Abstract
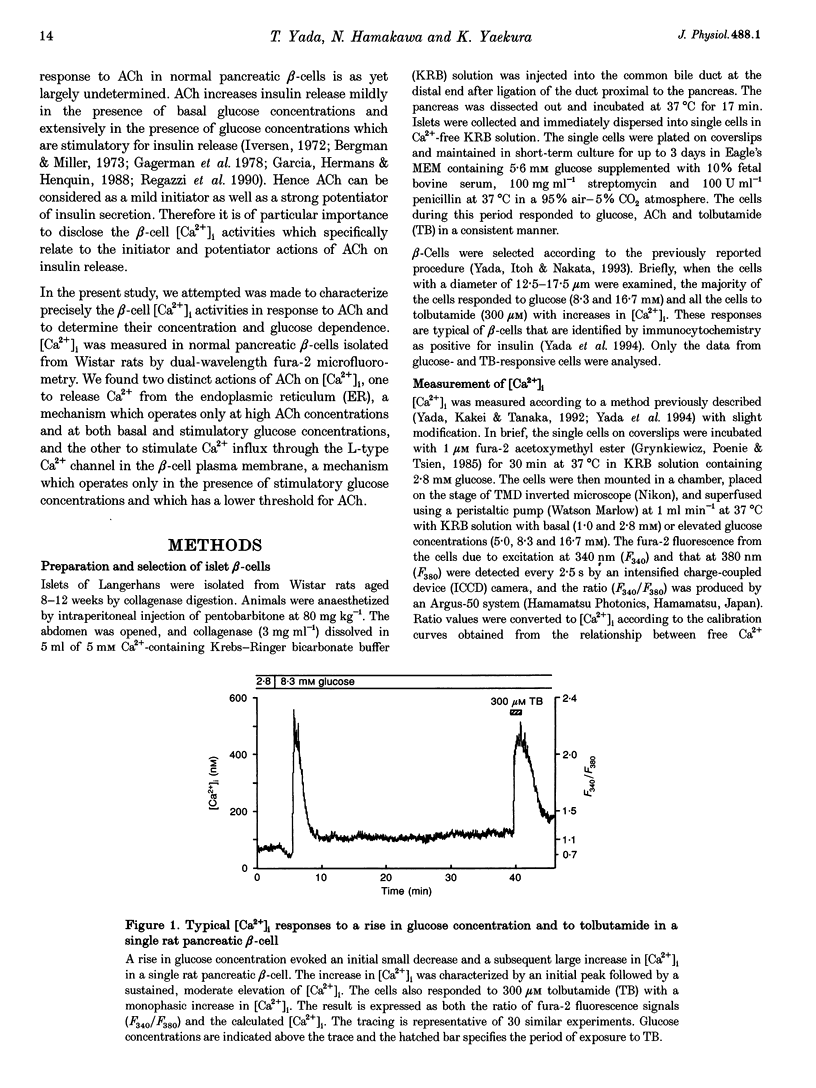
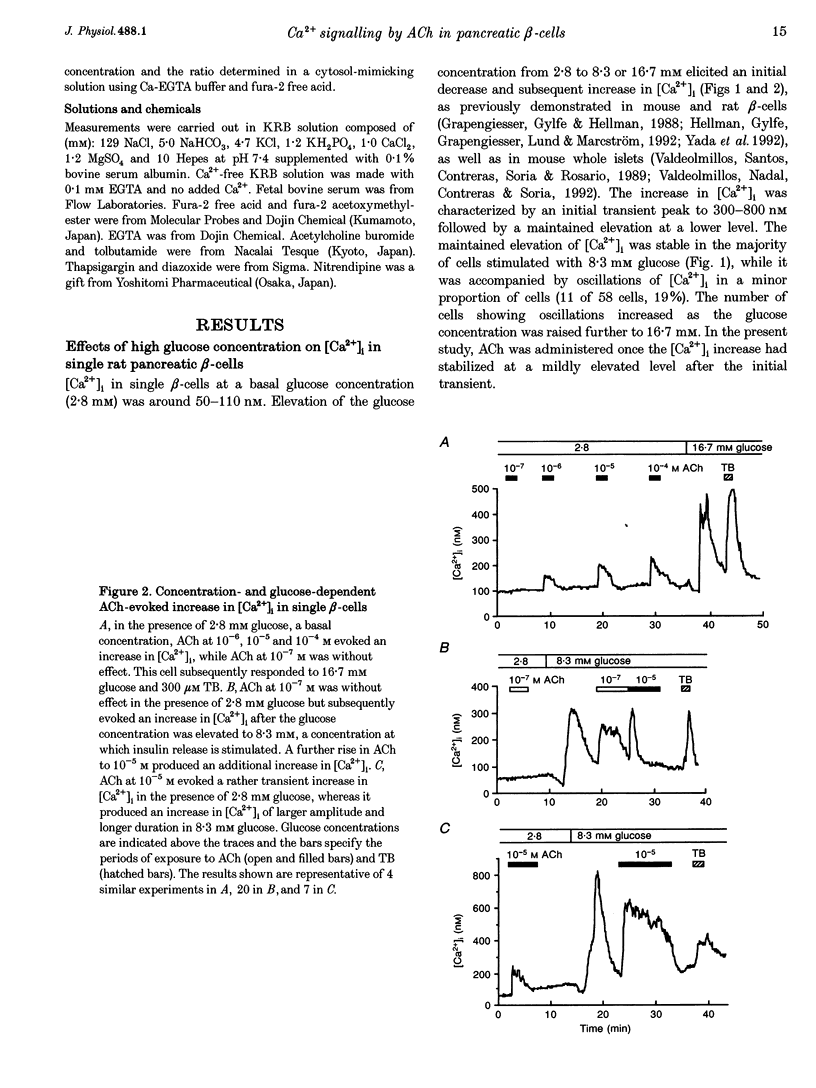
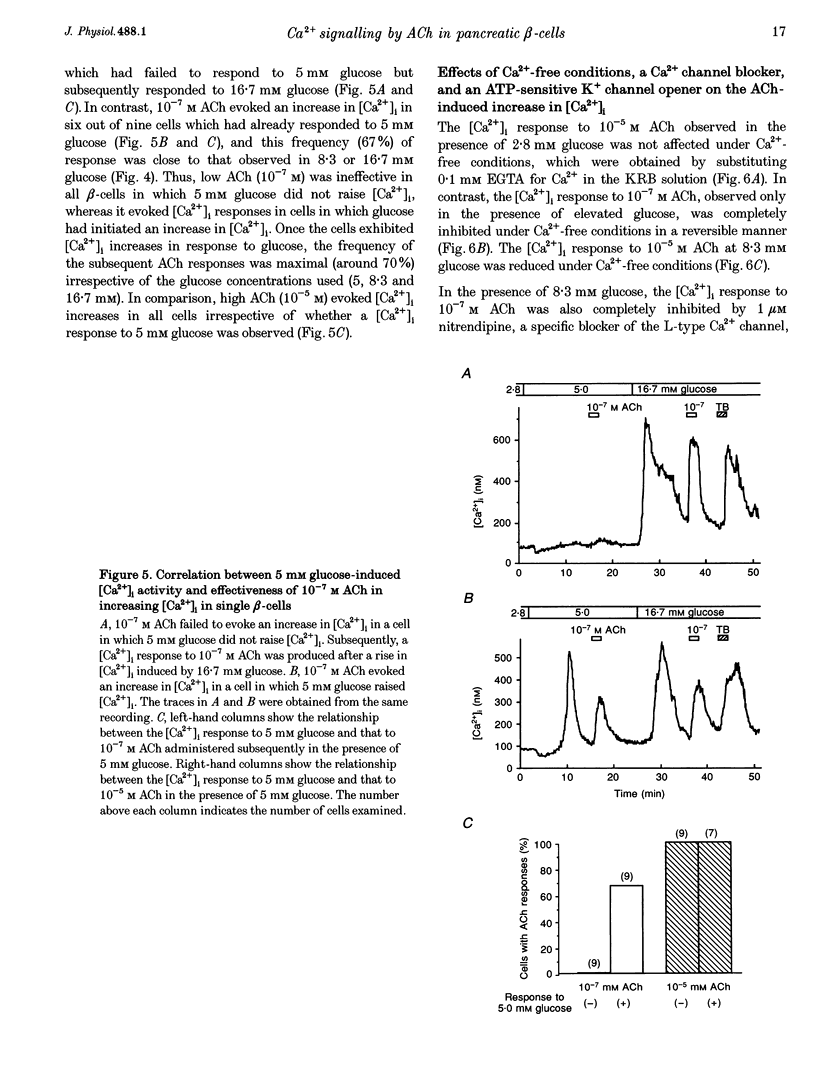
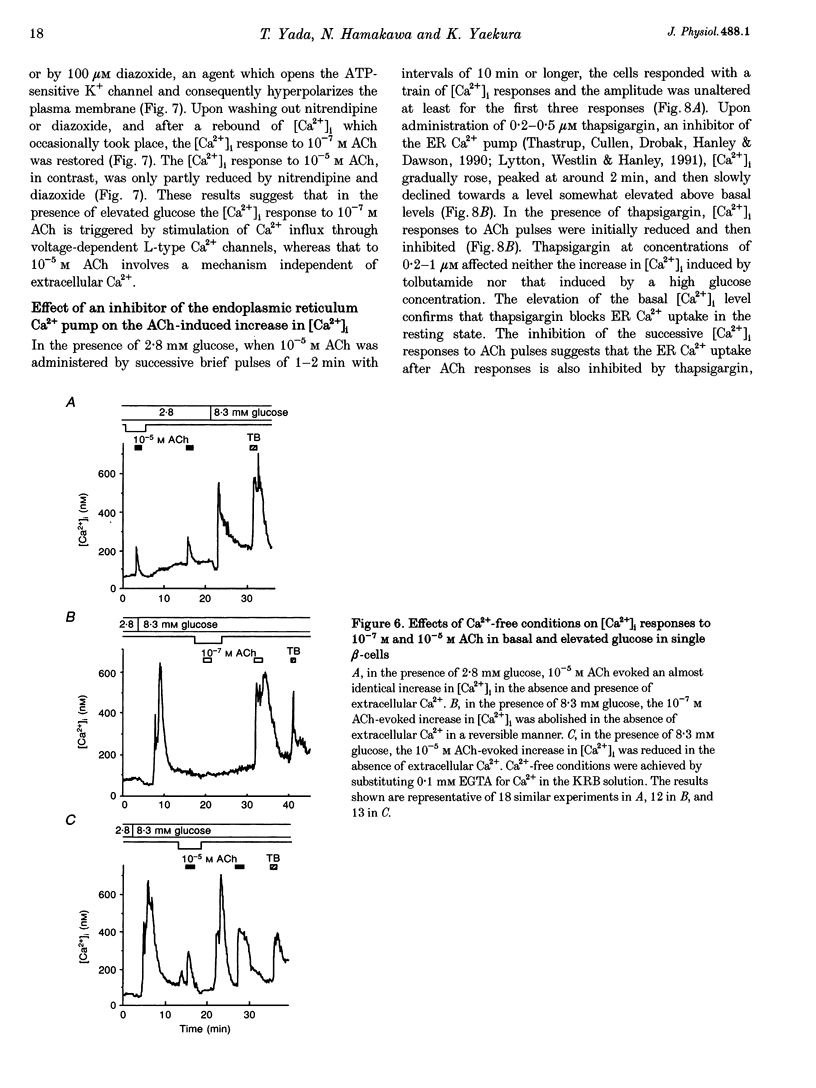
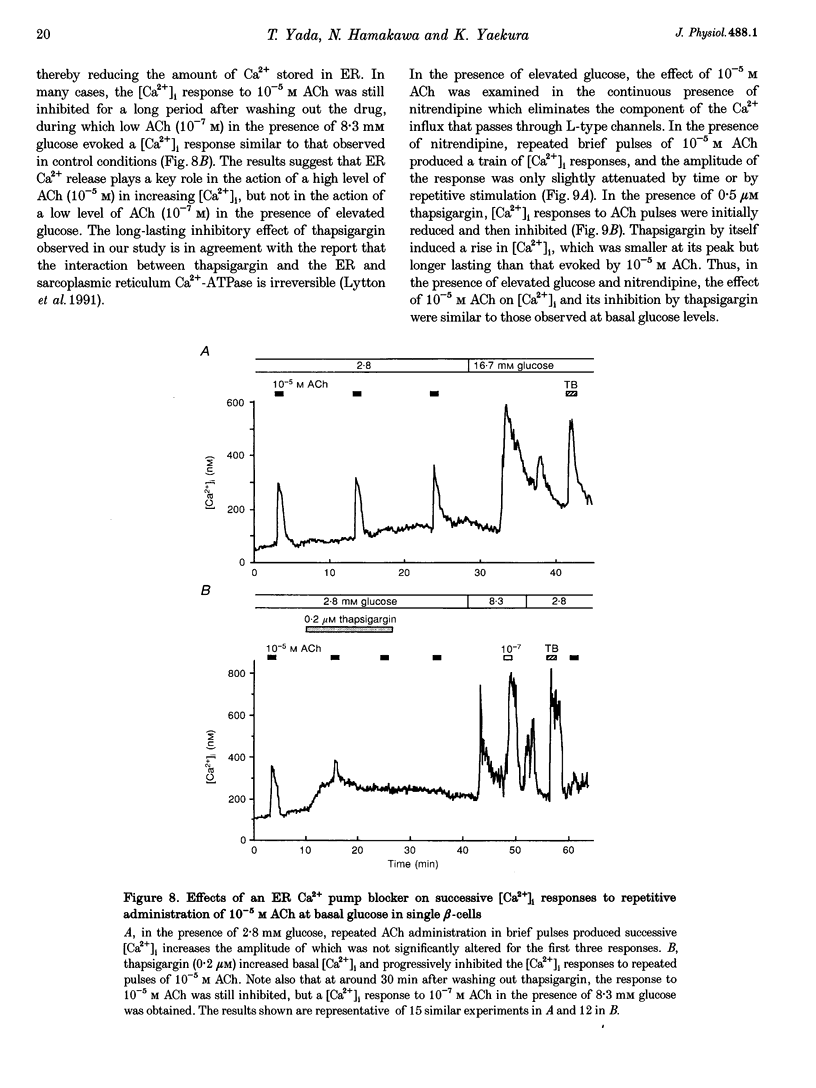
1. Calcium signalling by acetylcholine (ACh) in single rat pancreatic beta-cells was studied. The cytosolic free Ca2+ concentration ([Ca2+]i) was measured by dual-wavelength fura-2 microfluorometry. 2. In the presence of basal glucose (2.8 mM), 10(-6) to 10(-4) M ACh (high ACh) transiently increased [Ca2+]i. The [Ca2+]i response to 10(-5) M ACh was little altered under Ca(2+)-free conditions. Brief pulses of 10(-5) M ACh evoked successive [Ca2+]i responses, which were progressively inhibited by 0.2-0.5 microM thapsigargin, a specific inhibitor of the endoplasmic reticulum (ER) Ca2+ pump. 3. Elevation of glucose to 8.3 mM, a concentration which stimulates insulin release, increased [Ca2+]i to an initial peak followed by a sustained, moderate elevation. Addition of 10(-8) to 10(-7) M ACh (low ACh) evoked a further increase in [Ca2+]i. The [Ca2+]i response to 10(-7) M ACh was completely inhibited under Ca(2+)-free conditions by 1 microM nitrendipine, a blocker of L-type Ca2+ channels, and by 100 microM diazoxide, an opener of ATP-sensitive K+ channels. 4. In the presence of 8.3 mM glucose, [Ca2+]i responses to 10(-5) M ACh were reduced but not abolished by Ca(2+)-free conditions, nitrendipine and diazoxide. Successive [Ca2+]i transients induced by 10(-5) M ACh pulses in the presence of nitrendipine were progressively inhibited by thapsigargin. 5. The results revealed two distinct modes of Ca2+ signalling: low ACh increases [Ca2+]i by stimulating Ca2+ influx through voltage-dependent L-type Ca2+ channels only in the beta-cells in which glucose has already elevated [Ca2+]i, while high ACh increases [Ca2+]i at basal as well as stimulatory glucose concentrations by releasing Ca2+ from the ER. The former mechanism is likely to relate to the potentiator action and the latter to the initiator action of ACh on insulin release. High ACh and elevated glucose provoke both modes of Ca2+ signalling.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén B., Taborsky G. J., Jr, Porte D., Jr Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1986 Dec;29(12):827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman R. N., Miller R. E. Direct enhancement of insulin secretion by vagal stimulation of the isolated pancreas. Am J Physiol. 1973 Aug;225(2):481–486. doi: 10.1152/ajplegacy.1973.225.2.481. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Takemura H., Thastrup O., Putney J. W., Jr, Menniti F. S. Mechanisms of activated Ca2+ entry in the rat pancreatoma cell line, AR4-2J. Cell Calcium. 1992 Jan;13(1):49–58. doi: 10.1016/0143-4160(92)90029-r. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem. 1993 May 25;268(15):11356–11363. [PubMed] [Google Scholar]
- Cook D. L., Crill W. E., Porte D., Jr Glucose and acetylcholine have different effects on the plateau pacemaker of pancreatic islet cells. Diabetes. 1981 Jul;30(7):558–561. doi: 10.2337/diab.30.7.558. [DOI] [PubMed] [Google Scholar]
- Gagerman E., Idahl L. A., Meissner H. P., Täljedal I. B. Insulin release, cGMP, cAMP, and membrane potential in acetylcholine-stimulated islets. Am J Physiol. 1978 Nov;235(5):E493–E500. doi: 10.1152/ajpendo.1978.235.5.E493. [DOI] [PubMed] [Google Scholar]
- Gao Z. Y., Gilon P., Henquin J. C. The role of protein kinase-C in signal transduction through vasopressin and acetylcholine receptors in pancreatic B-cells from normal mouse. Endocrinology. 1994 Jul;135(1):191–199. doi: 10.1210/endo.135.1.8013353. [DOI] [PubMed] [Google Scholar]
- Garcia M. C., Hermans M. P., Henquin J. C. Glucose-, calcium- and concentration-dependence of acetylcholine stimulation of insulin release and ionic fluxes in mouse islets. Biochem J. 1988 Aug 15;254(1):211–218. doi: 10.1042/bj2540211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilon P., Henquin J. C. Activation of muscarinic receptors increases the concentration of free Na+ in mouse pancreatic B-cells. FEBS Lett. 1993 Jan 11;315(3):353–356. doi: 10.1016/0014-5793(93)81193-4. [DOI] [PubMed] [Google Scholar]
- Gilon P., Shepherd R. M., Henquin J. C. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem. 1993 Oct 25;268(30):22265–22268. [PubMed] [Google Scholar]
- Grapengiesser E., Gylfe E., Hellman B. Dual effect of glucose on cytoplasmic Ca2+ in single pancreatic beta-cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):419–425. doi: 10.1016/0006-291x(88)90537-2. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gylfe E. Carbachol induces sustained glucose-dependent oscillations of cytoplasmic Ca2+ in hyperpolarized pancreatic beta cells. Pflugers Arch. 1991 Dec;419(6):639–643. doi: 10.1007/BF00370308. [DOI] [PubMed] [Google Scholar]
- Hamakawa N., Yada T. Interplay of glucose-stimulated Ca2+ sequestration and acetylcholine-induced Ca2+ release at the endoplasmic reticulum in rat pancreatic beta-cells. Cell Calcium. 1995 Jan;17(1):21–31. doi: 10.1016/0143-4160(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Hellman B., Gylfe E. Mobilization of different intracellular calcium pools after activation of muscarinic receptors in pancreatic beta-cells. Pharmacology. 1986;32(5):257–267. doi: 10.1159/000138178. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Garcia M. C., Bozem M., Hermans M. P., Nenquin M. Muscarinic control of pancreatic B cell function involves sodium-dependent depolarization and calcium influx. Endocrinology. 1988 May;122(5):2134–2142. doi: 10.1210/endo-122-5-2134. [DOI] [PubMed] [Google Scholar]
- Herchuelz A., Lebrun P. A role for Na/Ca exchange in the pancreatic B cell. Studies with thapsigargin and caffeine. Biochem Pharmacol. 1993 Jan 7;45(1):7–11. doi: 10.1016/0006-2952(93)90370-c. [DOI] [PubMed] [Google Scholar]
- Hermans M. P., Schmeer W., Henquin J. C. Modulation of the effect of acetylcholine on insulin release by the membrane potential of B cells. Endocrinology. 1987 May;120(5):1765–1773. doi: 10.1210/endo-120-5-1765. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Chalk J. G., Ashcroft S. J. The role of cytosolic free Ca2+ and protein kinase C in acetylcholine-induced insulin release in the clonal beta-cell line, HIT-T15. Biochem J. 1990 Apr 1;267(1):227–232. doi: 10.1042/bj2670227. [DOI] [PMC free article] [PubMed] [Google Scholar]
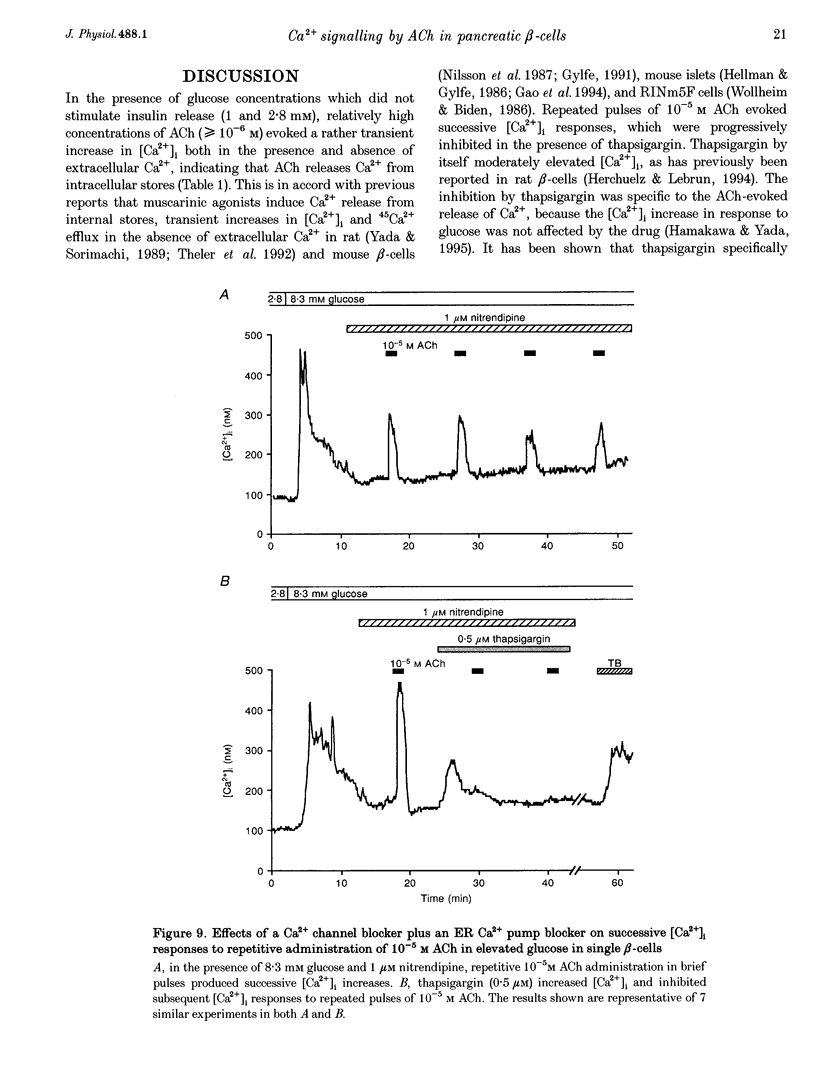
- Iversen J. Effect of acetyl choline on the secretion of glucagon and insulin from the isolated, perfused canine pancreas. Diabetes. 1973 May;22(5):381–387. doi: 10.2337/diab.22.5.381. [DOI] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Hallberg A., Hellman B., Berggren P. O. Characterization of the inositol 1,4,5-trisphosphate-induced Ca2+ release in pancreatic beta-cells. Biochem J. 1987 Dec 1;248(2):329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Persaud S. J., Jones P. M., Sugden D., Howell S. L. The role of protein kinase C in cholinergic stimulation of insulin secretion from rat islets of Langerhans. Biochem J. 1989 Dec 15;264(3):753–758. doi: 10.1042/bj2640753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Riesch B., Fathi M., Schlegel W., Wollheim C. B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988 Apr;81(4):1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Regazzi R., Li G. D., Deshusses J., Wollheim C. B. Stimulus-response coupling in insulin-secreting HIT cells. Effects of secretagogues on cytosolic Ca2+, diacylglycerol, and protein kinase C activity. J Biol Chem. 1990 Sep 5;265(25):15003–15009. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theler J. M., Mollard P., Guérineau N., Vacher P., Pralong W. F., Schlegel W., Wollheim C. B. Video imaging of cytosolic Ca2+ in pancreatic beta-cells stimulated by glucose, carbachol, and ATP. J Biol Chem. 1992 Sep 5;267(25):18110–18117. [PubMed] [Google Scholar]
- Valdeolmillos M., Nadal A., Contreras D., Soria B. The relationship between glucose-induced K+ATP channel closure and the rise in [Ca2+]i in single mouse pancreatic beta-cells. J Physiol. 1992 Sep;455:173–186. doi: 10.1113/jphysiol.1992.sp019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdeolmillos M., Santos R. M., Contreras D., Soria B., Rosario L. M. Glucose-induced oscillations of intracellular Ca2+ concentration resembling bursting electrical activity in single mouse islets of Langerhans. FEBS Lett. 1989 Dec 18;259(1):19–23. doi: 10.1016/0014-5793(89)81484-x. [DOI] [PubMed] [Google Scholar]
- Wang J., Baimbridge K. G., Brown J. C. Glucose- and acetylcholine-induced increase in intracellular free Ca2+ in subpopulations of individual rat pancreatic beta-cells. Endocrinology. 1992 Jul;131(1):146–152. doi: 10.1210/endo.131.1.1611994. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Yada T., Itoh K., Nakata M. Glucagon-like peptide-1-(7-36)amide and a rise in cyclic adenosine 3',5'-monophosphate increase cytosolic free Ca2+ in rat pancreatic beta-cells by enhancing Ca2+ channel activity. Endocrinology. 1993 Oct;133(4):1685–1692. doi: 10.1210/endo.133.4.8404610. [DOI] [PubMed] [Google Scholar]
- Yada T., Kakei M., Tanaka H. Single pancreatic beta-cells from normal rats exhibit an initial decrease and subsequent increase in cytosolic free Ca2+ in response to glucose. Cell Calcium. 1992 Jan;13(1):69–76. doi: 10.1016/0143-4160(92)90031-m. [DOI] [PubMed] [Google Scholar]
- Yada T., Sakurada M., Ihida K., Nakata M., Murata F., Arimura A., Kikuchi M. Pituitary adenylate cyclase activating polypeptide is an extraordinarily potent intra-pancreatic regulator of insulin secretion from islet beta-cells. J Biol Chem. 1994 Jan 14;269(2):1290–1293. [PubMed] [Google Scholar]



