Summary
Background
Prompt antibiotic administration for febrile neutropenia (FN) is standard of care, and targets of time to antibiotics (TTA) <60 min are common. We sought to determine the effect of TTA ≥60 versus <60 min on adverse outcomes (intensive care unit (ICU) admission or death) in children with cancer and FN. Effect modification by a decision rule that predicts infection (AUS-rule) and bacteraemia were also investigated.
Methods
The prospective, multi-centre (n = 8), Australian PICNICC study dataset was analysed. To control for confounding, we used outcome regression adjusted for propensity score modelled as restricted cubic spline with two degrees of freedom. The propensity score was estimated from a logistic regression model for the exposure on the confounders, identified a priori (age, sex, severely unwell, disease, chemotherapy intensity and site). TTA was defined as time from from emergency triage to first antibiotic dose.
Findings
1685 FN episodes in 976 patients were included. Median TTA was 53 min (IQR 37–77 min, 1542 (92%) <120 min). An adverse outcome occurred in 43 (2.6%) episodes (39 ICU; 5 deaths). The confounder-adjusted point estimate suggested a lower risk for adverse outcome associated with TTA ≥60 min (RR 0.62, 95% CI 0.32–1.21), but the wide 95% CI precluded definitive judgement about strength and direction of the effect (unadjusted RR 0.52; 95% CI 0.26, 1.05). Similarly, although the point estimates were suggestive of a null association or reduced risk for adverse outcome associated with TTA ≥60 min for all comparisons across bacteraemia or AUS-rule strata, the 95% CIs were imprecise.
Interpretation
For children with FN, there was no definite evidence that TTA ≥60 min from hospital triage (but within 2 h), increased risk of adverse outcome or prolonged hospital admission. This study has important implications for FN TTA mandates, suggesting a more nuanced approach is required.
Funding
National Health and Medical Research Council and Medical Research Future Fund.
Keywords: Febrile neutropenia (FN), Time to antibiotics (TTA), Children with cancer
Research in context.
Evidence before this study
Time to antibiotics (TTA) has become an important quality of care metric in the management of paediatric febrile neutropenia (FN) and many hospitals recommend antibiotic administration within 60 min of presentation. However, the most compelling evidence for the benefit of early administration of antibiotics comes from studies in immunocompetent adults with severe sepsis. We searched PubMed, without date restriction, using the terms: (febrile OR fever) AND (neutropenia OR neutropenic) AND (time to antibiotic). There are just six studies specifically exploring the effect of TTA on outcome in children with cancer and FN. Results of these studies are mixed; some suggest an association between delayed TTA and poor outcomes such as intensive care unit (ICU) admission and in-hospital mortality, while others have found no association. A recent systematic review and meta-analysis of paediatric FN studies found no clear association between TTA <60 versus ≥60 min and ICU admission (OR 1.43; 95% CI 0.57–3.60), with considerable heterogeneity. Important limitations to our interpretation of these data include the impact of triage bias and retrospective, observational study design.
Added value of this study
Our study is the largest prospective study to explore the causal effect of TTA (≥60 min versus <60 min) on adverse outcome, defined as ICU admission or death within 30 days, in children with cancer and outpatient onset FN. Patients were recruited from all eight tertiary paediatric cancer centres in Australia. The analysis of this observational study was designed to emulate a target trial and, to our knowledge, similar novel methodology has not been performed in this population. The point estimate and the values within the 95%CI ruled out major increases in risk of serious adverse event (RR 0.62, 95% CI 0.32–1.21) or prolonged hospital admission for antibiotic administration from hospital triage ≥60 min (but within 2 h) compared with <60 min. There was also no definitive evidence of effect modification by either AUS-rule or bacteraemia. In the episodes that received antibiotics after ≥180 min from fever onset at home, the point estimate was suggestive of an increased risk of adverse outcome, but the wide 95% CIs again precluded the possibility of making definitive judgments about direction and strength of the effect.
Implications of all the available evidence
Our study adds to the accumulating evidence that antibiotic administration after 60 min from hospital triage, but within 2 h, to children with cancer and FN does not increase risk of serious adverse event or prolong hospital admission. Collectively, these data should challenge current standards of care and inform a more nuanced approach to paediatric FN management. This will enable clinicians to pause, assess and observe patients and use the complete clinical picture to risk-stratify and inform antibiotic choices.
Introduction
Febrile neutropenia (FN) is a common complication of chemotherapy-induced neutropenia in children with cancer. While many children who present to hospital with FN do not have an identifiable infection, the 10%–15% incidence of bacteraemia underpins the importance of early recognition and treatment.1,2 To mitigate the consequences of serious bacterial infection, in particular bacteraemia, international guidelines recommend early administration of broad-spectrum intravenous antibiotics3,4 and many hospitals have focused their quality improvement initiatives on reducing time to first dose antibiotic.5
Time to antibiotics (TTA) is arbitrarily defined as the time from emergency department presentation to administration of the first dose of antibiotics.5 It has become an important quality of care metric in the management of paediatric FN and many hospitals recommend antibiotic administration within 60 min of presentation.6,7 However, the most compelling evidence for early administration of antibiotics comes from studies in immunocompetent adults with severe sepsis.8 Results of studies examining the clinical impact of TTA within 60 min in paediatric patients with cancer and FN are mixed.9 Some studies suggest an association between delayed TTA and poor outcomes such as intensive care unit (ICU) admission and mortality (variably defined)10,11 while others have found no association.12,13
Current TTA guidelines and quality improvement measures do not account for the heterogenous risk of infection and adverse outcome in children with cancer and FN. There are accumulating data on the role of risk stratification in this population and a range of available clinical decision rules (CDR) to assist clinicians in decision making.14,15 The AUS-rule is a CDR derived in Australia15 and validated internationally.16 Using three variables (chemotherapy intensity, platelets, and white cell count) it is designed to predict bacterial infection as is recommended as part of a formal low-risk FN pathway.14 How and if these rules may affect a more nuanced TTA approach remains an important evidence gap.
The primary objective of this study was to determine the clinical impact of TTA (≥60 min versus <60 min) on adverse outcome, defined as ICU admission or death within 30 days, in children undergoing chemotherapy for cancer with outpatient onset FN. The secondary objective was to investigate effect modification of a FN clinical decision rule (AUS-rule) and presence of bacteraemia.
Methods
The analysis of this observational study was designed to emulate a target trial.17 The process involved specifying the protocol components of a hypothetical trial that would have been done to address the question and emulating these components using the observational data. The target trial specification and emulation are described below and summarised in Supplementary Table S1.18
Prospectively collected data from the Australian Predicting Infectious Complications in Children with Cancer (PICNICC) and the ‘There is No Place Like Home’ studies (Australian and New Zealand Clinical Trials Registry ID ACTRN12616001440415) were used for the target trial emulation. The PICNICC study was a prospective, multicentre, observational study designed to validate clinical decision rules for the prediction of infection or adverse outcome in children with cancer and FN.2 The ‘There is No Place Like home’ study, an extension of the PICNICC study, was designed to implement and evaluate a paediatric low-risk FN program across Australia. All eight of Australia's paediatric tertiary hospitals participated in both studies including Royal Children's Hospital Melbourne, Monash Children's Hospital, John Hunter Children's Hospital, Children's Hospital Westmead, Sydney Children's Hospital Randwick, Perth Children's Hospital, Queensland Children's Hospital and Women and Children's Hospital Adelaide. Patients were enrolled from December 1, 2016 and censored at December 31, 2022.
Demographic (including age, sex, diagnosis, chemotherapy intensity, prior haematopoietic cell transplant (HCT)), clinical (including sepsis, fluid bolus, clinical instability, type and time of first dose antibiotic, infection diagnosis, antibiotic duration) and outcome (including length of stay (LOS), ICU admission, and readmission or death within 30 days) data were collected from patient records and entered into a REDCap database by site research assistants. Outcome data, including ICU admission and all-cause 30 day mortality, were defined according to an international FN research consensus statement.19 Data were collected at time of triage, end of FN episode (defined as afebrile >48 h, recovery of absolute neutrophil count (ANC) beyond nadir and antibiotic cessation2) and at day 30 post-presentation.2 During the study period, TTA <60 min was considered an important quality of care measure for paediatric FN at each participating site and therefore detailed data on time of first dose antibiotic and time of fever onset at home were also collected prospectively.
During the study period, children were managed according to local FN guidelines, using piperacillin-tazobactam or cefepime as first-line empiric therapy and risk stratification was not routinely used in the emergency department (ED).20 Microbiological investigations were performed according to site FN guidelines. Across all sites this included: at least one blood culture set (aerobic blood culture plus an anerobic blood culture if sufficient blood available) from the central venous catheter (all patients) and urine for culture; nasal swab for respiratory virus PCR; chest X-ray; stool for culture, Clostridioides difficile toxin assay and viral PCR; and skin or wound swab for culture and viral PCR (as indicated). Each site also had local recommendations for target TTA within 1 h of arriving to the ED and patients were typically admitted for intravenous antibiotics that were continued until fever resolution and ANC recovery (as judged by the treating physician). Antibacterial prophylaxis with fluoroquinolone was not routinely used.
The study had national human research ethics committee approval by the Royal Children's Hospital ethics committee, as well as site specific ethics and governance at each participating centre. Informed consent was obtained for patients that had additional blood samples, Medicare data or quality of life surveys completed as part of the PICNICC and ‘There is No Place Like Home’ studies. A waiver of consent was applied for the remaining patients as data were routinely available in the clinical record. The study was conducted in accordance with the Declaration of Helsinki.
The target trial specification
For the target trial, eligibility criteria were children under 19 years old with a solid organ cancer or leukaemia, who received chemotherapy in the preceding 30 days and presented to a participating hospital with outpatient-onset of fever (defined as temperature ≥38 °C) with neutropenia (defined as ANC <500/mm3 or <1000/mm3 with an expected decline to <500/mm3 within the next 48 h). Children who underwent HCT within three months or those who were already receiving antibiotics for treatment of an infection were excluded.
The strategies to be compared were TTA ≥60 min and TTA <60 min. The 60 min threshold was used due to its inclusion in many treatment guidelines for FN. TTA was defined as the date and time of triage in emergency department or equivalent to the date and time of administration of first dose of antibiotic.
The primary outcome was ‘adverse outcome,’ a binary composite of ICU admission or death within 30 days of triage in emergency department.19 The secondary outcome was hospital length of stay (LOS), measured as the date and time from triage in emergency department to the date and time of hospital discharge.
Follow-up started at the date and time of triage in emergency department and ended at day 30 post presentation. Complete study participant follow up after hospital discharged was possible as all patients received their cancer treatment at one of the eight study sites and therefore had frequent outpatient appointments or admissions for chemotherapy.
The effect of interest was the average causal effect of TTA ≥60 versus TTA <60 min on the outcomes of interest (risk ratio scale for the primary outcome and median difference for the secondary outcome). For the primary outcome, the effect modifiers of interest were (i) AUS-rule score and (ii) bacteraemia. The AUS-rule incorporates three variables (chemotherapy intensity, platelets <50 g/L, and total white cell count <300/mm3) with a maximum score of three. In the derivation study, scores of 0, 1, 2 and 3 were associated with <5%, 7%, 12% and 25% rate of bacteraemia, respectively.15 Bacteraemia was defined as a recognised pathogen (including organisms associated with mucosal barrier injury in the setting of mucositis or neutropenia) from one or more blood culture sets or a common commensal from two or more blood cultures sets.19
The emulation of the target trial
The target trial was emulated as closely as possible using data from the PICNICC and ‘There is No Place Like Home’ studies (Supplementary Table S1). Eligibility criteria were the same as for the target trial, although neutropenia was pragmatically defined as ANC <1000/mm3 to replicate routine clinical practice across Australia. Of the total study participants (n = 1973), those with inpatient onset FN (n = 282), those who did not receive antibiotics (n = 4), with unknown time to antibiotic (n = 1), and with sex recorded as other (n = 1) were excluded from the analytic sample (n = 1685). One further participant with missing data on hospital length of stay was excluded from analysis for the secondary outcome.
Random allocation to TTA (≥60 or <60 min) was emulated by adjusting for the following confounders, identified a priori (Supplementary Figure S1): age, sex, severely unwell appearance on presentation to the emergency department, disease and prior chemotherapy intensity and study site. In all analyses age was modelled as a restricted cubic spline with two degrees of freedom. ‘Severely unwell’ was defined as presence of any of severe sepsis or septic shock,21 altered conscious state, respiratory rate or blood pressure in the mandatory emergency review criteria, inotrope requirement, fluid bolus ≥40 ml/kg requirement or documented as ‘severely unwell’ or equivalent.2 Disease and chemotherapy intensity were grouped into (i) acute leukaemia or lymphoma and chemotherapy more intensive than acute lymphocytic leukaemia (ALL) maintenance-style therapy; (ii) acute leukaemia or lymphoma and chemotherapy equivalent to ALL maintenance-style therapy, or (iii) solid organ/other cancers. Previous infective episodes or ICU admissions were also identified as potential confounders, but this information was not captured in the original data-base. Due to the small number of events at some sites, hospital sites were combined into three state-based categories: (i) Victoria; (ii) New South Wales and (iii) Other (Western Australia, Queensland and South Australia).
For effect modification analysis, AUS-rule scores of 0 and 1 were combined categorically to ensure an adequate number of events were available in each subgroup. For this analysis, chemotherapy intensity was no longer included in the confounder adjustment set as it contributed to the AUS-rule score. For bacteraemia, blood cultures collected as part of routine FN management and prior to first dose antibiotic were used, acknowledging this information is not routinely available for up to 48 h.
Additional analysis
We repeated the primary analysis for FN episodes who were known to have an ANC count <500/mm3 at presentation (n = 977). For a subset of FN episodes with available data (n = 701), we analysed the effect of TTA from time of fever onset at home (≥180 versus <180 min) on the primary outcome. The 180 min threshold was used due to this timeframe being recommended in the 2012 surviving sepsis campaign.22 For both of the additional analyses, all other considerations regarding the target trial specification and emulation were the same as described above. Disease and chemotherapy intensity was not included as a confounder in analysis of time of fever onset at home because there were no outcomes (i.e., ICU admission or death) in two categories of the variable.
Statistical analysis
Patient characteristics per FN episode were described for the overall cohort, by TTA groups, and by the primary outcome, as median and interquartile range for continuous variables and frequency and percentage for categorical variables.
The effect of TTA on the primary outcome was estimated using regression on propensity score to adjust for confounding.23 For this approach, first, the propensity score (the probability being in the TTA ≥60 or TTA <60 min group given the identified confounders) was estimated from a logistic regression model for the exposure on the confounders. Then a logistic regression model for the outcome on the exposure and the propensity score was used to predict the outcome for all episodes under TTA ≥60 and TTA <60 min. The propensity score was modelled as a restricted cubic spline with two degrees of freedom to allow for a more flexible outcome model. The ratio of the average predicted outcomes under TTA ≥60 versus TTA <60 min was taken as the estimate of the average causal effect risk ratio. The same procedure was followed to estimate the average causal effect for TTA ≥60 versus TTA <60 min for the subgroup of episodes with known ANC count <500/mm3 and to estimate the average causal effect comparing TTA from time of fever onset at home ≥180 versus TTA <180 min.
For the secondary outcome, multivariable quintile regression was used to estimate the median difference in TTA ≥60 versus TTA <60 min. No exposure-confounder interactions were included in the model and it was assumed that the effect of TTA ≥60 versus TTA <60 min on each outcome was constant across confounder strata.
Because some patients included in the dataset contributed multiple FN episodes, for both outcomes, the 95% confidence intervals (CIs) were obtained using clustered normal-interval bootstrapping (2000 bootstrap samples) to account for correlation between repeated episodes per patient.24
For effect modification analysis, risk ratios for each stratum of AUS-rule score or presence of bacteraemia were estimated from outcome models on the exposure and the propensity score (modelled as a restricted cubic spline with two degrees of freedom as described above) that additionally included an interaction term between the exposure and the effect modifier. All participants included in the analytic sample had complete data. Analyses were performed in Stata version 18.25
Role of funding source
The study was funded by a National Health and Medical Research Council (NHMRC) and Medical Research Future Fund (MRFF) grant. The funders did not influence study design, analysis or interpretation of results.
Results
The analytic sample comprised 1685 FN episodes from 976 patients (one episode in 570 patients, two in 462 patients, three in 294 patients and ≥ four in 359 patients). The median TTA was 53 min (interquartile range (IQR) 37–77 min) with 969 (58%) receiving antibiotics <60 min and 1542 (92%) <120 min. The median TTA from onset of fever at home was 135 min (IQR 98–208 min) in the 701 (42%) FN episodes with these data available.
Demographic characteristics, cause of fever and outcome data are presented in Table 1 (overall and by TTA) and in Supplementary Table S2 (by adverse outcome). Baseline characteristics were broadly similar between the two exposure groups. The median age of patients was 6 years (IQR 3–11 years) and 937 (56%) had acute leukaemia or lymphoma. Only 82 (5%) episodes were considered ‘severely unwell’ at presentation to hospital, and a bacteraemia was documented in 171 (10%) episodes (25 (31%) that were severely unwell and 146 (9%) that were not severely unwell). There were 43 (3%) episodes that were admitted to ICU and 5 (0.4%) patients died within 30 days of FN episode. The median time from triage to ICU admission was 0.4 days (IQR 0.2–5.6 days) and to death was 7.2 days (IQR 6.4–11.4 days). No deaths were directly attributed to infection. The median hospital length of stay (LOS) was five days (IQR 3–8 days).
Table 1.
Descriptive statistics for characteristics and clinical outcomes of the eligible study participants by time to antibiotic (<60 min and ≥60 min) and overall.
| Time to antibiotic |
|||
|---|---|---|---|
| <60 min | ≥60 min | Total | |
| N | 969 (57.5%) | 716 (42.5%) | 1685 (100%) |
| Time to antibiotic (mins), median (IQR) | 39 (27–49) | 84 (69–109) | 53 (37–77) |
| Time to antibiotic from fever onset at home, n (%) | |||
| <60 min | 32 (3.3%) | 0 (0.0%) | 32 (1.9%) |
| ≥60 min–<180 | 274 (28.3%) | 164 (22.9%) | 438 (26.0%) |
| ≥180 min | 90 (9.3%) | 141 (19.7%) | 231 (13.7%) |
| Missing | 573 (59.1%) | 411 (57.4%) | 984 (58.4%) |
| Time to antibiotic from fever onset at home (mins), median (IQR) | 110 (80–165) | 170 (128–236) | 135 (98–208) |
| Age (years), median (IQR) | 6 (3–11) | 6 (4–11) | 6 (3–11) |
| Sex, n (%) | |||
| Female | 463 (47.8%) | 319 (44.6%) | 782 (46.4%) |
| Male | 506 (52.2%) | 397 (55.4%) | 903 (53.6%) |
| Absolute Neutrophil Count at presentation | |||
| <1000 but not <500 cells/mm3 | 237 (24.5%) | 159 (22.2%) | 396 (23.5%) |
| <500 cells/mm3 | 560 (57.8%) | 417 (58.2%) | 977 (58.0%) |
| <1000 but unknown if 500 cells/mm3 | 172 (17.8%) | 140 (19.6%) | 312 (18.5%) |
| Chemo intensity, n (%) | |||
| Intense (leukaemia/lymphoma) | 431 (44.5%) | 303 (42.3%) | 734 (43.6%) |
| Non-intense (leukaemia/lymphoma) | 100 (10.3%) | 103 (14.4%) | 203 (12.0%) |
| Solid organ/other | 438 (45.2%) | 310 (43.3%) | 748 (44.4%) |
| Severely unwell appearance,a n (%) | |||
| No | 915 (94.4%) | 688 (96.1%) | 1603 (95.1%) |
| Yes | 54 (5.6%) | 28 (3.9%) | 82 (4.9%) |
| Previous stem cell transplant, n (%) | |||
| No | 956 (98.7%) | 703 (98.2%) | 1659 (98.5%) |
| Yes | 13 (1.3%) | 13 (1.8%) | 26 (1.5%) |
| Study site (state), n (%) | |||
| VIC | 459 (47.4%) | 367 (51.3%) | 826 (49.0%) |
| NSW | 148 (15.3%) | 63 (8.8%) | 211 (12.5%) |
| Other | 362 (37.4%) | 286 (39.9%) | 648 (38.5%) |
| Cancer diagnosis, n (%) | |||
| ALL/AML | 457 (47.2%) | 348 (48.6%) | 805 (47.8%) |
| Lymphoma | 70 (7.2%) | 58 (8.1%) | 128 (7.6%) |
| Other leukaemia | 4 (0.4%) | 0 (0.0%) | 4 (0.2%) |
| Solid organ tumour | 434 (44.8%) | 302 (42.2%) | 736 (43.7%) |
| Other | 4 (0.4%) | 8 (1.1%) | 12 (0.7%) |
| AUS-rule score, n (%) | |||
| 0 (very low risk) or 1 (low risk) | 443 (45.7%) | 349 (48.7%) | 792 (47.0%) |
| 2 (moderate risk) | 292 (30.1%) | 207 (28.9%) | 499 (29.6%) |
| 3 (higher risk) | 234 (24.1%) | 160 (22.3%) | 394 (23.4%) |
| Cause of fever, n (%) | |||
| MDI–bacteraemia | 118 (12.2%) | 53 (7.4%) | 171 (10.1%) |
| MDI—non-bacteraemia infection | 188 (19.4%) | 172 (24.0%) | 360 (21.4%) |
| Clinically documented infection | 64 (6.6%) | 63 (8.8%) | 127 (7.5%) |
| Fever of unknown cause | 599 (61.8%) | 428 (59.8%) | 1027 (60.9%) |
| ICU admission, n (%) | |||
| No | 941 (97.1%) | 705 (98.5%) | 1646 (97.7%) |
| Yes | 28 (2.9%) | 11 (1.5%) | 39 (2.3%) |
| Death, n (%) | |||
| No | 965 (99.6%) | 714 (99.7%) | 1679 (99.6%) |
| Yes | 4 (0.4%) | 1 (0.1%) | 5 (0.3%) |
| ICU admission or death, n (%) | |||
| No | 938 (96.8%) | 704 (98.3%) | 1642 (97.4%) |
| Yes | 31 (3.2%) | 12 (1.7%) | 43 (2.6%) |
| Hospital length of stay (days), median (IQR) | 5 (3–9) | 4 (3–8) | 5 (3–8) |
| Missing | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) |
IQR, interquartile range; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; MDI, microbiologically defined infection.
Yes if any of Glasgow Coma Scale (GCS) < 15 (not secondary to sleep), Alert, Voice, Pain, Unresponsive (AVPU)=V, P or U (not secondary to sleep), documented as ‘severely unwell’, severe sepsis/septic shock or blood pressure (BP) or respiratory rate (RR) in Medical Emergency Team (MET) criteria or fluid bolus >40 ml/kg.
The median age of patients with adverse outcome (ICU admission or death) was 12 years (IQR 6–15 years). Of the 43 FN episodes with adverse outcome, 31 (72% of the total episodes with adverse outcome) were in the TTA <60 min group (compared with 938 (57%) of the 1642 episodes without adverse outcome), 23 (54%) were considered ‘severely unwell’ at presentation (versus 3.6% (n = 59) of those without adverse outcome), and 34 (79%) received intense chemotherapy for leukaemia or lymphoma (versus 43% (n= 700) of those without adverse outcome).
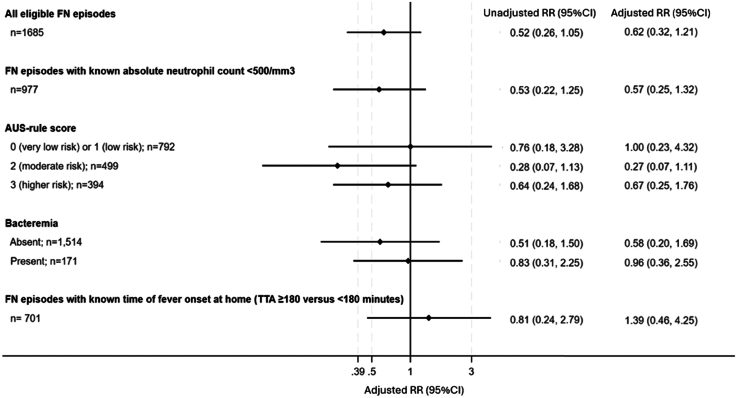
After adjusting for age, sex, study site, disease and chemotherapy intensity, and clinically unwell appearance at presentation, FN episodes with TTA ≥60 min had 0.62 times lower risk of ICU admission or death compared with those with TTA <60 min. However, the 95% CI was wide, and inclusive of values that were compatible with a reduced risk as low as 0.32 or an increased risk as high as 1.21 (Fig. 1). The results were consistent when analyses were limited to the subgroup of FN episodes (n = 977) with known ANC <500/mm3 at presentation (Fig. 1). For completeness, and in keeping with the STROBE guidelines, unadjusted RRs and corresponding 95% CIs for the assoication between TTA and primary outcome are also reported in Fig. 1; but we note that these are not the target trial emulation estimates.
Fig. 1.
Estimated average causal effect on primary outcome (ICU admission or death) for time to antibiotics (≥60 versus <60 min) overall, for the subset with known absolute neutrophil count, and by AUS-rule score or presence of bacteraemia, and for time to antibiotics since fever onset at home (≥180 versus <180 min) for the subset with available data. FN, febrile neutropenia; TTA, time to antibiotics; RR, risk ratio; CI, confidence interval.
Across all AUS-rule categories and for FN episodes with or without a documented bacteraemia, the point estimates were suggestive of a null effect or reduced risk of ICU admission or death for TTA ≥60 versus <60 min, although the 95% CIs remained wide (Fig. 1).
Taking into consideration the time from fever onset at home, and adjusting for confounders, FN episodes with TTA ≥180 min had 1.39 times higher risk of ICU admission or death compared with those with TTA <180 min, but as for the primary analysis the 95% confidence interval was wide (0.46, 4.25) (Fig. 1).
The median hospital LOS, estimated from adjusted analysis was 0.27 days fewer with TTA ≥60 versus TTA <60 min. The 95% CI was inclusive of values that were compatible with both fewer (up to 0.72 days) and more (up to 0.17 days) days of hospital stay (unadjusted difference in median hospital LOS −0.46; 95% CI −0.96, 0.04). Similarly, for the 977 FN episodes with known ANC <500/mm,3 the adjusted median difference in hospital LOS was −0.21 days (95% CI −0.82, 0.40 days; unadjusted median difference −1.05; 95% CI −1.69, 0.40).
Discussion
In our analysis of a large, prospectively collected database of consecutive episodes of outpatient onset FN, whereby a small proportion of patients (n = 43, 2.6%) experienced adverse outcomes, the point estimate and the values within the 95% CI ruled out major increases in risk of serious adverse event or prolonged hospital admission for antibiotic administration ≥60 min (but within 2 h) compared with <60 min. There was also no definitive evidence of effect modification by either AUS-rule or bacteraemia. In the episodes that received antibiotics after ≥180 min from fever onset at home, the point estimate was suggestive of an increased risk of adverse outcome, but the wide 95% CIs again precluded the possibility of making definitive judgments about direction and strength of the effect.
Our findings are in keeping with the key results of two recent paediatric FN studies.12,13 In a prospectively collected dataset of inpatient and outpatient onset FN (n = 266), TTA ≥60 min was found to be unrelated to ‘safety related events’ (death, admission to ICU, severe sepsis and bacteraemia) (OR 0.6, 95% CI 0.29–1.22). This study was notable for the calculation of TTA from onset of fever at home, rather than triage time, and had an overall median TTA of 120 min. Likewise, in a retrospective study of inpatient and outpatient onset FN (n = 2335), there was no evidence of an association between TTA (≥60 min) and ‘major complications’ (infection-related mortality, ICU admission, vasopressor support or endotracheal intubation) within 6 h, 24 h, or 7 days from triage time (7 days: adjusted OR 0.96, 95% CI, 0.49–1.89).13 Similar to our study, the mean TTA in this cohort was 56 min (IQR, 37–90). Across both studies, there was also no evidence of an association between TTA and length of hospital stay.
To date, only two paediatric FN studies have shown an adverse outcome associated with TTA greater than 60 min. In a retrospective, single site study (n = 1628) a TTA interval of 61–120 min compared with less than 60 min was associated with an adverse outcome (in-hospital mortality, ICU admission or fluid bolus requirement ≥40 ml/kg) within the first 24 h (OR 1.81, 95% CI 1.01–3.26).11 Unlike other TTA studies, this study included fluid bolus requirement as part of the primary composite outcome and, when considered separately, TTA was not associated with ICU admission alone, or prolonged hospital LOS. A smaller quality improvement study from the USA (n = 220) noted a significant reduction in need for ICU-level care in patients with FN who received antibiotics within 60 min (13% versus 30%), although these ICU admission rates were substantially higher than in our study (2.3%).10 A subsequent meta-analysis of these and two other paediatric studies similarly did not find evidence of an association between TTA ≥60 min and ICU admission (OR 1.43, 95% CI 0.57–3.60).9
It is conceivable that the patient population most likely to benefit from early TTA are those with a bacterial infection. Any bacterial infection has previously been documented in up to 25% of all FN episodes,2 with bacterial bloodstream infections documented in between 10 and 15%.2,13 In our study, we did not find evidence for a causal effect of TTA on adverse outcome when stratified by either AUS-rule,15 designed to predict bacterial infection, or the subsequent confirmation of bacteraemia.
To our knowledge a similar target trial emulation analysis has not been performed in this population. The specification of the hypothetical randomised trial to address the question of interest, and its explicit emulation using the large prospectively collected data, assisted with improving the quality of the causal inference using these data.17,26 Another strength of our study was that it used data from a prospective FN study involving all eight tertiary cancer centres in Australia and therefore results are applicable at a national level. We also explored the impact of a tighter definition of neutropenia (ANC <500/mm3 at presentation) by excluding episodes with ANC between 500 and 1000/mm3 where a decline to <500/mm3 within 48 h was unknown. This increases the applicability of our data to centres that use this definition, rather than the more pragmatic ANC <1000/mm3.
Our estimates of the effect of TTA on adverse outcomes relied on no unmeasured confounding, no measurement bias, and no selection bias assumptions. To address confounding, accounting for triage bias in studies investigating the effect of TTA is critically important. With increased focus on sepsis recognition, and almost universal TTA <60 min recommendations, patients who are severely unwell on presentation to hospital are more likely to receive antibiotics early but may already have a trajectory requiring ICU-level support. To limit the impact of this, ‘severely unwell’ was identified a priori as a confounder and adjusted for accordingly in our analyses. While our definition was broad, it is plausible that other factors (e.g., clinician gestalt) were unable to be accounted for and this, in part, may have explained why a higher proportion of episodes with documented bacteraemia received antibiotics within 60 min (12% versus 7%). Data on compliance with other components of FN or sepsis management, including volume and timing of fluid boluses and lactate levels, were unavailable in >50% of episodes. These factors may have also impacted patient outcome, but it is unlikely that they would have led to confounding as their use occurs alongside of, or after receiving, antibiotics. Risk of selection bias was low in our study as all children admitted to participating hospitals with FN during the study period were included. Measurement bias was also minimised by having trained research staff collect data on TTA and other analysis variables. Despite the large sample size, we cannot rule out sparse data bias in our estimates due to the rarity of the primary outcome. To account for this we used regression adjustment with nonlinear splines for propensity scores to estimate the RRs, which has been shown to perform well in terms of bias in the presence of rare outcomes.23 However despite this method of analysis, the small number of adverse outcomes (n = 43, 2.6%) is an important limitation of our study and therefore our estimates and their corresponding 95% CIs need to be interpreted with caution.
Although our data support re-evaluation of current TTA mandates, it must be interpreted in the context of an overall median TTA of just 53 min and upper quartile of 77 min. This is an important limitation of our study as it is possible that adverse outcomes were infrequent as the majority of the patients (92%) received antibiotics within 2 h. This may explain the suggestion for an increased risk of adverse outcome when dichotomised at 180 min in the episodes where data for time of fever onset at home was available. This is in keeping with an adult FN study (n = 3219) that found delays of between three and 6 h, as compared to less than 2 h, increased the risk of ICU admission and 30-day mortality.27 Our analysis was also restricted to outpatient-onset FN and therefore may not be applicable to higher-risk patients, such as those with acute myeloid leukaemia, who are more likely to be admitted at time of fever onset. Finally, as we did not collect detailed information about type of non-bacteraemia MDI (bacterial or viral) we were unable to further stratify our analysis into any-bacterial versus non-bacterial infection.
Unintended consequences of TTA targets of 60 min are reflected in the increased exposure of anti-pseudomonal FN antibiotics to non-neutropenic cancer patients.28 Many centres across Australia and internationally recommend administration of FN antibiotics prior to availability of ANC results to meet these targets. Winding back on this approach will require multi-disciplinary involvement, senior clinician engagement and safety-net criteria to ensure that paediatric cancer patients with sepsis or clinical instability remain prioritised in the emergency department and outpatient areas.
Our study adds to the accumulating evidence that antibiotic administration after 60 min from hospital triage, but within 2 h, to children with cancer and FN does not increase risk of serious adverse event or prolong hospital admission. These data should challenge current standards of care and inform a more nuanced approach to FN management locally and abroad. This will enable clinicians to pause, assess and observe patients and use the complete clinical picture to risk-stratify and inform antibiotic choices.
Contributors
All authors conceived the analysis and GMH, FEB, MLB, JEC, HT, FA, TW, LS, LH, BM, RDAL, MAS, KAT contributed to funding acquisition. GMH, JEC, BP, HT, FA, TW, LS and BM oversaw data collection. GMH, SGD and FJ performed the analysis and all authors provided clinical interpretation of findings. GMH and SGD drafted the manuscript. All authors reviewed, edited and confirmed the acceptance of the final submitted version. The corresponding author (GMH) has full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
De-identified participant data and data dictionary will be made available to researchers after appropriate human research ethics approvals and following a signed data access agreement.
Declaration of interests
There are no conflicts of interest to disclose.
Acknowledgements
We gratefully acknowledge the contribution of our project manager Marijana Vanevski as well as our research assistants Claudia Corrente, Rhiannon Barrow, Sara Cook, Sheree Westhorpe, Joanne Abbotsford, Nikki Lennox, Kaitlin Todd, Felicity Wright and Jamie Chase.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101226.
Appendix A. Supplementary data
References
- 1.Morgan J.E., Phillips B., Haeusler G.M., Chisholm J.C. Optimising antimicrobial selection and duration in the treatment of febrile neutropenia in children. Infect Drug Resist. 2021;14:1283–1293. doi: 10.2147/IDR.S238567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haeusler G.M., Thursky K.A., Slavin M.A., et al. Risk stratification in children with cancer and febrile neutropenia: a national, prospective, multicentre validation of nine clinical decision rules. eClinicalMedicine. 2020;18 doi: 10.1016/j.eclinm.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehrnbecher T., Averbuch D., Castagnola E., et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021;22(6):e270–e280. doi: 10.1016/S1470-2045(20)30725-7. [DOI] [PubMed] [Google Scholar]
- 4.Lehrnbecher T., Robinson P.D., Ammann R.A., et al. Guideline for the management of fever and neutropenia in pediatric patients with cancer and hematopoietic cell transplantation recipients: 2023 update. J Clin Oncol. 2023 doi: 10.1200/JCO.22.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig C., Schneider C., Morgan J.E., Ammann R.A., Sung L., Phillips B. Interventions aiming to reduce time to antibiotics (TTA) in patients with fever and neutropenia during chemotherapy for cancer (FN), a systematic review. Support Care Cancer. 2020;28(5):2369–2380. doi: 10.1007/s00520-019-05056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Royal Children's Hospital . 2018. Melbourne, Australia, Clinical Practice Guideline on Fever and suspected or confirmed neutropenia in children with cancer.https://www.rch.org.au/clinicalguide/guideline_index/Fever_and_suspected_or_confirmed_neutropenia/ [Google Scholar]
- 7.Morgan J., Deyo J., Cox J., Fasipe F., Mohamed A., Russo C. Quality improvement interventions across a network of pediatric hematology-oncology clinics. Pediatr Qual Saf. 2019;4(2) doi: 10.1097/pq9.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Roberts D., Wood K.E., et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 9.Koenig C., Schneider C., Morgan J.E., Ammann R.A., Sung L., Phillips B. Association of time to antibiotics and clinical outcomes in patients with fever and neutropenia during chemotherapy for cancer: a systematic review. Support Care Cancer. 2020;28(3):1369–1383. doi: 10.1007/s00520-019-04961-4. [DOI] [PubMed] [Google Scholar]
- 10.Salstrom J.L., Coughlin R.L., Pool K., et al. Pediatric patients who receive antibiotics for fever and neutropenia in less than 60 min have decreased intensive care needs. Pediatr Blood Cancer. 2015;62(5):807–815. doi: 10.1002/pbc.25435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher M., Hodgkiss H., Zhang S., et al. Prompt administration of antibiotics is associated with improved outcomes in febrile neutropenia in children with cancer. Pediatr Blood Cancer. 2013;60(8):1299–1306. doi: 10.1002/pbc.24485. [DOI] [PubMed] [Google Scholar]
- 12.Koenig C., Kuehni C.E., Bodmer N., et al. Time to antibiotics is unrelated to outcome in pediatric patients with fever in neutropenia presenting without severe disease during chemotherapy for cancer. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-18168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Castro G.C., Slatnick L.R., Shannon M., et al. Impact of time-to-antibiotic delivery in pediatric patients with cancer presenting with febrile neutropenia. JCO Oncol Pract. 2024;20(2):228–238. doi: 10.1200/OP.23.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haeusler G.M., Gaynor L., Teh B., et al. Home-based care of low-risk febrile neutropenia in children-an implementation study in a tertiary paediatric hospital. Support Care Cancer. 2023;41(9):1774–1785. doi: 10.1007/s00520-020-05654-z. [DOI] [PubMed] [Google Scholar]
- 15.Haeusler G.M., Phillips R., Slavin M.A., et al. Re-evaluating and recalibrating predictors of bacterial infection in children with cancer and febrile neutropenia. eClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips B., Morgan J.E. Meta-analytic validation of new 'AUS' febrile neutropenia risk score. Pediatr Blood Cancer. 2020 doi: 10.1002/pbc.28580. [DOI] [PubMed] [Google Scholar]
- 17.Hernan M.A., Wang W., Leaf D.E. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328(24):2446–2447. doi: 10.1001/jama.2022.21383. [DOI] [PubMed] [Google Scholar]
- 18.Downes M., Shepherd D., Wijesuriya R., et al. 2023. Statistical analysis plan template for observational studies.https://melbourne.figshare.com/articles/online_resource/Analysis_plan_template_for_life-course_cohort_studies/12471380 [Google Scholar]
- 19.Haeusler G.M., Phillips R.S., Lehrnbecher T., Thursky K.A., Sung L., Ammann R.A. Core outcomes and definitions for pediatric fever and neutropenia research: a consensus statement from an international panel. Pediatr Blood Cancer. 2015;62(3):483–489. doi: 10.1002/pbc.25335. [DOI] [PubMed] [Google Scholar]
- 20.Singh N., Douglas A.P., Slavin M.A., Haeusler G.M., Thursky K.A. Antimicrobial use and appropriateness in neutropenic fever: a study of the Hospital National Antimicrobial Prescribing Survey data. J Antimicrob Chemother. 2024;79(3):632–640. doi: 10.1093/jac/dkae015. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein B., Giroir B., Randolph A., International Consensus Conference on Pediatric S International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 22.Levy M.M., Evans L.E., Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 23.Franklin J.M., Eddings W., Austin P.C., Stuart E.A., Schneeweiss S. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat Med. 2017;36(12):1946–1963. doi: 10.1002/sim.7250. [DOI] [PubMed] [Google Scholar]
- 24.Naimi A.I., Whitcomb B.W. Simple approaches for dealing with correlated data. Am J Epidemiol. 2023;192(4):507–509. doi: 10.1093/aje/kwac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp . StataCorp LLC; College Station, TX: 2023. Stata statistical software: release 18. [Google Scholar]
- 26.Hernan M.A. Methods of public Health research - strengthening causal inference from observational data. N Engl J Med. 2021;385(15):1345–1348. doi: 10.1056/NEJMp2113319. [DOI] [PubMed] [Google Scholar]
- 27.Daniels L.M., Durani U., Barreto J.N., et al. Impact of time to antibiotic on hospital stay, intensive care unit admission, and mortality in febrile neutropenia. Support Care Cancer. 2019;27(11):4171–4177. doi: 10.1007/s00520-019-04701-8. [DOI] [PubMed] [Google Scholar]
- 28.Walker H., Esbenshade A.J., Dale S., et al. Non-neutropenic fever in children with cancer: management, outcomes and clinical decision rule validation. Pediatr Blood Cancer. 2022;69(12) doi: 10.1002/pbc.29931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.