Abstract
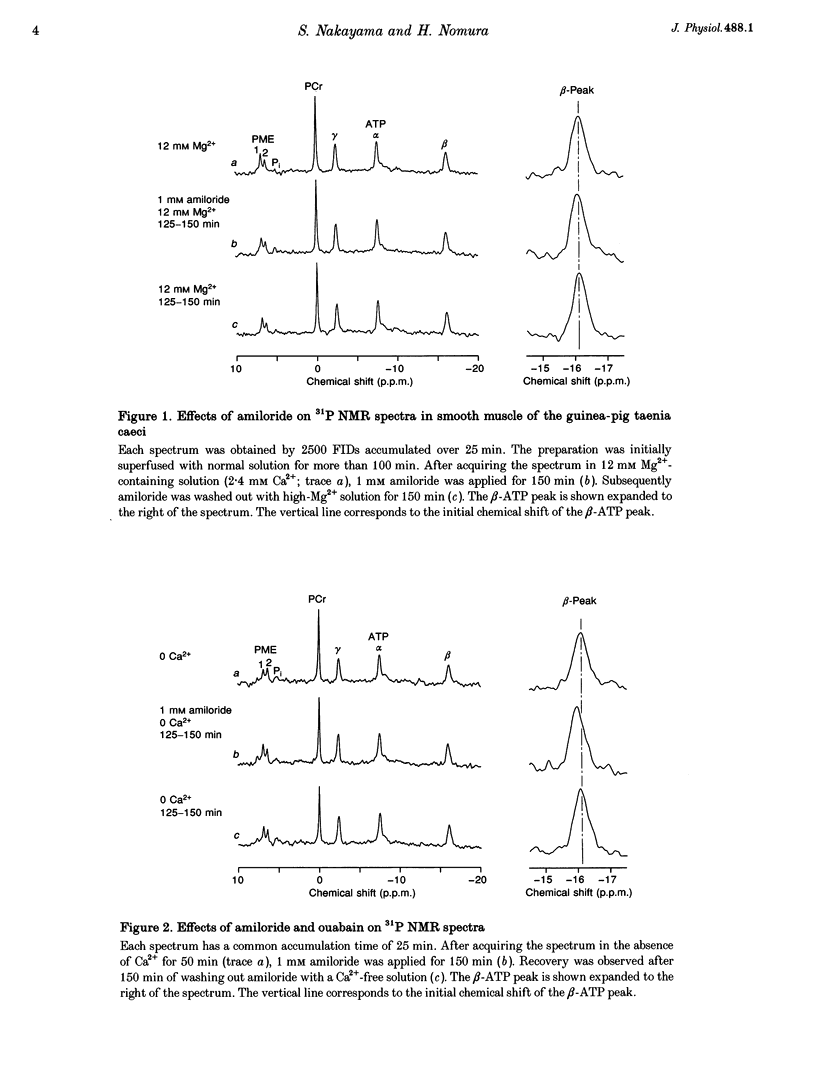
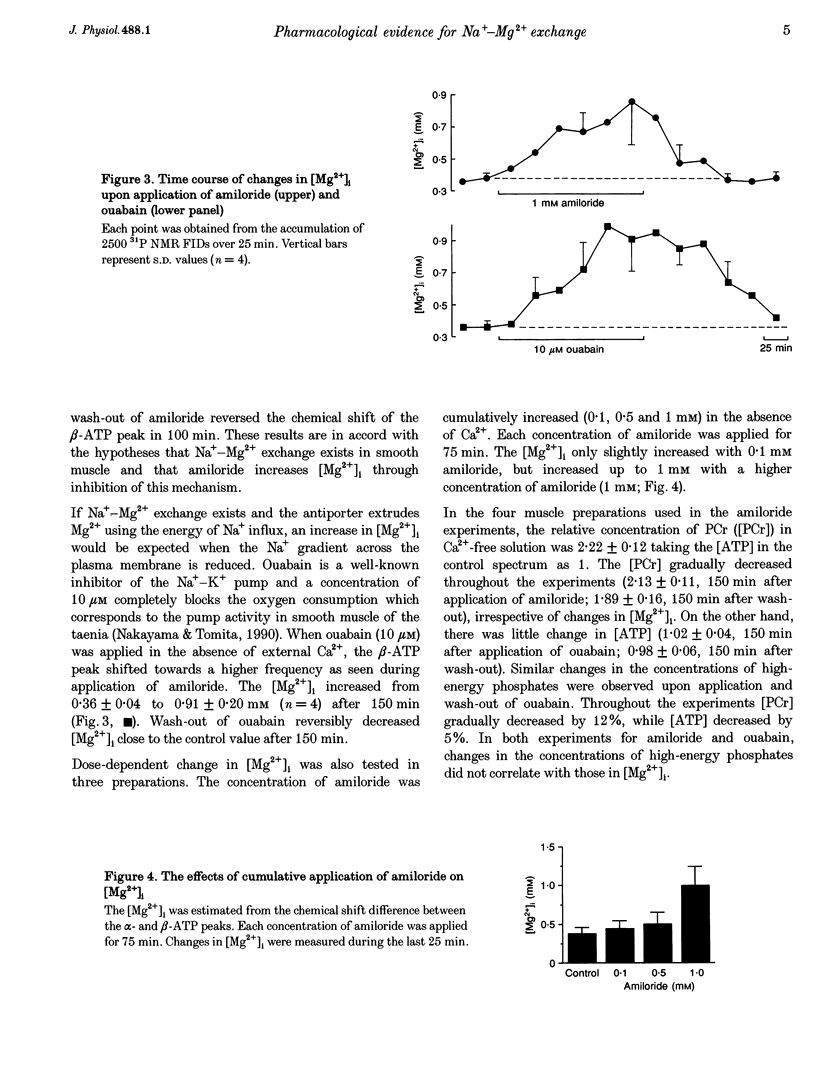
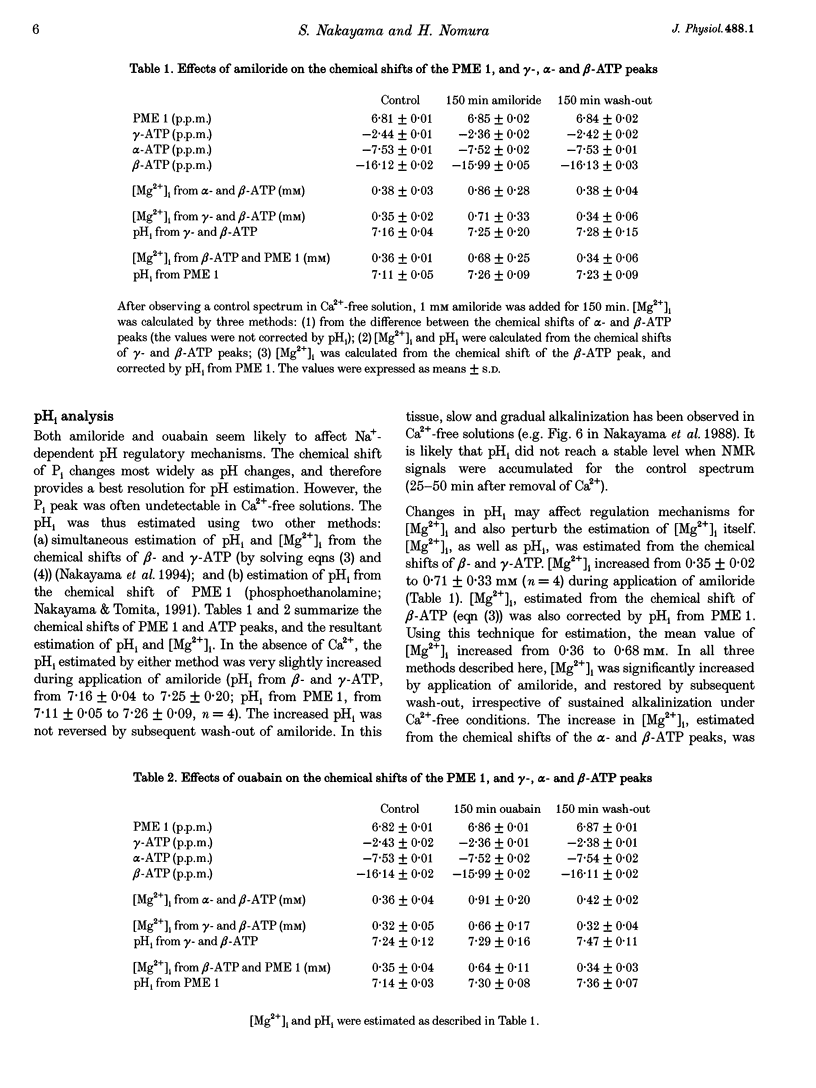
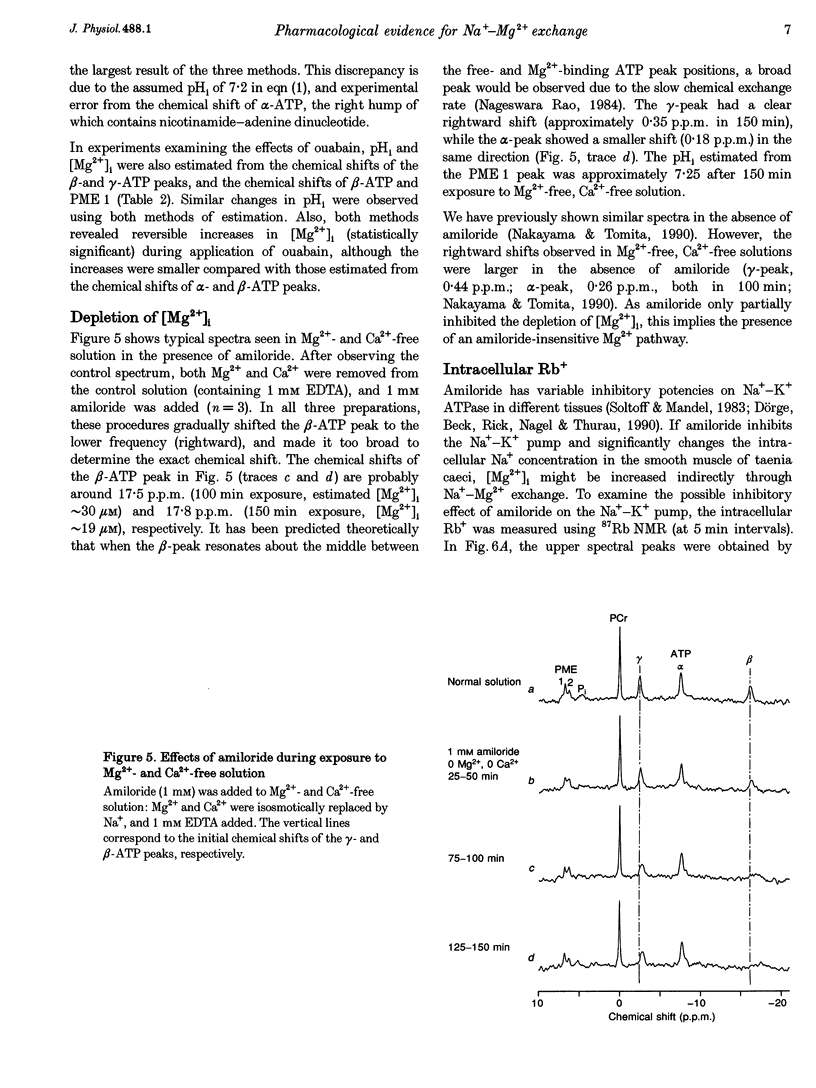
1. The effects of amiloride and ouabain on the regulation of the intracellular, free Mg2+ concentration ([Mg2+]i) were investigated in the taenia isolated from the guinea-pig caecum, using nuclear magnetic resonance (NMR) techniques. 2. [Mg2+]i were mainly estimated from the separation of the alpha- and beta-ATP peaks observed in 31P NMR spectra. In normal (physiological) and nominally Ca(2+)-free solutions, [Mg2+]i was approximately 0.3-0.4 mM. Application of either amiloride or ouabain in Ca(2+)-free solutions significantly increased [Mg2+]i, with only a small change in ATP content. Washout of the drugs reversed the changes in [Mg2+]i. 3. Changes in pHi were estimated from: (1) the chemical shift of phosphoethanolamine, and (2) solving two relational equations of pHi and [Mg2+]i obtained from the beta- and gamma-ATP peaks. Both estimations revealed some intracellular alkalosis during application of these two drugs. After correction for pHi, a significant increase in [Mg2+]i was still obtained 150 min after application of either drug. 4. In the presence of amiloride, simultaneous removal of extracellular Mg2+ and Ca2+ significantly depleted intracellular Mg2+. This result suggests the presence of an amiloride-insensitive (or less sensitive) pathway which passively transports Mg2+ across the plasma membrane. 5. The intracellular Rb+ concentration was monitored as an index of Na(+)-K+ pump activity, using 87Rb NMR. In Ca(2+)-free solutions containing 5 mM Rb+, the intracellular Rb+ concentration was hardly changed by amiloride, but was depleted by additional applications of ouabain. Wash-out of ouabain restored the intracellular Rb+ in the presence of amiloride. 6. These results are consistent with the presence of Na(+)-Mg2+ exchange as an effective Mg(2+)-extruding mechanism in smooth muscle. Although many other factors may cause changes in [Mg2+]i, it seems likely that amiloride directly inhibits the Na(+)-Mg2+ exchanger, whilst ouabain does so indirectly through reduction of the Na+ gradient across the plasma membrane.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Brading A. F. Effect of Na+ and K+ on Cl- distribution in guinea-pig vas deferens smooth muscle: evidence for Na+, K+, Cl- co-transport. J Physiol. 1990 Feb;421:13–32. doi: 10.1113/jphysiol.1990.sp017931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aickin C. C. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- Aickin C. C. Regulation of intracellular pH in the smooth muscle of guinea-pig ureter: Na+ dependence. J Physiol. 1994 Sep 1;479(Pt 2):301–316. doi: 10.1113/jphysiol.1994.sp020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J. L., Wenz B., Gupta R. K. Changes in intracellular Mg adenosine triphosphate and ionized Mg2+ during blood storage: detection by 31P nuclear magnetic resonance spectroscopy. Blood. 1985 Jun;65(6):1526–1530. [PubMed] [Google Scholar]
- Bountra C., Vaughan-Jones R. D. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989 Nov;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Somlyo A. V., Hilgemann D. W. The giant cardiac membrane patch method: stimulation of outward Na(+)-Ca2+ exchange current by MgATP. J Physiol. 1992 Aug;454:27–57. doi: 10.1113/jphysiol.1992.sp019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. An ATP-dependent Na+/Mg2+ countertransport is the only mechanism for Mg extrusion in squid axons. Biochim Biophys Acta. 1988 Dec 22;946(2):424–428. doi: 10.1016/0005-2736(88)90418-x. [DOI] [PubMed] [Google Scholar]
- Dörge A., Beck F. X., Rick R., Nagel W., Thurau K. Effect of amiloride on electrolyte concentrations and rubidium uptake in principal and mitochondria-rich cells of frog skin. Pflugers Arch. 1990 May;416(3):335–338. doi: 10.1007/BF00392070. [DOI] [PubMed] [Google Scholar]
- Flatman P. W. Mechanisms of magnesium transport. Annu Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Smith L. M. Magnesium transport in ferret red cells. J Physiol. 1990 Dec;431:11–25. doi: 10.1113/jphysiol.1990.sp018318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W., Smith L. M. Sodium-dependent magnesium uptake by ferret red cells. J Physiol. 1991 Nov;443:217–230. doi: 10.1113/jphysiol.1991.sp018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel E. J., Graziani M., Schatzmann H. J. ATP requirement of the sodium-dependent magnesium extrusion from human red blood cells. J Physiol. 1989 Jul;414:385–397. doi: 10.1113/jphysiol.1989.sp017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenrich C. C., Murphy E., Levy L. A., London R. E., Lieberman M. Intracellular pH modulates cytosolic free magnesium in cultured chicken heart cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1024–C1030. doi: 10.1152/ajpcell.1992.262.4.C1024. [DOI] [PubMed] [Google Scholar]
- Féray J. C., Garay R. Demonstration of a Na+: Mg2+ exchange in human red cells by its sensitivity to tricyclic antidepressant drugs. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):332–337. doi: 10.1007/BF00173409. [DOI] [PubMed] [Google Scholar]
- Headrick J. P., Willis R. J. Effect of inotropic stimulation on cytosolic Mg2+ in isolated rat heart: a 31P magnetic resonance study. Magn Reson Med. 1989 Dec;12(3):328–338. doi: 10.1002/mrm.1910120305. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W. Regulation and deregulation of cardiac Na(+)-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 1990 Mar 15;344(6263):242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Paul R. J. Effects of hypoxia on high-energy phosphagen content, energy metabolism and isometric force in guinea-pig taenia caeci. J Physiol. 1990 May;424:41–56. doi: 10.1113/jphysiol.1990.sp018054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. M., Cragoe E. J., Jr, Allen J. C., Halligan R. D., Shelat H. Na(+)-H+ and Na(+)-dependent Cl(-)-HCO3- exchange control pHi in vascular smooth muscle. Am J Physiol. 1990 Jul;259(1 Pt 1):C134–C143. doi: 10.1152/ajpcell.1990.259.1.C134. [DOI] [PubMed] [Google Scholar]
- Kaila K., Vaughan-Jones R. D. Influence of sodium-hydrogen exchange on intracellular pH, sodium and tension in sheep cardiac Purkinje fibres. J Physiol. 1987 Sep;390:93–118. doi: 10.1113/jphysiol.1987.sp016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdi H., Schatzmann H. J. Some properties of a system for sodium-dependent outward movement of magnesium from metabolizing human red blood cells. J Physiol. 1987 Sep;390:367–382. doi: 10.1113/jphysiol.1987.sp016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Nomura H., Tomita T. Intracellular-free magnesium in the smooth muscle of guinea pig taenia caeci: a concomitant analysis for magnesium and pH upon sodium removal. J Gen Physiol. 1994 May;103(5):833–851. doi: 10.1085/jgp.103.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Seo Y., Takai A., Tomita T., Watari H. Phosphorous compounds studied by 31P nuclear magnetic resonance spectroscopy in the taenia of guinea-pig caecum. J Physiol. 1988 Aug;402:565–578. doi: 10.1113/jphysiol.1988.sp017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Tomita T. Depletion of intracellular free Mg2+ in Mg2(+)- and Ca2(+)-free solution in the taenia isolated from guinea-pig caecum. J Physiol. 1990 Feb;421:363–378. doi: 10.1113/jphysiol.1990.sp017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S., Tomita T. Regulation of intracellular free magnesium concentration in the taenia of guinea-pig caecum. J Physiol. 1991 Apr;435:559–572. doi: 10.1113/jphysiol.1991.sp018525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Moriyama T., Karaki H., Kohama K., Cragoe E. J., Jr Direct inhibition of contractile apparatus by analogues of amiloride in the smooth muscle of guinea-pig taenia caecum and chicken gizzard. Biochem Pharmacol. 1989 Mar 15;38(6):915–922. doi: 10.1016/0006-2952(89)90280-3. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Amiloride directly inhibits the Na,K-ATPase activity of rabbit kidney proximal tubules. Science. 1983 May 27;220(4600):957–958. doi: 10.1126/science.6302840. [DOI] [PubMed] [Google Scholar]
- Spurway N. C., Wray S. A phosphorus nuclear magnetic resonance study of metabolites and intracellular pH in rabbit vascular smooth muscle. J Physiol. 1987 Dec;393:57–71. doi: 10.1113/jphysiol.1987.sp016810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward M. C., Seo Y., Murakami M., Watari H. NMR relaxation characteristics of rubidium-87 in perfused rat salivary glands. Proc Biol Sci. 1991 Feb 22;243(1307):115–120. doi: 10.1098/rspb.1991.0019. [DOI] [PubMed] [Google Scholar]
- Sturek M., Hermsmeyer K. Calcium and sodium channels in spontaneously contracting vascular muscle cells. Science. 1986 Jul 25;233(4762):475–478. doi: 10.1126/science.2425434. [DOI] [PubMed] [Google Scholar]
- Taggart M. J., Wray S. Occurrence of intracellular pH transients during spontaneous contractions in rat uterine smooth muscle. J Physiol. 1993 Dec;472:23–31. doi: 10.1113/jphysiol.1993.sp019933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. G., Lakatta E. G., Gerstenblith G. Effects of amiloride on metabolism and contractility during reoxygenation in perfused rat hearts. Circ Res. 1990 Apr;66(4):1012–1022. doi: 10.1161/01.res.66.4.1012. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]


