. Mortality remains the most scrutinized clinical outcome due to its strong correlation with the disease burden resulting from exacerbation and death[14]. Hospitalization-related outcomes are also significant, as they not only increase healthcare costs but also elevate the risk of nosocomial infections and other complications[15]. Developing predictive models for hospitalization can optimize resource allocation and improve bed turnover rates, providing more efficient treatment for patients. However, there is limited focus on specific symptomatic prognostic issues, such as the high incidence of cardiovascular events post-discharge[16]. This study synthesizes results from different research to reflect the overall effectiveness of the model regarding the same outcomes in combined AUC. However, high heterogeneity also indicates that the geographical distribution, departmental limitations, and study outcomes of these studies lead to variations in the construction and application of AECOPD prognostic risk prediction models across different countries and healthcare settings, potentially influenced by research design, patient characteristics, types of prediction models, national policies, and healthcare resources.
The generalizability of the model refers to its implementation in different settings. Although there are numerous external validation studies demonstrating the effectiveness of DECAF, BAP65[17], and PEARL[18], the methods and conditions used during model development can impact their performance. Therefore, it is essential to provide detailed and cautious descriptions of the research methods to facilitate better dissemination. By integrating the bias risk factors from modeling studies and considering their generalizability, we have summarized the following issues:
Many studies may exclude patients with comorbidities and complications to emphasize the prognosis of adverse outcomes in AECOPD, which can affect the results to some extent. We believe that comorbidities or complications can serve as predictive factors, or the reasons for exclusion should be clearly stated and explained; otherwise, it may be challenging to generalize under comparable conditions[19].
Numerous multicenter studies do not explicitly detail the measurement, evaluation methods, and standards for predictive factors across different research centers, making it difficult to unify and compare the reliability of results in various environments. It is essential to strictly standardize and clarify the assessment criteria for predictive factors, ensuring that all study subjects utilize the same definitions and measurement methods. This highlights a limitation of integrated databases; while studies may employ cohort designs[20], prospective methodologies better meet the requirements for this research.
Many studies fail to provide clear definitions for outcome determinations, such as ICU admission or intubation. Objective indicators and thresholds should be emphasized in outcome assessments to avoid subjective physician judgments; otherwise, widespread adoption may be hindered.
In terms of the rigor in statistical methodology and result presentation, we have summarized the following issues:
A larger sample size contributes to a more robust model. When developing predictive models for binary outcomes or event time results, the required sample size should ensure that each candidate variable has at least 10 events. It is important to note that the candidate variables represent the total number of predictive factors considered at any stage of the modeling process, not just those included in the final model. Many studies overlook this requirement. Future research should place greater emphasis on the importance of sample size in the development of predictive models and select appropriate statistical methods for calculation and analysis, minimizing the loss of modeling sample size as much as possible.
During the model development process, careful evaluation and control of the number and complexity of variable selection are needed. Relying solely on univariate analyses to filter and refine the most predictive variables may overlook contributions from variable combinations[21]. It is advisable to further optimize the variable selection strategy during model development, utilizing prior experience where possible and employing backward elimination of redundant predictors or forward selection of promising predictors[22].
Most models are at risk of overfitting or instability when the sample size is relatively small or when there are numerous and complex candidate predictors[23]. In cases where there may be heightened optimism or overfitting, internal and external validation becomes particularly important. However, studies that simultaneously develop and validate models are limited. Future research should adopt stricter methodologies to validate model stability, and it is recommended to use bootstrap methods rather than random split techniques for this purpose[24].
To be applicable in routine clinical practice, model coefficients are often simplified to numbers that are easy for clinicians to score. The interpretability and visual representation of models are crucial for acceptance by healthcare professionals, especially for machine learning models[25]. However, some models fail to provide detailed presentation formats, which may hinder the understanding and application of model results in actual clinical decision-making.
The predictive factors included in the models of this review include factors such as medical history, clinical manifestation, treatment condition, comorbidities, and laboratory results. Different models focus on various aspects, utilizing a wide range of indicators. Notably, age, dyspnea assessment via the MRC scale, and PaCO2 are frequently emphasized. Age, as the most commonly used factor, reflects the higher social and clinical burden faced by the elderly population [26]. Dyspnea, being one of the primary symptoms during acute exacerbation, is effectively assessed using the MRC dyspnea scale, which is widely used in COPD patients [27]. PaCO2 is a crucial laboratory indicator for evaluating ventilation status. Future research should continue to screen and compare the accuracy of these factors, paying particular attention to the performance and applicability of models across different age groups. Additionally, new clinical findings, such as those related to microbiome ecology [28], novel metabolites [29], and sputum biomarkers [30], should be considered for their potential value in improving or developing new models.
The PEARL score is the only predictive model assessed as having low bias, predicting 90-day readmission or non-readmission mortality [31]. Compared to other models, this study explicitly stated that all sites adhered to data collection guidelines, had a sufficient sample size, and avoided using univariate analysis for predictor selection, ultimately presenting the model results as a score. In previous studies [32], the DECAF score, despite issues of insufficient sample size and the use of univariate analysis for predictor selection, was recommended as a better model for reducing hospital admissions and 90-day mortality due to its simplicity and external validation in multiple countries. From the perspective of predictive factors, both models are simple in structure. The PEARL score emphasizes the patient's medical history and cardiac function, while the DECAF score focuses more on acute physiological changes and lung pathology. Currently, both scores have considerable application ranges [33], but DECAF has been more extensively studied in clinical practice. This also highlights that, although preliminary assessments of modeling studies have been conducted, summarizing and evaluating external validation studies is equally important. Future research should further compare these two scoring models, particularly in different clinical settings and patient populations.
The study did not limit the outcomes of the models, allowing for a broader understanding of the current focus and quality of research on prognostic risk in patients with AECOPD. Using assessment tools, the study provided a comprehensive breakdown and extraction of information on the bias and applicability of the models, offering insights for future research improvements and clinical application testing. Additionally, valuable models were analyzed for their current status and future prospects. However, this study has some limitations. Restricting the literature search to English databases may introduce some limitations regarding population and regional characteristics. Furthermore, as this study only included information from studies that developed models, it did not comprehensively analyze the external validation of these models and conduct a meta-analysis of similar models to calculate summary estimates of model performance and calibration.
Conclusion
This study reviews 53 prognostic risk prediction models for AECOPD, highlighting differences and limitations in their predictive performance. The PEARL score shows a lower degree of bias but requires further validation across diverse populations. Future research should focus on methodological rigor, multi-center validation, and simplifying models for better clinical utility, ultimately advancing AECOPD risk prediction and individualized patient treatment.
Supplementary Information
Supplementary Material 1. Study Information: Basic information on all included studies, including authors, year, country, and study design.
Supplementary Material 2. Model Information: Information on all included predictive models, including study outcomes, sample size, and modeling methods.
Supplementary Material 3. ROB and Applicability: Assessment results of bias risk and applicability for all included predictive models.
Supplementary Material 4. Model Evaluation Information: Specific items related to the bias risk and applicability assessment results for all included predictive models.
Acknowledgements
Not applicable.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- AECOPD
Acute exacerbations of chronic obstructive pulmonary disease
- CHARMS
Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies
- PROBAST
Prediction model Risk Of Bias Assessment Tool
- MRC
Medical Research Council
- eMRC
Extended Medical Research Council
- PCO2
Partial Pressure of Carbon Dioxide
- BUN
Blood urea nitrogen
- GCS
Glasgow Coma Scale
- CAT Score
COPD Assessment Test Score
- AM/AR
Use inspiratory assistance muscle/accessory breathing
- PH
Potential of hydrogen
- PLT
Platelet count
- WBC
White blood cell
- EOS
Eosinophil count
- MC/BSO
Metastatic cancer/malignant tumors of the blood or solid organs
- LT-HOT
Long-term home oxygen therapy
- NIMV
Non-invasive mechanical ventilation
Author contributions
XZH conducted the literature search and screening, data extraction and analysis, and drafted the manuscript. WY assisted with the literature search and screening, and provided comprehensive editing. LF contributed to data extraction and analysis, and write sections of the manuscript. XY polished and revised the article. WYP supervised the study, providing guidance and critical revisions to the manuscript.
Funding
CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number: 2021-I2M-1-049).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ye Wang, Email: wangye_pumc@163.com.
Yuping Wang, Email: wyppumc@163.com.
References
- 1.Committees GG. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024. https://goldcopd.org/2024-gold-report/.
- 2.Blanco I, Diego I, Bueno P, Casas-Maldonado F, Miravitlles M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur Respir J. 2019;54(1):1900610. [DOI] [PubMed] [Google Scholar]
- 3.(WHO) WHO. Chronic obstructive pulmonary disease 2023. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Accessed 15 Jun 2024.
- 4.Halpin DM, Miravitlles M, Metzdorf N, Celli B. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenoir A, Whittaker H, Gayle A, Jarvis D, Quint JK. Mortality in non-exacerbating COPD: a longitudinal analysis of UK primary care data. Thorax. 2023;78(9):904–11. [DOI] [PubMed] [Google Scholar]
- 6.Jones P, Alzaabi A, Casas Herrera A, Polatli M, Rabahi MF, Cortes Telles A, et al. Understanding the Gaps in the Reporting of COPD Exacerbations by Patients: A Review. COPD. 2024;21(1):2316594. [DOI] [PubMed] [Google Scholar]
- 7.Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10): e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Felix BM, Lopez-Alcalde J, Roque M, Muriel A, Zamora J. CHARMS and PROBAST at your fingertips: a template for data extraction and risk of bias assessment in systematic reviews of predictive models. BMC Med Res Methodol. 2023;23(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170(1):W1–33. [DOI] [PubMed] [Google Scholar]
- 10.Bellou V, Belbasis L, Konstantinidis AK, Tzoulaki I, Evangelou E. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ (Clinical research ed). 2019;367: l5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IfHMa E. GBD Compare 2019. http://vizhub.healthdata.org/gbd-compare.
- 12.Medicine NCfR. Introduction of early screening and comprehensive intervention project for COPD high-risk population 2023. https://www.zryhyy.com.cn/gjhxzx/c104815/202305/7ffe5311fe074b1eb73740bf98782076.shtml.
- 13.Amin AN, Cornelison S, Woods JA, Hanania NA. Managing hospitalized patients with a COPD exacerbation: the role of hospitalists and the multidisciplinary team. Postgrad Med. 2022;134(2):152–9. [DOI] [PubMed] [Google Scholar]
- 14.Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD–a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prediletto I, Giancotti G, Nava S. COPD exacerbation: why it is important to avoid ICU admission. J Clin Med. 2023;12(10):3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto T, Shimada YJ, Faridi MK, Camargo CA Jr, Hasegawa K. Incidence of acute cardiovascular event after acute exacerbation of COPD. J Gen Intern Med. 2018;33(9):1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sv S. Prediction of Outcomes in Acute Exacerbation of COPD with Decaf Score and BAP 65 Scores in a Rural Population. J Assoc Phys India. 2020;68(1):80. [PubMed] [Google Scholar]
- 18.Kishor MN, Khippal N, Rathore YS, Jain S, Joshi V. Evaluation of PEARL score in assessingprognosis among patients with acute exacerbation of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2020;69(4):627–30. [Google Scholar]
- 19.MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reps JM, Ryan PB, Rijnbeek PR, Schuemie MJ. Design matters in patient-level prediction: evaluation of a cohort vs. case-control design when developing predictive models in observational healthcare datasets. J Big Data. 2021. 10.1186/s40537-021-00501-2. [Google Scholar]
- 21.Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Commun Health. 2020;8(1): e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendriksen JMT, Geersing GJ, Moons KGM, de Groot JAH. Diagnostic and prognostic prediction models. J Thromb Haemost. 2013;11(s1):129–41. [DOI] [PubMed] [Google Scholar]
- 23.Wynants L, Bouwmeester W, Moons KGM, Moerbeek M, Timmerman D, et al. A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. J Clin Epidemiol. 2015;68(12):1406–14. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81. [DOI] [PubMed] [Google Scholar]
- 25.Vellido A. The importance of interpretability and visualization in machine learning for applications in medicine and health care. Neural Comput Appl. 2020;32(24):18069–83. [Google Scholar]
- 26.Crisafulli E, Manco A, Guerrero M, Ceccato A, Huerta A, Gabarrús A, et al. Age is a determinant of short-term mortality in patients hospitalized for an acute exacerbation of COPD. Intern Emerg Med. 2021;16(2):401–8. [DOI] [PubMed] [Google Scholar]
- 27.Williams N. The MRC breathlessness scale. Occup Med (Oxford, England). 2017;67(6):496–7. [DOI] [PubMed] [Google Scholar]
- 28.Kou Z, Liu K, Qiao Z, Wang Y, Li Y, Li Y, et al. The alterations of oral, airway and intestine microbiota in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Front Immunol. 2024;15:1407439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding HZ, Wang H, Wu D, Zhou FC, Zhu J, Tong JB, et al. Serum metabolomics analysis of patients with chronic obstructive pulmonary disease and “frequent exacerbator” phenotype. Mol Med Rep. 2024. 10.3892/mmr.2024.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budroni S, Taccone M, Stella M, Aprea S, Schiavetti F, Bardelli M, et al. Cytokine biomarkers of exacerbations in sputum from Chronic Obstructive Pulmonary Disease patients: a prospective cohort study. J Infect Dis. 2024. 10.1093/infdis/jiae232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echevarria C, Steer J, Heslop-Marshall K, Stenton SC, Hickey PM, Hughes R, et al. The PEARL score predicts 90-day readmission or death after hospitalisation for acute exacerbation of COPD. Thorax. 2017;72(8):686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Z, Li X, Lei S, Xu J, Xie Y. A pooled analysis of the risk prediction models for mortality in acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2023;17(8):707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane ND, Gillespie SM, Steer J, Bourke SC. Uptake of clinical prognostic tools in COPD exacerbations requiring hospitalisation. COPD. 2021;18(4):406–10. [DOI] [PubMed] [Google Scholar]
- 34.Tabak YP, Sun X, Johannes RS, Gupta V, Shorr AF. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–602. [DOI] [PubMed] [Google Scholar]
- 35.Tabak YP, Sun X, Johannes RS, Hyde L, Shorr AF, Lindenauer PK. Development and validation of a mortality risk-adjustment model for patients hospitalized for exacerbations of chronic obstructive pulmonary disease. Med Care. 2013;51(7):597–605. [DOI] [PubMed] [Google Scholar]
- 36.Lindenauer PK, Grosso LM, Wang C, Wang Y, Krishnan JA, Lee TA, et al. Development, validation, and results of a risk-standardized measure of hospital 30-day mortality for patients with exacerbation of chronic obstructive pulmonary disease. J Hosp Med. 2013;8(8):428–35. [DOI] [PubMed] [Google Scholar]
- 37.Batzlaff CM, Karpman C, Afessa B, Benzo RP. Predicting 1-year mortality rate for patients admitted with an acute exacerbation of chronic obstructive pulmonary disease to an intensive care unit: an opportunity for palliative care. Mayo Clin Proc. 2014;89(5):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonomo M, Hermsen MG, Kaskovich S, Hemmrich MJ, Rojas JC, Carey KA, et al. Using machine learning to predict likelihood and cause of readmission after hospitalization for chronic obstructive pulmonary disease exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:2701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S, Shi Y, Hu B, Huang J. A prediction model for in-hospital mortality of acute exacerbations of chronic obstructive pulmonary disease patients based on red cell distribution width-to-platelet ratio. Int J Chron Obstruct Pulmon Dis. 2023;18:2079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildman MJ, Sanderson C, Groves J, Reeves BC, Ayres J, Harrison D, et al. Predicting mortality for patients with exacerbations of COPD and Asthma in the COPD and Asthma Outcome Study (CAOS). QJM. 2009;102(6):389–99. [DOI] [PubMed] [Google Scholar]
- 41.Asiimwe AC, Brims FJ, Andrews NP, Prytherch DR, Higgins BR, Kilburn SA, et al. Routine laboratory tests can predict in-hospital mortality in acute exacerbations of COPD. Lung. 2011;189(3):225–32. [DOI] [PubMed] [Google Scholar]
- 42.Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–6. [DOI] [PubMed] [Google Scholar]
- 43.Hartley T, Lane ND, Steer J, Elliott MW, Sovani MP, Curtis HJ, et al. The Noninvasive Ventilation Outcomes (NIVO) score: prediction of in-hospital mortality in exacerbations of COPD requiring assisted ventilation. Eur Respirat J. 2021;58(2):2004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mekanimitdee P, Morasert T, Patumanond J, Phinyo P. The MAGENTA model for individual prediction of in-hospital mortality in chronic obstructive pulmonary disease with acute exacerbation in resource-limited countries: a development study. PLoS ONE. 2021;16(8): e0256866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Gutiérrez S. Development of a severity scale for acute exacerbation of chronic obstructive pulmonary disease in hospital emergency departments. Emergencias 2014.
- 46.Quintana JM, Esteban C, Unzurrunzaga A, Garcia-Gutierrez S, Gonzalez N, Barrio I, et al. Predictive score for mortality in patients with COPD exacerbations attending hospital emergency departments. BMC Med. 2014;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintana JM, Esteban C, Unzurrunzaga A, Garcia-Gutierrez S, Gonzalez N, Lafuente I, et al. Prognostic severity scores for patients with COPD exacerbations attending emergency departments. Int J Tuberc Lung Dis. 2014;18(12):1415–20. [DOI] [PubMed] [Google Scholar]
- 48.Almagro P, Soriano JB, Cabrera FJ, Boixeda R, Alonso-Ortiz MB, Barreiro B, et al. Short- and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145(5):972–80. [DOI] [PubMed] [Google Scholar]
- 49.Esteban C, Arostegui I, Garcia-Gutierrez S, Gonzalez N, Lafuente I, Bare M, et al. A decision tree to assess short-term mortality after an emergency department visit for an exacerbation of COPD: a cohort study. Respir Res. 2015;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Rivero JL, Esquinas C, Barrecheguren M, Bonnin-Vilaplana M, Garcia-Sidro P, Herrejon A, et al. Risk factors of poor outcomes after admission for a COPD exacerbation: multivariate logistic predictive models. COPD. 2017;14(2):164–9. [DOI] [PubMed] [Google Scholar]
- 51.Alameda C, Matia AC, Casado V. Predictors for mortality due to acute exacerbation of COPD in primary care: derivation of a clinical prediction rule in a multicentre cohort study. Eur J Gen Pract. 2021;27(1):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Stavem K, Dahl FA, Humerfelt S, Haugen T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Chron Obstruct Pulmon Dis. 2014;9:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakamoto Y, Yamauchi Y, Yasunaga H, Takeshima H, Hasegawa W, Jo T, et al. Development of a nomogram for predicting in-hospital mortality of patients with exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiroshita A, Kimura Y, Shiba H, Shirakawa C, Sato K, Matsushita S, et al. Predicting in-hospital death in pneumonic COPD exacerbation via BAP-65, CURB-65 and machine learning. ERJ Open Res. 2022. 10.1183/23120541.00452-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng JC, Gong WW, Wu Y, Yan TY, Jiang XY. Development and validation of a prognostic nomogram among patients with acute exacerbation of chronic obstructive pulmonary disease in intensive care unit. BMC Pulm Med. 2022;22(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roche N, Zureik M, Soussan D, Neukirch F, Perrotin D, Urgence BSC. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur Respir J. 2008;32(4):953–61. [DOI] [PubMed] [Google Scholar]
- 57.Ramaraju K, Kaza AM, Balasubramanian N, Chandrasekaran S. Predicting Healthcare Utilization by Patients Admitted for COPD Exacerbation. J Clin Diagn Res. 2016;10(2):OC13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamantea F, Kostikas K, Bartziokas K, Karakontaki F, Tsikrika S, Pouriki S, et al. Prediction of hospitalization stay in COPD exacerbations: the AECOPD-F score. Respir Care. 2014;59(11):1679–86. [DOI] [PubMed] [Google Scholar]
- 59.Roche N. A clinical in-hospital prognostic score for acute exacerbations of COPD. Respir Res. 2014. 10.1186/s12931-014-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Li LIQ, Ge YL, Hu XY, Zhang Q, Zhang HF, et al. Procalcitonin (PCT) improves the accuracy and sensitivity of dyspnea, eosinopenia, consolidation, acidemia and atrial fibrillation (DECAF) score in predicting AECOPD patients admission to ICU. Clin Lab. 2020. 10.7754/Clin.Lab.2019.190612. [DOI] [PubMed] [Google Scholar]
- 61.Fan L, Zhao Q, Liu Y, Zhou L, Duan J. Semiquantitative cough strength score and associated outcomes in noninvasive positive pressure ventilation patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2014;108(12):1801–7. [DOI] [PubMed] [Google Scholar]
- 62.Liu D, Peng SH, Zhang J, Bai SH, Liu HX, Qu JM. Prediction of short term re-exacerbation in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi QF, Sheng Y, Zhu N, Tan Y, Xie XH, Wang SY, et al. The v-DECAF score can predict 90-day all-cause mortality in patients with COPD exacerbation requiring invasive mechanical ventilation. Clin Respir J. 2019;13(7):438–45. [DOI] [PubMed] [Google Scholar]
- 64.Hu WP, Lhamo T, Liu D, Hang JQ, Zhang FY, Zuo YH, et al. Development of a nomogram to predict the risk of 30-day re-exacerbation for patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease. COPD. 2019;16(2):160–7. [DOI] [PubMed] [Google Scholar]
- 65.Zhou M, Chen C, Peng J, Luo C-H, Feng DY, Yang H, et al. Fast prediction of deterioration and death risk in patients with acute exacerbation of chronic obstructive pulmonary disease using vital signs and admission history: retrospective cohort study. JMIR Med Info. 2019;7(4):e13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X, Zhu GP, Cai TF, Zheng JY. Establishment of risk prediction model and risk score for in-hospital mortality in patients with AECOPD. Clin Respir J. 2020;14(11):1090–8. [DOI] [PubMed] [Google Scholar]
- 67.Peng J, Chen C, Zhou M, Xie X, Zhou Y, Luo CH. A machine-learning approach to forecast aggravation risk in patients with acute exacerbation of chronic obstructive pulmonary disease with clinical indicators. Sci Rep. 2020;10(1):3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bi W, Sun Y, Ma LQ, Wu CJ. Predictive role of interleukin-6 and CAT score in mechanical ventilation in patients with chronic obstructive pulmonary disease at the acute exacerbation stage in the emergency department. World J Emerg Med. 2020;11(2):93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong F, Ren X, Huang K, Wang Y, Jiao J, Yang T. Development and validation of risk prediction model for in-hospital mortality among patients hospitalized with acute exacerbation chronic obstructive pulmonary disease between 2015 and 2019. Front Med (Lausanne). 2021;8: 630870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L, Chen L, Zheng H, Wu S, Wang S. Emergency admission parameters for predicting in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease with hypercapnic respiratory failure. BMC Pulm Med. 2021;21(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, Chen S. Prediction of readmission in patients with acute exacerbation of chronic obstructive pulmonary disease within one year after treatment and discharge. BMC Pulm Med. 2021;21(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang L, Li M, Shu J, Yang Y, Huang Q. A risk prediction model for prolonged length of stay in patients with acute exacerbations of chronic obstructive pulmonary disease: a retrospective study of 225 patients in a Single Center in Kunming. China Med Sci Monit. 2022;28: e934392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen D, Chen C, Zhang P, Zhang F, Zhang H, Sun Q, et al. The arrival ward requiring help by wheelchair or medical cart, arterial oxygenation index, age, albumin and neutrophil count score: Predicting in-hospital mortality in Chinese patients with acute exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis. 2023;20:14799731231197226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu L, Ruan X, Huang W, Huang N, Zeng J, He J, et al. Machine learning-based prediction of in-hospital mortality in patients with pneumonic chronic obstructive pulmonary disease exacerbations. J Asthma. 2024;61(3):212–21. [DOI] [PubMed] [Google Scholar]
- 75.He S, Wu S, Chen T, Huang W, Yu A, Cao C. The predictive value of baseline symptom score and the peripheral CD4CD8 double-positive T cells in patients with AECOPD. BMC Pulm Med. 2023;23(1):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan LN, Chen M, Wei H, Ma HR. Construction and validation of nomogram prediction model for risk of acute heart failure in patients with acute exacerbation of chronic obstructive pulmonary disease. Medicine (Baltimore). 2024;103(1): e36840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fakhraei R, Matelski J, Gershon A, Kendzerska T, Lapointe-Shaw L, Kaneswaran L, et al. Development of multivariable prediction models for the identification of patients admitted to hospital with an exacerbation of COPD and the prediction of risk of readmission: a retrospective cohort study using electronic medical record data. COPD. 2023;20(1):274–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Study Information: Basic information on all included studies, including authors, year, country, and study design.
Supplementary Material 2. Model Information: Information on all included predictive models, including study outcomes, sample size, and modeling methods.
Supplementary Material 3. ROB and Applicability: Assessment results of bias risk and applicability for all included predictive models.
Supplementary Material 4. Model Evaluation Information: Specific items related to the bias risk and applicability assessment results for all included predictive models.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.