Abstract
1. The role of supra-brainstem structures in the ventilatory response to inhaled CO2 is unknown. The present study uses positron emission tomography (PET), with infusion of H2(15)O, to measure changes in relative regional cerebral blood flow (rCBF) in order to identify sites of increased neuronal activation during CO2-stimulated breathing (CO2-SB) in awake man. 2. Five male volunteers were scanned during CO2-SB (mean +/- S.E.M.; end-tidal PCO2, 50.3 +/- 1.7 mmHg; respiratory frequency, 16.4 +/- 2.7 min-1; tidal volume, 1.8 +/- 0.2 l). As control, scans were performed during 'passive' isocapnic (elevated fraction of inspired CO2) positive pressure ventilation (end-tidal PCO2, 38.4 +/- 1.0 mmHg; respiratory frequency, 15.5 +/- 2.2 min-1; tidal volume, 1.6 +/- 0.2 l). With CO2-SB, all subjects reported dyspnoea. 3. The anatomical locations of the increases in relative rCBF (CO2-SB versus control) were obtained using magnetic resonance imaging. 4. Group analysis identified neuronal activation within the upper brainstem, midbrain and hypothalamus, thalamus, hippocampus and parahippocampus, fusiform gyrus, cingulate area, insula, frontal cortex, temporo-occipital cortex and parietal cortex. No neuronal activation was seen within the primary motor cortex (at sites previously shown to be associated with volitional breathing). 5. These results suggest neuronal activation within the limbic system; this activation may be important in the sensory and/or motor respiratory responses to hypercapnia in awake man.
Full text
PDF


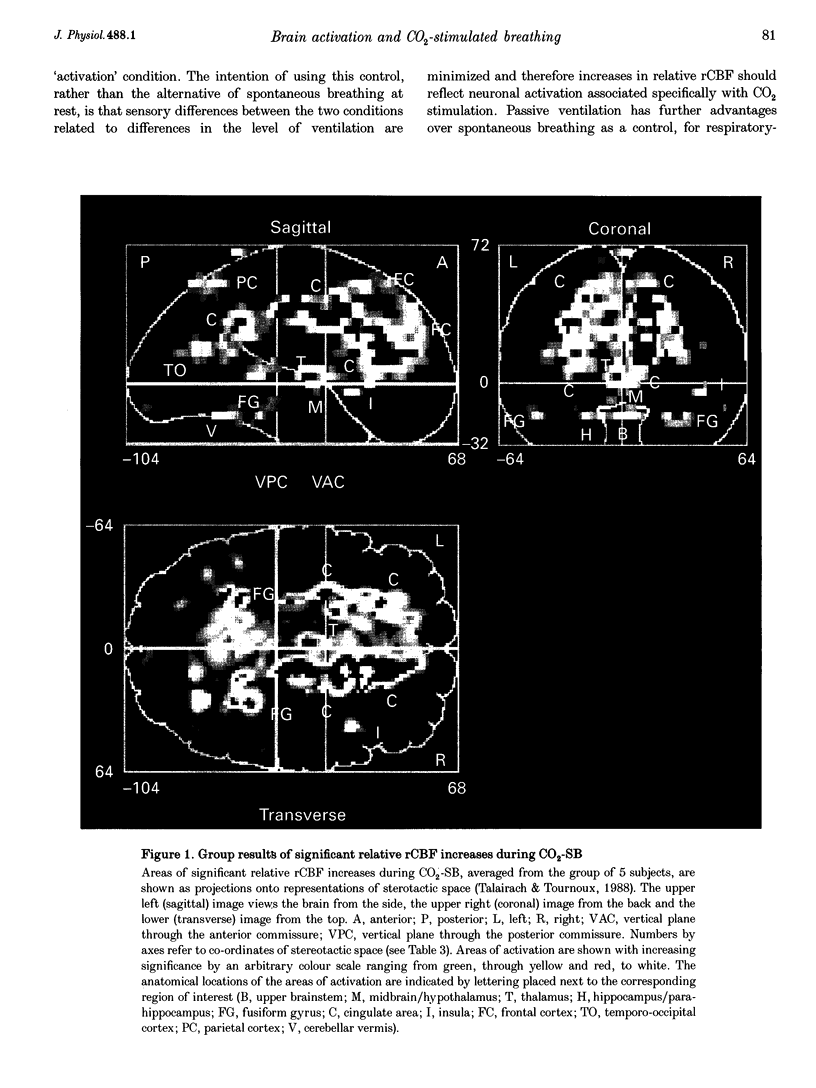



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Z., Eldridge F. L., Wagner P. G. Respiratory-associated rhythmic firing of midbrain neurones in cats: relation to level of respiratory drive. J Physiol. 1991 Jun;437:305–325. doi: 10.1113/jphysiol.1991.sp018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Eldridge F. L., Wagner P. G. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol. 1992 Oct;90(1):99–113. doi: 10.1016/0034-5687(92)90137-l. [DOI] [PubMed] [Google Scholar]
- Colebatch J. G., Adams L., Murphy K., Martin A. J., Lammertsma A. A., Tochon-Danguy H. J., Clark J. C., Friston K. J., Guz A. Regional cerebral blood flow during volitional breathing in man. J Physiol. 1991 Nov;443:91–103. doi: 10.1113/jphysiol.1991.sp018824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med. 1989 Feb;30(2):141–149. [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Dolan R. J., Lammertsma A. A., Frackowiak R. S. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990 Jul;10(4):458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Gandevia S. C., Rothwell J. C. Activation of the human diaphragm from the motor cortex. J Physiol. 1987 Mar;384:109–118. doi: 10.1113/jphysiol.1987.sp016445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D., Hathout G. M., Kirlew K. A., Tang H., Woo M. S., Zhang J., Lufkin R. B., Harper R. M. Localization of putative neural respiratory regions in the human by functional magnetic resonance imaging. J Appl Physiol (1985) 1994 May;76(5):2076–2083. doi: 10.1152/jappl.1994.76.5.2076. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Brown W. D., Friston K. J., Qi L. Y., Frackowiak R. S. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991 Apr 22;244(1309):39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Maskill D., Murphy K., Mier A., Owen M., Guz A. Motor cortical representation of the diaphragm in man. J Physiol. 1991 Nov;443:105–121. doi: 10.1113/jphysiol.1991.sp018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J. C., Huang S. C., Phelps M. E., Carson R. E., MacDonald N. S., Mahoney K. A noninvasive positron computed tomography technique using oxygen-15--labeled water for the evaluation of neurobehavioral task batteries. J Cereb Blood Flow Metab. 1985 Mar;5(1):70–78. doi: 10.1038/jcbfm.1985.10. [DOI] [PubMed] [Google Scholar]
- Murphy K., Mier A., Adams L., Guz A. Putative cerebral cortical involvement in the ventilatory response to inhaled CO2 in conscious man. J Physiol. 1990 Jan;420:1–18. doi: 10.1113/jphysiol.1990.sp017898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinard E., Mazoyer B., Verrey B., Pappata S., Crouzel C. Rapid measurement of regional cerebral blood flow in the baboon using 15O-labelled water and dynamic positron emission tomography. Med Biol Eng Comput. 1993 Sep;31(5):495–502. doi: 10.1007/BF02441985. [DOI] [PubMed] [Google Scholar]
- Ramsay S. C., Adams L., Murphy K., Corfield D. R., Grootoonk S., Bailey D. L., Frackowiak R. S., Guz A. Regional cerebral blood flow during volitional expiration in man: a comparison with volitional inspiration. J Physiol. 1993 Feb;461:85–101. doi: 10.1113/jphysiol.1993.sp019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay S. C., Murphy K., Shea S. A., Friston K. J., Lammertsma A. A., Clark J. C., Adams L., Guz A., Frackowiak R. S. Changes in global cerebral blood flow in humans: effect on regional cerebral blood flow during a neural activation task. J Physiol. 1993 Nov;471:521–534. doi: 10.1113/jphysiol.1993.sp019913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop T. G. Posterior hypothalamic modulation of the respiratory response to CO2 in cats. Pflugers Arch. 1991 Mar;418(1-2):7–13. doi: 10.1007/BF00370445. [DOI] [PubMed] [Google Scholar]