Abstract
Purpose
The purpose of this study was to investigate the clinical characteristics of patients with corneal Mooren's ulcers (MU) and explore potential risk factors for recurrence after keratoplasty.
Methods
The study retrospectively analyzed 87 patients (101 eyes) diagnosed with MU. Factors associated with recurrence after keratoplasty were identified using correlation analysis, and a clinical scoring system was developed based on the magnitude of the univariate and multivariate logistic regression analysis.
Results
The average age of onset for the 87 patients was 55.2 years old, and the male-to-female ratio was 1:0.74. Of the 101 eyes diagnosed with MU, 73 cases (83.9 %) were Unilateral. Eleven eyes (10.9 %) had a history of ocular surgery, and five (5.0 %) had a history of ocular trauma. The perforation rate was 18.8 %, and the recurrence rate after keratoplasty was 37.3 %. Ulcer depth (P < 0.05, R = -0.252), corneal perforation status (P < 0.05, R = 0.238), and history of ocular surgery or trauma (P < 0.05, R = −0.238) were associated with recurrence. Based on these findings, a clinical scoring system was constructed to evaluate the recurrence of MU.
Conclusion
MU mostly occurs in elderly men, often with monocular onset. The recurrence rate after keratoplasty is high at 37.3 %. Deep ulcer infiltration, corneal perforation, and a history of ocular surgery or trauma are associated with recurrence after keratoplasty. A scoring system has been established based on these clinical characteristics and can be used to predict the risk of recurrence after keratoplasty.
Keywords: Mooren's ulcer, Clinical characteristics, Treatment, Corneal perforation, Clinical scoring system, Recurrence
1. Introduction
Mooren's ulcer (MU) is a painful, progressive, spontaneous peripheral ulcerative keratitis (PUK) that can affect one or both eyes. Typically, the ulceration starts in the periphery and progresses centrally or circumferentially, sometimes involving the entire cornea. The signs of MU are varied and can include ulcers, neovascularization, and perforation. The incidence, clinical features, and prognosis of MU can vary by ethnicity and geography [1,2]. The incidence is relatively low in most regions, particularly in the Northern Hemisphere, while it is slightly higher than the global average in southern Africa and India [3,4]. According to literature, the incidence of MU in China is about 0.03 % [5]. However, due to the low sample size and inconsistent diagnosis methods, research data on the clinical features of MU in China are scattered, especially in Southeast China (see Fig. 1).
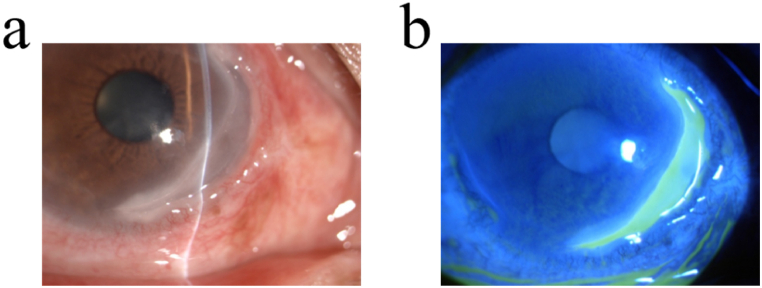
Fig. 1.
The typical characteristic of MU. Slit-lamp microscopic photograph (a) and fluorescein staining (b) of MU shows characteristic steep, overhanging central and leading edge with thin and vascularized cornea base.
The exact cause of MU is unknown, but many studies have suggested that it is an autoimmune reaction induced by external stimuli, such as corneal trauma or surgery, in individuals with genetic susceptibility [3,4,[6], [7], [8]]. When diagnosing MU, other causes of PUK, such as rheumatoid arthritis, Granulomatous polyangiitis, and systemic lupus erythematosus, must be ruled out. The management of MU is difficult and includes pharmacological, surgical, and other adjuvant therapies. Pharmacological treatment based on immunomodulation is used, with a “stepwise” approach of topical or systemic corticosteroids and immunosuppressants [9]. Surgical treatment includes conjunctival excision, corneal cryopreservation, amniotic membrane transplantation [10,11], keratoplasty [12], and artificial cornea [13,14]. Keratoplasty is the most common therapeutic method for MU, but it has a high risk of recurrence [15,16]. A previous study reported a recurrence rate of 9.7 % after keratoplasty [17]. Therefore, in the clinical management of MU, it is important to identify high-risk cases for keratoplasty and consider more suitable therapeutic treatments, such as artificial cornea.
This study retrospectively analyzed the clinical characteristics of 101 eyes of 87 patients with MU who were admitted to the Affiliated Xiamen Eye Centre of Xiamen University in southern China over the past decade. The aim of this study was to investigate the clinical characteristics of patients with MU and establish a clinical scoring system to predict the recurrence of MU after keratoplasty.
2. Materials and methods
2.1. Diagnosis of MU
The diagnosis of MU was based on the patient's clinical presentation, the typical clinical features: a painful, crescent-shaped, peripheral corneal ulcer which starts at the limbus with a gray overhanging infiltrated margin. Slit lamp examination of a typical ulcer pattern (figures [1(a, b)]), and the results of comprehensive laboratory tests [18]. The patients exhibited typical characteristics of MU, such as severe ocular pain, redness, photophobia, and lacrimation, with the ulceration starting in the peripheral part of the cornea and gradually progressing to the periphery or central cornea. Additionally, systemic immune diseases that can cause PUK, such as rheumatoid arthritis, Granulomatous polyangiitis, and systemic lupus erythematosus, were excluded. All patients included in the study underwent scraping, stain microscopy, and culture before treatment. Patients with infectious factors causing corneal ulcers were excluded.
2.2. Ethical approval
Ethical approval for this study was obtained from Ethics Committee of Xiamen Eye Centre of Xiamen University (XMYKZX-KY-2024-022) and all the procedures were in accordance with the declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
2.3. Data collection
We collected medical records from 101 eyes of 87 MU patients who were hospitalized at the Affiliated Xiamen Eye Centre of Xiamen University in southern China between January 2010 and December 2020. Basic data were collected, including age, gender, laterality of eye, ulcer location, ulcer extent, depth of the ulcer, history of corneal perforation, history of ocular surgery or trauma, treatment, pre-treatment visual acuity, post-treatment visual acuity, and recurrence status. Patients were divided into two groups based on age of onset: patients aged ≤35 years were categorized as the young group, and those aged >35 years were categorized as the old group [19].
The extent of ulceration was expressed as the number of clocks involved in the limbus. We evaluated the depth of the ulcer by measuring the corneal thickness (CT) using anterior segment OCT. This involved determining the infiltration depth and vertical total corneal thickness at the point of maximum infiltration. We then created a scoring system by categorizing the ratio of infiltration depth to total corneal depth. To determine the factors affecting recurrence after keratoplasty, patients were divided into the recurrence and non-recurrence groups, with all patients followed-up for 3 years. Patients with recurrence were considered outcome event end follow-up and treatment (minimum was 2 months). The clinical characteristics were assessed by observing symptoms such as eye redness and eye pain, as well as the size of the ulcer after keratoplasty. The recurrence group was defined as patients experiencing re-emergence of irritation after treatment, such as ocular redness and pain, as well as the reappearance of conjunctival congestion and submerged ulcers at the corneal rim [20].
2.4. The procedure of keratoplasty
Informed consent was obtained from the patient or their kinship family.
The corneal implant bed was constructed according to the pathology of the diseased/perforated area, and the lamellar graft was made with corneal shear. The size and thickness were consistent with the implanted bed, and the epithelial surface was placed on the implant bed. The corneal side of the implant bed and the corneal side of the corneal graft were sutured with 10-0 nylon thread.
2.5. Statistical analysis
The data were statistically analyzed using SPSS v. 21.0. The clinical characteristics of the patients were analyzed descriptively. A chi-square test or rank sum test was chosen to compare the differences between the two groups based on normality analysis. Our selection of predictive variables comes from literature and clinical practice. The risk score was developed based on the final logistic regression model. In order to construct the risk score, we selected the optimal model based on the c-index, while also considering the actual situation and clinical significance. Independent samples t-test was used to assess the difference in clinical scores between the non-recurrence group and the recurrence group. Univariate logistic regression was used to analyse the degree of influence of clinical scores on relapse. The level of significance was set at p < 0.05.
3. Results
3.1. The demography and clinical characteristics of MU
This study included 87 patients, with 14 in the youth group and 73 in the old group. The age of onset ranged from 20 to 79 years, with an average age of 55.2 years. In the total population, there were 50 male and 37 female patients, resulting in a male to female ratio of 1:0.74. The male to female ratio in the young group was 1:1, while in the old group it was 1:0.7. These details are summarized in Table 1.
Table 1.
Demography and clinical characteristics of Mooren' s ulcer.
| Total | Group |
||
|---|---|---|---|
| Young | Old | ||
| Total (cases, eyes) | 87,101 | 14,17 | 73,84 |
| Age (years), median (range) | 55.2(20–79) | ||
| Sex (Male: Female) | 1:0.74(50:37) | 1:1 | 1:0.7 |
| Eye category | |||
| Left(eyes) | 50(49.5 %) | 7(41.2 %) | 43(51.2 %) |
| Right(eyes) | 51(50.5 %) | 10(58.8) | 41(49.8 %) |
| Unilateral (cases, eyes) | 73,73 (83.9 %) | 11,11(78.6 %) | 62, 62(84.5 %) |
| Bilateral (cases, eyes) | 14,28(16.1 %) | 3,6 (21.4 %) | 11, 22 (15.5 %) |
| Location | |||
| superior(eyes) | 41 (40.6 %) | 7(41.2 %) | 34(40.5 %) |
| temporal(eyes) | 29 (28.7 %) | 6(35.3 %) | 23(27.4 %) |
| inferior(eyes) | 25 (24.8 %) | 6(35.3 %) | 19(22.2) |
| nasal(eyes) | 47 (46.5 %) | 8(47.1 %) | 39(46.4 %) |
| Clock hours of ulcer involved cornea | |||
| −3 clock hours | 13(12.9 %) | 0 | 13(15.5 %) |
| −6 clock hours | 44(43.6 %) | 9(52.9 %) | 35(41.7 %) |
| −9 clock hours | 32(31.6 %) | 3(17.6 %) | 29(34.5 %) |
| −12 clock hours | 12(11.9 %) | 2(11.8 %) | 10(11.9 %) |
| Depth of the ulcer | |||
| −1/3CT | 60(59.4 %) | 6(35.3 %) | 54(64.3 %) |
| −2/3CT | 21(20.8 %) | 5(29.4 %) | 16(19.0 %) |
| -1CT | 20(19.8 %) | 3(17.6 %) | 17(20.2 %) |
| Perforation(eyes) | 19(18.8 %) | 6(35.3 %) | 13(15.5 %) |
| History | |||
| First clinical course(Moth) (mean, SD) | 14.8,11.6 | 13.7,11.8 | 14.7, 41.5 |
| Ocular trauma | 11(10.9 %) | 1(5.9 %) | 10(11.9 %) |
| Cornea involved surgery | 7(6.9 %) | 0 | 7(8.3 %) |
CT, Corneal thickness
83.9 % of the affected eyes were unilaterally involved, while 16.1 % were bilaterally involved. Bilateral lesions were more common in the old group (78.6 %) than in the young group (21.4 %). The site of ulcer appearance was recorded in 101 eyes, with the nasal cornea being the most commonly affected (46.5), followed by superior (40.6 %), the temporal (28.7 %), and inferior (24.8 %) cornea. The number of clock hours of ulcer involvement at 0–3 clock hours; 0–6 clock hours; 0–9 clock hours; and 0–12 clock hours were 12.9 %, 43.6 %, 31.6 %, and 11.9 %, respectively. The depth of the ulcer varied, with 59.4 % being 0–1/3 CT, 20.8 % being 0–2/3 CT, and 19.8 % being 0–1 CT. Corneal perforation occurred in 18.8 % of cases, 35.2 % in the young group and 15.5 % in the old group. The first clinical course was 14.8 ± 11.6 months. Of the 101 affected eyes, 11 (10.9 %) had a history of surgery with corneal involvement, including 3 eyes that underwent cataract surgery, 5 eyes that underwent pterygium excision surgery, 1 eye that underwent amniotic membrane transplantation (AMT), 1 eye underwent antiglaucoma surgery, and 1 eye underwent antiglaucoma surgery combined with AMT combined with cataract surgery. 7 eyes (6.9 %) had a history of ocular trauma (all in the old group), including 2 eyes with a history of corneal foreign body splash, 1 eye with a history of ocular chemical injury, and 2 eyes with a history of blunt corneal contusion (Table 1).
3.2. Treatment and prognosis of MU
In this study, 72.3 % of the affected eyes received surgical treatment, with keratoplasty being the most common procedure (66.3 %), especially among the young age group. The visual acuity of affected eyes before and after treatment was analyzed (Table 2). Among the patients who underwent keratoplasty, 37.3 % experienced recurrence. The characteristics and treatment of patients with and without recurrence were compared, and it was found that the age and history of corneal perforation or ocular trauma/surgery were significant factors in predicting recurrence (Table 3, p = 0.029 and 0.006, respectively). The depth of the ulcer was also found to be a significant factor. (Table 3, p = 0.010).
Table 2.
Treatment of MU and pre-treatment/post-treatment BCVA.
| Total | Group |
||
|---|---|---|---|
| Young | Old | ||
| Medicine | 27(26.7 %) | 1(6.7 %) | 26(30.2 %) |
| Surgery# | |||
| KP | 67(66.3 %) | 13(86.7 %) | 54(62.8 %) |
| AMT | 4(4.0 %) | 0 | 4(4.7 %) |
| CF | 2(2.0 %) | 1(6.7 %) | 1(1.2 %) |
| other∗ | 1(1.0 %) | 0 | 1(1.2 %) |
| Pre-treatment BCVA | |||
| −0.05 | 33(32.7 %) | 6(35.2 %) | 27(32.1 %) |
| −0.3 | 24(23.8 %) | 5(29.4 %) | 19(22.6 %) |
| −0.8 | 22(21.8 %) | 4(23.6 %) | 18(21.3 %) |
| 0.8- | 22(21.8 %) | 2(11.8 %) | 20(23.8 %) |
| Post-treatment BCVA | |||
| −0.05 | 34(33.7 %) | 6(35.2 %) | 28(33.3 %) |
| −0.3 | 25(24.8 %) | 4(23.6 %) | 21(25.0 %) |
| −0.8 | 26(25.7 % | 6(35.2 %) | 20(23.8 %) |
| 0.8- | 16(15.8 %) | 1(5.9 %) | 15(17.9 %) |
#KP, Keratoplasty; AMT, Amniotic membrane transplantation; CF, Conjunctiva flap. ∗Such as eye removes; BCVA, Best corrected visual acuity.
Table 3.
Prognosis of MU after keratoplasty.
| Recurrent group (n = 25) (37.3 %) | Nonrecurrent group (n = 42) (62.7 %) | X2 | P | ||
|---|---|---|---|---|---|
| Eye category | 0.44004 | 0.507 | |||
| Right | 11 (44.0 %) | 22 (52.4 %) | |||
| Left | 14 (56.0 %) | 20 (47.6 %) | |||
| Age | 49.5 ± 15.2 | 56.1 ± 16.1 | |||
| Sex | 0.3614 | 0.548 | |||
| Male | 13 (52.0 %) | 25 (69.5 %) | |||
| Female | 12 (48.0 %) | 17 (40.5 %) | |||
| Ocular trauma or surgery | 4.751 | 0.029∗ | |||
| Yes | 2 (8.0 %) | 13(30.1 %) | |||
| No | 23 (92.0 %) | 29 (69.9 %) | |||
| Perforation | 7.57 | 0.006∗ | |||
| Yes | 12 (48.0 %) | 7(16.7 %) | |||
| No | 13(52.0 %) | 35(83.3 %) | |||
| Clock hours of ulcer involved cornea | 2.017 | 0.569 | |||
| −3 clock hours | 2(8.0 %) | 5(11.9 %) | |||
| −6 clock hours | 12(48.0 %) | 18(42.9 %) | |||
| −9 clock hours | 7(28.0 %) | 15(35.7 %) | |||
| −12 clock hours | 4(16.0 %) | 4 (9.5 %) | |||
| Depth of the ulcer(CT) | 9.299 | 0.010∗ | |||
| −1/3 | 6(24.0 %) | 21(50.0 %) | |||
| −2/3 | 6(24.0 %) | 13(31.0 %) | |||
| −1 | 13(52.0 %) | 8(19.0 %) | |||
CT, Corneal thickness; ∗, P < 0.05, there was statistically significant differences.
3.3. Establishing a clinical scoring system
To explore potential risk factors for recurrence after keratoplasty, clinical data were analyzed for correlation with recurrence. There was no significant correlation with age(P > 0.05, R = −0.119), sex(P > 0.05, R = −0.081), eye category(P > 0.05, R = 0.089), and clock hours of ulcer involved cornea(P > 0.05, R = 0.171). The recurrence of MU was positively correlated with ulcer depth and corneal perforation (P < 0.05, R = 0.252, R = 0.238, respectively), while negatively correlated with the history of ocular surgery or trauma (P < 0.05, R = −0.238). Based on these findings, a clinical scoring system was established using the R-value size of ulcer depth, corneal perforation, and history of ocular surgery or trauma.
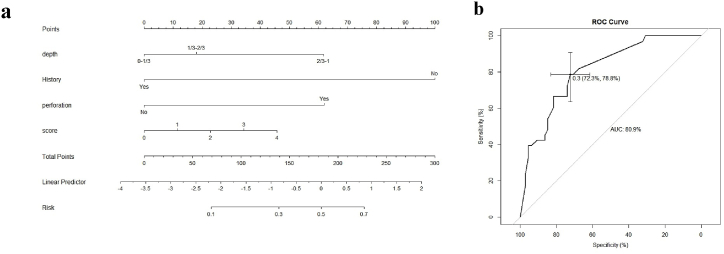
We used multivariate logistic regression analysis to fit the combined predictors of MU recurrence and build a predictive model for the following covariates: depth, history, and perforation. The model accounted for the presence of MU recurrence, and the area under the curve (AUC) was 80.9 %, with a sensitivity of 78.8 % and a specificity of 72.3 %. These results indicate that the model has some reference value for predicting the risk of MU recurrence after keratoplasty (figures [2(a, b)]) (see Fig. 2).
Fig. 2.
The establishing of MU's clinical scoring system. a was the Nomogram model. b was the receiver operating characteristic (ROC) curve analysis. The AUC was 80.9 %, with a sensitivity of 78.8 % and a specificity of 72.3 %.
3.4. The clinically applied scoring system
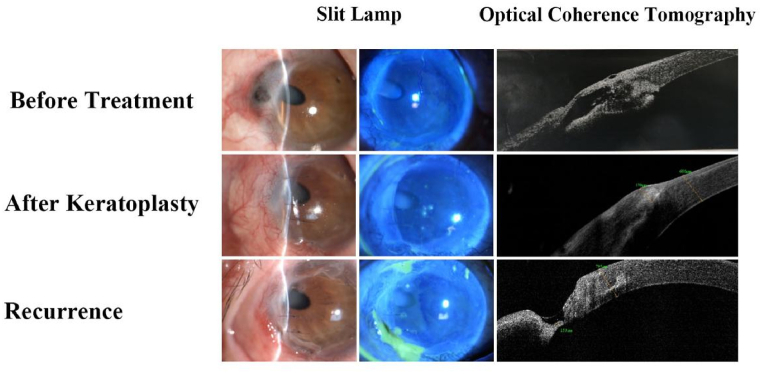
The score system based on the Nomogram model (figures [2(a, b)]) accumulates scores obtained from various independent variables to generate a total score, which corresponds to the total points in figures [2(a)]. The probability of recurrence can be predicted by aligning the total points vertically with the values in the risk section. In Fig. 3, we demonstrated the application of this scoring system by closely monitoring high-risk patients and intensifying intervention measures accordingly.
Fig. 3.
The recurrence patient after keratoplasty. A patient with Mooren's ulcer presented with a 2-year history of redness and pain in the right eye. Examination revealed deep corneal infiltration with perforation. The keratoplasty was performed. The risk assessment indicated a high likelihood of recurrence (70 %). Despite close monitoring, signs of recurrence appeared 9 months later.
4. Discussion
This retrospective study found that MU is more common in elderly men, typically occurring at the nasal corneal limbus and often with monocular onset. A history of ocular surgery or trauma may be a causative factor. Additionally, the study found that ulcer infiltration, corneal perforation, and a history of ocular surgery or trauma are associated with an increased risk of MU recurrence (Fig. 3). Based on these clinical characteristics, a scoring system was successfully established to predict the risk of MU recurrence.
This study found that almost all MU patients experienced eye redness, pain, photophobia, and lacrimation. The mean age of onset was 55.2 years, and the condition was more common in males. The male to female ratio in the total population was 1:0.74, which is consistent with previous reports of MU in China [5]. In other regions such as southern India and Nigeria, the proportion of male patients was even greater, with a male to female ratio of 1:0.21 and 1:0.28, respectively [7]. However, a study in Korea found that female patients were more common, with a male to female ratio of 1:1.18 [2]. In our opinion, it is speculated that the higher incidence in males may be due to a higher incidence of ocular trauma or cultural practices discouraging women from seeking medical care.
Corneal surgery or trauma may increase the risk of developing MU. A previous study reported that 10.7 % of MU patients had a history of corneal trauma or surgery [21]. In this study, 17.8 % of patients had a history of ocular surgery or trauma, including cataract surgery, pterygium excision, AMT, antiglaucoma surgery, and a combination of these surgeries. History of trauma included corneal foreign body splash injury, ocular chemical injury, and blunt corneal contusion. The corneal epithelium is the outermost structure of the cornea and serves to protect it from pathogenic microorganisms. It is hypothesized that corneal surgery or trauma may damage the corneal epithelium, leading to tissue-specific corneal antigen expression and increased sensitivity of the corneal tissue to immune-related cells, ultimately resulting in autoimmune injury [3,8]. However, further studies are needed to investigate the pathogenesis of MU in patients without a clear cause.
The statistical analysis in this study revealed that ulcers after keratoplasty were with a recurrence rate of 37.3 %. A previous retrospective case study of 173 eyes with MU reported a recurrence rate of 28.28 % after keratoplasty [17]. Identifying risk factors for recurrence is important in guiding the treatment of post-keratoplasty cases. In this study, a clinical scoring system was developed based on the clinical characteristics of patients with and without recurrence to predict the risk of recurrence.
Previous studies have identified bilateral onset, history of corneal infection, corneal perforation, male gender, and surgical treatment as risk factors for MU recurrence [22]. In this study, the deep ulcer infiltration, corneal perforation, and a history of ocular surgery or trauma were found to be factors involved in the recurrence of MU post-keratoplasty. Deep ulcer infiltration and corneal perforation were identified as risk factors, while the history of ocular surgery or trauma may be a protective factor for ulcer recurrence, possibly due to immune tolerance established by previous corneal antigen exposure. Based on these clinical characteristics, a clinical scoring system was established using the logistic regression model. This system had a sensitivity of 78.8 % and a specificity of 72.3 %, which could be useful in assessing the risk prediction of MU recurrence.
There were several limitations to this study. Firstly, the database was collected retrospectively, and a prospective study would be necessary to confirm the findings. Secondly, the sample size was limited due to the low prevalence of the disease. Additionally, the clinical scoring system developed in this study may not necessarily apply to different regions and races, as the study sample was collected from a single centre. Therefore, multi-centre and large sample clinical validation with more valuable auxiliary inspections would be needed to extend the model and achieve a more accurate and valid scoring system.
In summary, this study analyzed the clinical characteristics of a large cohort of MU patients in southeast China and found a high recurrence rate of 37.3 % after keratoplasty. Ulcer depth, corneal perforation, and history of ocular surgery or trauma were found to be highly related to MU recurrence. A clinical scoring system was established to assess the risk factors for recurrence after keratoplasty. With the improvement of risk assessment, the prospects for better treatment strategies for MU will be promising.
CRediT authorship contribution statement
Shangkun Ou: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Yujie Zhang: Writing – original draft, Visualization, Formal analysis, Data curation, Conceptualization. Yu'an Lin: Methodology, Formal analysis, Data curation. Xie Fang: Formal analysis, Data curation. Zhiwen Xie: Methodology, Data curation. Ke Shi: Software, Data curation. Minqing Cai: Methodology, Data curation. Shengqi Su: Methodology. Huping Wu: Writing – review & editing, Visualization, Validation, Conceptualization.
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of Xiamen University affiliated Xiamen Eye Centre (XMYKZX-KY-2024-022).
Consent for publication
Not applicable.
Data availability statement
Data associated with our study has not been deposited into a publicly available repository.
Data included in article/supp. material/referenced in article.
The data are not publicly available due to restrictions that apply to the availability of the data (e.g., privacy or ethics). Datasets from this study may be available upon request from the corresponding author and provided upon approval from the sponsor and by data privacy and ethical provisions.
Funding
This study was supported by the Fujian Provincial Science Fund for Distinguished Young Scholars (2023J06053), Guizhou Provincial Science and Technology Projects (QKHJC-ZK[2024]ZD043), the National Natural Science Foundation of China (82101084), and funding from the Xiamen Science and Technology Program for Public Wellbeing (3501Z20214 ZD1209, 3502Z20224ZD1208, 3502Z20224ZD1209, 3502Z20224ZD1210), Huaxia Eye Hospital Group Research Funding (HXKY202305D005). The funders had no role in the study design, data collection and analysis, publishing decision, or preparation of the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Shangkun Ou reports article publishing charges and writing assistance were provided by he Fujian Provincial Science Fund for Distinguished Young Scholars (2023J06053). NA If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Shangkun Ou, Email: shangkun_ou@126.com.
Huping Wu, Email: wuhuping123@163.com.
References
- 1.M E Zegans M.S. Mooren's ulcer. Int. Ophthalmol. Clin. 1998;(38):4. doi: 10.1097/00004397-199803840-00008. [DOI] [PubMed] [Google Scholar]
- 2.Dong Hyun Kim M., et al. Mooren's ulcer in a cornea referral practice in Korea. Ocul. Immunol. Inflamm. 2016;2016:55–59. doi: 10.3109/09273948.2014.926938. [DOI] [PubMed] [Google Scholar]
- 3.Taylor C.J., et al. HLA and Mooren's ulceration. Br. J. Ophthalmol. 2000;84(1):72–75. doi: 10.1136/bjo.84.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrysanthi Kafkala M., et al. Mooren ulcer: an immunopathologic study. Cornea. 2006;2006(25):667–673. doi: 10.1097/01.ico.0000214216.75496.7e. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., et al. Mooren's ulcer in China: a study of clinical characteristics and treatment. Br. J. Ophthalmol. 2000:1244–1249. doi: 10.1136/bjo.84.11.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju Lee Hyun, et al. Interplay of immune cells in mooren ulcer. Cornea. 2015:1164–1167. doi: 10.1097/ICO.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky J.R., et al. Hookworm infestation as a risk factor for Mooren's ulcer in South India. Ophthalmology. 2007;114(3):450–453. doi: 10.1016/j.ophtha.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Shinomiya K., et al. Immunohistochemical analysis of inflammatory limbal conjunctiva adjacent to Mooren's ulcer. Br. J. Ophthalmol. 2013;97(3):362–366. doi: 10.1136/bjophthalmol-2012-302631. [DOI] [PubMed] [Google Scholar]
- 9.Brown S.I., Mondino B.J. Therapy of Mooren's ulcer. Am. J. Ophthalmol. 1984:1–6. doi: 10.1016/0002-9394(84)90179-x. [DOI] [PubMed] [Google Scholar]
- 10.Ngan N.D., Chau H.T. Amniotic membrane transplantation for Mooren's ulcer. Clin. Exp. Ophthalmol. 2011;39(5):386–392. doi: 10.1111/j.1442-9071.2010.02479.x. [DOI] [PubMed] [Google Scholar]
- 11.Lavaju P., et al. Use of amniotic membrane and autologous serum eye drops in Mooren's ulcer. Nepal. J. Ophthalmol. 2013:120–123. doi: 10.3126/nepjoph.v5i1.7839. [DOI] [PubMed] [Google Scholar]
- 12.Gao H., et al. Partial lamellar keratoplasty for peripheral corneal disease using a graft from the glycerin-preserved corneoscleral rim. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252(6):963–968. doi: 10.1007/s00417-014-2642-2. [DOI] [PubMed] [Google Scholar]
- 13.Basu S., Taneja M., Sangwan V.S. Boston type 1 keratoprosthesis for severe blinding vernal keratoconjunctivitis and Mooren's ulcer. Int. Ophthalmol. 2011;31(3):219–222. doi: 10.1007/s10792-011-9438-8. [DOI] [PubMed] [Google Scholar]
- 14.Jerez-Pena M., et al. Bilateral Boston keratoprosthesis type 1 in a case of severe Mooren's ulcer. Eur. J. Ophthalmol. 2020 doi: 10.1177/1120672120909768. [DOI] [PubMed] [Google Scholar]
- 15.Gottsch J.D., Liu S.H., Stark W.J. Mooren's ulcer and evidence of stromal graft rejection after penetrating keratoplasty. Am. J. Ophthalmol. 1992;113(4):412–417. doi: 10.1016/s0002-9394(14)76164-1. [DOI] [PubMed] [Google Scholar]
- 16.Mondino B.J., Hofbauer J.D., Foos R.Y. Mooren's ulcer after penetrating keratoplasty. Am. J. Ophthalmol. 1987;103(1):53–56. doi: 10.1016/s0002-9394(14)74169-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu J., et al. Modified lamellar keratoplasty and immunosuppressive therapy guided by in vivo confocal microscopy for perforated Mooren's ulcer. Br. J. Ophthalmol. 2015;99(6):778–783. doi: 10.1136/bjophthalmol-2014-306012. [DOI] [PubMed] [Google Scholar]
- 18.Hatou S., et al. The application of in vivo confocal scanning laser microscopy in the diagnosis and evaluation of treatment responses in Mooren's ulcer. Invest. Ophthalmol. Vis. Sci. 2011;52(9):6680–6689. doi: 10.1167/iovs.10-5906. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y., et al. Risk factors, clinical features, and treatment outcomes of recurrent mooren ulcers in China. Cornea. 2017;36(2):202–209. doi: 10.1097/ICO.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 20.Guindolet D., et al. Management of severe and refractory Mooren's ulcers with rituximab. Br. J. Ophthalmol. 2017;101(4):418–422. doi: 10.1136/bjophthalmol-2016-308838. [DOI] [PubMed] [Google Scholar]
- 21.Chen J., et al. Mooren's ulcer in China: a study of clinical characteristics and treatment. Br. J. Ophthalmol. 2000;84(11):1244–1249. doi: 10.1136/bjo.84.11.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L., et al. Clinical characteristics and risk factors of recurrent Mooren's ulcer. J Ophthalmol. 2017;2017 doi: 10.1155/2017/8978527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with our study has not been deposited into a publicly available repository.
Data included in article/supp. material/referenced in article.
The data are not publicly available due to restrictions that apply to the availability of the data (e.g., privacy or ethics). Datasets from this study may be available upon request from the corresponding author and provided upon approval from the sponsor and by data privacy and ethical provisions.