Abstract
Introduction and importance
Persistent Mullerian duct syndrome is an exceptional genetic condition that occurs secondary to mutations in AMH and AMHR-II. The individuals with this condition exhibit well-developed secondary sexual characteristics despite having a uterus and fallopian tubes. The case mentioned here was worth reporting due to the scarcity of prevalence of PMDS. Secondarily, it is important that the patient had retained MD derivatives in his inguinal canal for 27 years without any malignant change.
Case presentation
This case report features a 27-year-old male who presented with complaints of right-sided scrotal swelling for 3 years, an empty left scrotal pouch since birth, infertility, and off-and-on hematospermia. Clinical examination revealed a right-sided indirect, complete, reducible hernia and bilateral cryptorchidism. Investigations confirmed the presence of both testes in a right inguinal canal along with a partially developed uterus and fallopian tube. Hernioplasty and orchidopexy were done under spinal anesthesia. Remnants of MD were excised and sent for histopathology.
Clinical discussion
PMDS is a rare genetic syndrome with a variety of clinical features. This unique presentation highlights the need for awareness of such rare causes of infertility, hematospermia, and malignancy.
Conclusion
PMDS often goes unnoticed in childhood and early teenage resulting in drastic consequences. A well intricated multidisciplinary approach is required to identify and manage such exceptional conditions.
Keywords: Persistent Mullerian duct syndrome, Mullerian duct remnants, Hematospermia
Highlights
-
•
A 27-year-old male with prolonged inguinoscrotal swelling; case highlights PMDS diagnostic challenges and the need for better management.
1. Introduction
PMDS is a very rare autosomal recessive disorder of male pseudo-hermaphroditism caused by a mutation in genes producing anti-Mullerian hormone. This condition is characterized by the retention of female reproductive organs in otherwise normally virilized 46-XY males. The Mullerian ducts (para-mesonephric ducts) are embryonic precursors of the female genital system. In males, these ducts regress under the influence of anti-Mullerian hormone produced by the Sertoli cells during the 7th week of gestation [1]. The testosterone produced from the Leydig cells promotes differentiation of Wolffian ducts which results in the development of the male genitourinary system. In some individuals, due to mutation in AMH or AMHR-II genes, there is a deficiency in the production of AMH or tissue resistance to its action secondary to receptor abnormalities. Consequently, the Mullerian duct fails to regress. This genetic mutation is the root cause of persistent Mullerian duct syndrome [2]. In this condition, a man appears genotypically and phenotypically normal (because the secretion and action of testosterone is undisturbed) but also possesses Mullerian duct derivatives i.e. uterus and fallopian tubes [3]. This disorder was first described by Nelson in 1939 [4]. PMDS has a variety of presentations e.g. female type, Uteri Inguinal, and Transverse Testicular Ectopia. The case described here was having Transverse Testicular Ectopia i.e. his both testes were crossing the right-sided deep inguinal ring and were lying in the right inguinal canal along with a partially developed uterus and fallopian tube. PMDS is often found incidentally in the patients being investigated for inguinal hernia, undescended testes, or infertility. In the current scenario, the patient presented to the surgical outpatient department for the management of a right-sided inguinal hernia and an empty left scrotal pouch. The diagnosis of this disorder requires a set of investigations including endocrine assessment, analysis for gene mutations and radiologic evidence, and histopathology.
PMDS is treated by adopting a multidisciplinary approach. The management team must include a general surgeon, urologist, endocrinologist, clinical cytogeneticist, radiologist, and histopathologist. Inguinal hernias are treated by performing hernioraphy or hernioplasty. Orchidopexy is required for undescended testes and the patient is asked for regular follow-up visits. Orchidopexy has been found to maintain fertility in around 14 % of cases [5]. If the spermatic cord appears to be short or if the intra-abdominal testis appears to be non-viable, an orchiectomy is planned. MD remnants are also removed to prevent malignant transformation.
Persistent Mullerian duct syndrome is an exceptionally rare disorder. Medical knowledge is scarce regarding the details of this syndrome due to the limited number of cases and variety of presentations. This case was important to report because the patient survived for almost three decades of his life carrying female reproductive organs in his inguinal canal without any malignant transformation as was confirmed on histopathology.
2. Case presentation
A 27-year-old male presented to the surgical outpatient department with a complaint of swelling in the right inguinoscrotal region for the last 3 years. This swelling was initially small and gradually increased over the duration of the previous 2–3 years. He also describes the sensation of dragging and heaviness in the right inguinal region. He explained that the swelling becomes more prominent after standing for prolonged periods and exertion and it disappears on lying down. He didn't describe any risk factor for the development of hernia e.g. chronic cough, chronic constipation or heavy weight lifting, etc. Moreover, he said that his left scrotal pouch has felt empty since childhood and he, sometimes, experiences blood in his semen. His past medical and surgical history came out to be unremarkable. Family history revealed that he was issue-less. He used to remain psychologically upset due to infertility. Clinical Examination revealed soft, non-tender, and reducible right scrotal swelling with positive cough reflex and negative trans-illumination test. It was not possible to get above the swelling. The ring occlusion test was also positive. The right testis was not separately palpable. The left scrotal pouch was also found to be empty. No urethral discharge or urethral stricture was noted. There was no other significant finding on the relevant examination.
On scrotal ultrasound, there was an omentum in the hernia sac but both of the testes were absent from the scrotum. Ultrasound abdomen revealed a 7 × 2 cm solid-looking cylindrical mass inside the right inguinal canal. MRI confirmed the presence of a rudimentary uterus and both testes in the right inguinal canal.
Testicular color Doppler showed mild bilateral testicular atrophy. Semen analysis revealed oligoasthenoteratozoospermia. Hernioplasty, orchidopexy, and removal of MD remnants were planned. The supra-inguinal incision was given. After dissecting the camper's and Scarpa's fascia, the inguinal canal was opened as shown in Fig. 1. Both testes were fixed in the scrotal pouch. Remnants of MD were removed as shown in Fig. 2 and sent for histopathology. Polypropylene mesh was placed to repair the hernial rent. Post-operative recovery was uneventful and the patient was discharged on the 3rd postoperative day with the advice of regular follow-up visits with the operating surgeon. He was reassured about his sexual identity and was referred to an endocrinologist for management of infertility.
Fig. 1.
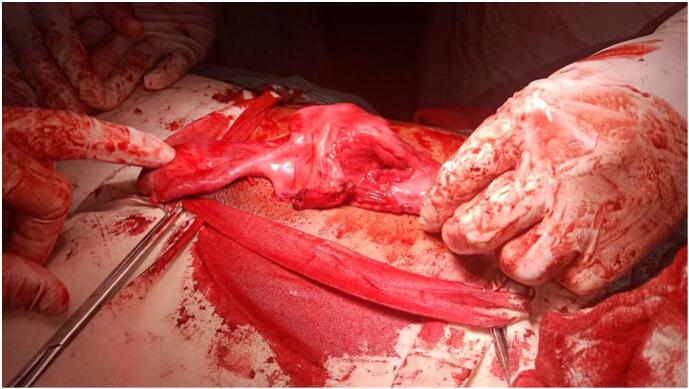
Per Op pic of Rudimentary uterus.
Fig. 2.
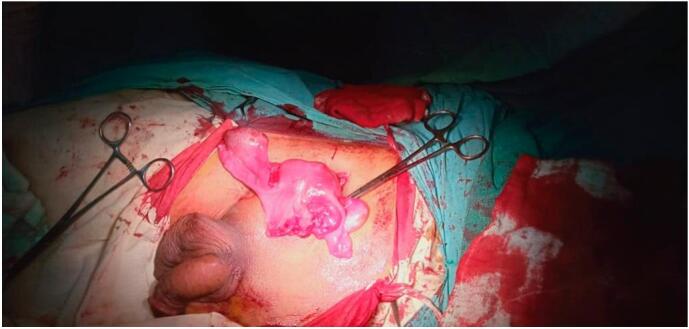
Remnants of persistant mullerian duct.
3. Discussion
Persistent Mullerian Duct Syndrome is a sexual developmental disorder observed in the male population. It is a rare phenomenon with less than 300 cases described in the literature [6,7]. Males affected with this disorder tend to have well-developed secondary sexual characteristics and 46-XY chromosome patterns on karyotype analysis [8]. But they also have female reproductive organs i.e. uterus and fallopian tube. Embryologically, the male urogenital system develops from Wolffian ducts (pro and mesonephric ducts) which need androgens to differentiate into the epididymis, vas deferens, and seminal vesicles. In 6th week of embryologic development, these Wolffian ducts induce the formation of Mullerian ducts. After the fetal sex determination, hormones produced by fetal testes i.e. anti-Mullerian hormone (AMH), testosterone, and insulin like-3 (INSL3) trigger the differentiation of WDs and regression of MDs. Anti Mullerian hormone production is instructed by AMH gene (located on chromosome 19p13) while AMHR-II gene (located on chromosome 12q13) instructs for making a protein called anti Mullerian hormone receptor [9]. Thus, AMH and AMHR-II genes play a fundamental role in the regression of MDs and differentiation of WDs resulting in the development of male sexual characteristics. In some individuals, there are mutations in AMH and AMHR-II genes and subsequent defective production of hormones and receptors that limit the differentiation of MDs in the male population. As a result, these males develop a uterus, fallopian tubes, and vagina, in addition to well-developed male sex organs. However, in 12 % of cases, mutations in AMH and AMHR-II have not been detected. In these cases, disruption, in other pathways of Mullerian duct regression is thought to be the probable cause of PMDS [10]. PMDS is a complex disorder. It has three anatomical variations depending upon the site of the uterus and testes. First, the female type in which bilateral testes are located in the pelvic cavity and they are found to be connected to the fallopian tube. The second type is known as Uteri Inguinal. This type presents with one testis in the hernia sac or in the scrotal pouch and the other one in the abdominal cavity. The third variety of PMDS is also known as Transverse Testicular Ectopia, in which both testes are located in the same hernia sac along with the uterus and uterine tubes [11] [12]. Clinically, PMDS presents with Cryptorchidism, inguinoscrotal swellings, oligospermia, and infertility. Cryptorchidism is secondary to insulin-like 3 (INSL-3) deficiency or mechanical pull by the Mullerian duct construct [13]. An intricate set of investigations is needed to diagnose PMDS including hormonal assay, genetic analysis, radiography, and histopathology. The treatment strategy of PMDS is focused on surgical management of inguinal hernia i.e. hernioraphy or hernioplasty, maintaining the fertility of the patient by orchidopexy and preventing or curing malignant changes in derivatives of Mullerian duct and ectopic testes by removing MD derivatives or planning orchiectomy. The work has been reported in line with the SCARE criteria [14].
4. Conclusion
In conclusion, PMDS is a rare genetic disorder that is diagnosed incidentally while investigating other disorders like inguinal hernia, hematospermia, infertility, or cryptorchidism. A variety of anatomical presentations highlights the complexity of PMDS. Its management requires a wide range of investigations and an inter-departmental approach. Reporting such cases will help clinicians keep PMDS in mind while dealing with inguinal swellings, infertility, or undescended testes.
Ethical approval
Not required, as the study is exempt from ethical approval for case reports in our institution, Allied Hospital Faisalabad.
Sources of funding
There are no sources of funding in this study.
Author contribution
All authors contributed to the study design, writing of manuscript, editing, and proof reading. Each author gave approval of the final manuscript.
Guarantor
Muhammad Shair Ismail.
Registration of research studies
None.
Consent
Informed consent was taken from patient.
Declaration of competing interest
There are no conflicts of interest in this case report.
Contributor Information
Muhammad Shair Ismail, Email: shairismail@gmail.com.
Ahmad Ismail, Email: ahmadismail@kemu.edu.pk.
References
- 1.Anti-Müllerian hormone in disorders of sex determination and differentiation. Divya Renu, B. G. R. K. R. N. [DOI] [PubMed]
- 2.Mutations of the anti-mullerian hormone gene in patients with persistent mullerian duct syndrome: biosynthesis, secretion, and processing of the abnormal proteins and analysis using a three-dimensional model. [DOI] [PubMed]
- 3.Persistent mullerian duct syndrome: A 24-year experience. [DOI] [PubMed]
- 4.Anti-Müllerian hormone in disorders of sex determination and differentiation. Divya Renu, B. G. R. K. R. N. [DOI] [PubMed]
- 5.Shao F.M., Mao Q.L., Yang H.M., Hou L.J. Persistent Mullerian duct syndrome: a report of two cases and literature review. Chin J Pediateic Surg. 2011;32:468–470. [Google Scholar]
- 6.Faria IM de, Souza AM de, Júnior LRP, Leite GGVR. Surgical resection therapy of a rare presentation of persistent Mullerian duct syndrome: a case review. Therapeutic Advances in Rare Disease. 2023;4. [DOI] [PMC free article] [PubMed]
- 7.Da Aw L., Zain M.M., Esteves S.C., et al. Persistent Mullerian duct syndrome: a rare entity with a rare presentation in need of multidisciplinary management. Int. Braz J Urol. 2016;42:1237–1243. doi: 10.1590/S1677-5538.IBJU.2016.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith-Harrison L.I., Patel M.S., Smith R.P., Schenkman N.S. Persistent Mullerian duct structures presenting as hematuria in an adult: case report of robotic surgical removal and review of the literature. Urol Ann. 2015;7:544–546. doi: 10.4103/0974-7796.164859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picard J.Y., Cate R.L., Racine C., Josso N. The persistent Müllerian duct syndrome: an update based upon a personal experience of 157 cases. Sex. Dev. 2017;11(3):109–125. doi: 10.1159/000475516. [DOI] [PubMed] [Google Scholar]
- 10.di Clemente N., Belville C. Anti-Mullerian hormone receptor defect. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20:599–610. doi: 10.1016/j.beem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Clarnette T.D., Sugita Y., Hutson J.M. Genital anomalies in human and animal models reveal the mechanisms and hormones governing testicular descent. Br. J. Urol. 1997;79:99–112. doi: 10.1046/j.1464-410x.1997.25622.x. [DOI] [PubMed] [Google Scholar]
- 12.Shalaby M.M., Kurkar A., Zarzour M.A., Faddan A.A., Khalil M., Abdelhafez M.F. The management of the persistent Mullerian duct syndrome. Arab. J. Urol. 2014;12:239–244. doi: 10.1016/j.aju.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann S., Steding G., Emmen J.M., Brinkmann A.O., Nayernia K., Holstein A.F., et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- 14.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]


