Abstract
Sleep is a fundamental requirement of life and is integral to health. Deviation from optimal sleep associates with numerous diseases including those of the cardiovascular system. Studies, spanning animal models to humans, show that insufficient, disrupted or inconsistent sleep contribute to poor cardiovascular health by disrupting body systems. Fundamental experiments have begun to uncover the molecular and cellular links between sleep and heart health while large-scale human studies have associated sleep with cardiovascular outcomes in diverse populations. Here, we review preclinical and clinical findings that demonstrate how sleep influences the autonomic nervous, metabolic and immune systems to affect atherosclerotic cardiovascular disease.
As a fundamental and necessary biological process, sleep plays a pivotal role in maintaining health. A growing body of both clinical and preclinical research underscores the intricate interplay between sleep and essential biological processes, including those of the nervous, metabolic and immune systems1–3. Sleep insufficiency or disruption has been linked to a myriad of deleterious outcomes, encompassing impaired neuronal function, metabolic dysregulation, a compromised immune system and an increased susceptibility to atherosclerotic cardiovascular disease (ASCVD)4–6. As we learn more about the nuanced molecular, cellular and tissue mechanisms governing the relationship between sleep and health, it becomes increasingly evident that prioritizing adequate and quality sleep is key for fostering cardiovascular health.
Sleep deficiencies are an important public health concern as two-thirds of US adults report insufficient or poor sleep7,8. Recently, the American Heart Association (AHA) included sleep as one of ‘life’s essential 8’ factors for cardiovascular health9 and the American College of Cardiology’s top recommendation for primary prevention of heart disease is to adopt a healthful lifestyle that includes sufficient sleep10. These guidelines are based on numerous associative studies that have consistently demonstrated the importance of adequate sleep to cardiovascular health. However, limited causal evidence exists for sleep’s impact on ASCVD. Experimental and randomization studies in humans and rodents have begun to fill this knowledge gap uncovering mechanistic associations that invariably include the complex interplay between sleep and several bodily systems11,12.
In this Review, we interweave results from clinical, translational and fundamental studies exploring the role of sleep in ASCVD. The Review synthesizes studies spanning the scientific spectrum, from animal models to humans, highlights the advantages and hurdles of animal models in sleep research (Box 1) and is organized around the idea that sleep affects cardiovascular health by engaging three core systems: the nervous, metabolic and immune (Fig. 1).
BOX 1.
Animal models in experimental sleep studies
Mice and rats are the most widely used animals in biomedical research. There is extensive understanding of their physiology, genetics and behavior with numerous genetically manipulated strains available. Technologies and reagents have been developed for their specific use and the animals reproduce rapidly, expediting experimental results and knowledge. However, their use in experimental sleep science and extrapolation of data to human sleep biology faces unique hurdles. Zeitgebers (ZTs), external rhythmic cues including light and food, entrain circadian biological rhythms of all animals. Unlike humans, rodents are nocturnal as approximately 80% of their sleep occurs during the light (inactive, ZT0-ZT12 in experimental laboratory settings) phase and 20% during the dark (active, ZT12-ZT24) phase. Rodent sleep is more fragmented compared to humans and they cycle through sleep stages more rapidly. Even in well-controlled settings, rodent sleep is highly sensitive to diverse genetic and environmental factors. Nonetheless, animal studies enable causal mechanistic investigations that uncover biological details at a scale that is not achievable in humans.
Multiple methods of experimental rodent sleep manipulation have been developed. The most common approaches are by physical or tactile sleep disruption with a rotating wheel or platform, an automated sweep bar or gentle handling. Such interventions have the advantage of compromising sleep in any animal model or strain, can be incorporated with additional experimental interventions and have more subtle and nuanced impact on stress responses. Chronic mild physical sleep fragmentation with a sweep bar does not cause overt systemic stress or result in circadian and molecular clock changes11,12, allowing researchers to distinguish between the effects of sleep and circadian rhythm. Physical models of frequent sleep disruption, such as rotating or shaking platform or forced locomotion, may stimulate systemic stress responses depending on the setting215–217. Pharmacological and genetic interventions offer organ-specific and tissue-specific manipulation of sleep-mediating genes and pathways218–221; however, influences on circadian timing and developmental effects must be carefully considered. Optogenetic and chemogenetic tools allow for the most sophisticated and precise manipulation of sleep-licensing neural circuits with rapid and precise spatiotemporal control over specific neuronal structures and populations222,223. Finally, intermittent hypoxia recapitulates sleep apnea where hypoxic episodes cause awakenings224,225.
Experimental sleep manipulation in rodents has enabled profound insights into the links between sleep and atherosclerosis. Animal models allow unfettered access and manipulation of relevant cells, tissues and organs enabling the discovery of complex and intertwining biological pathways including the autonomic nervous, metabolic and immune systems. Insights gained from rodent models of experimental sleep manipulations are robust and rigorous and have led to new mechanistic discoveries advancing our knowledge on the role of sleep in cardiovascular health.
Fig. 1 |. Sleep and atherosclerotic cardiovascular disease are connected through the integration of the nervous, immune and metabolic systems.
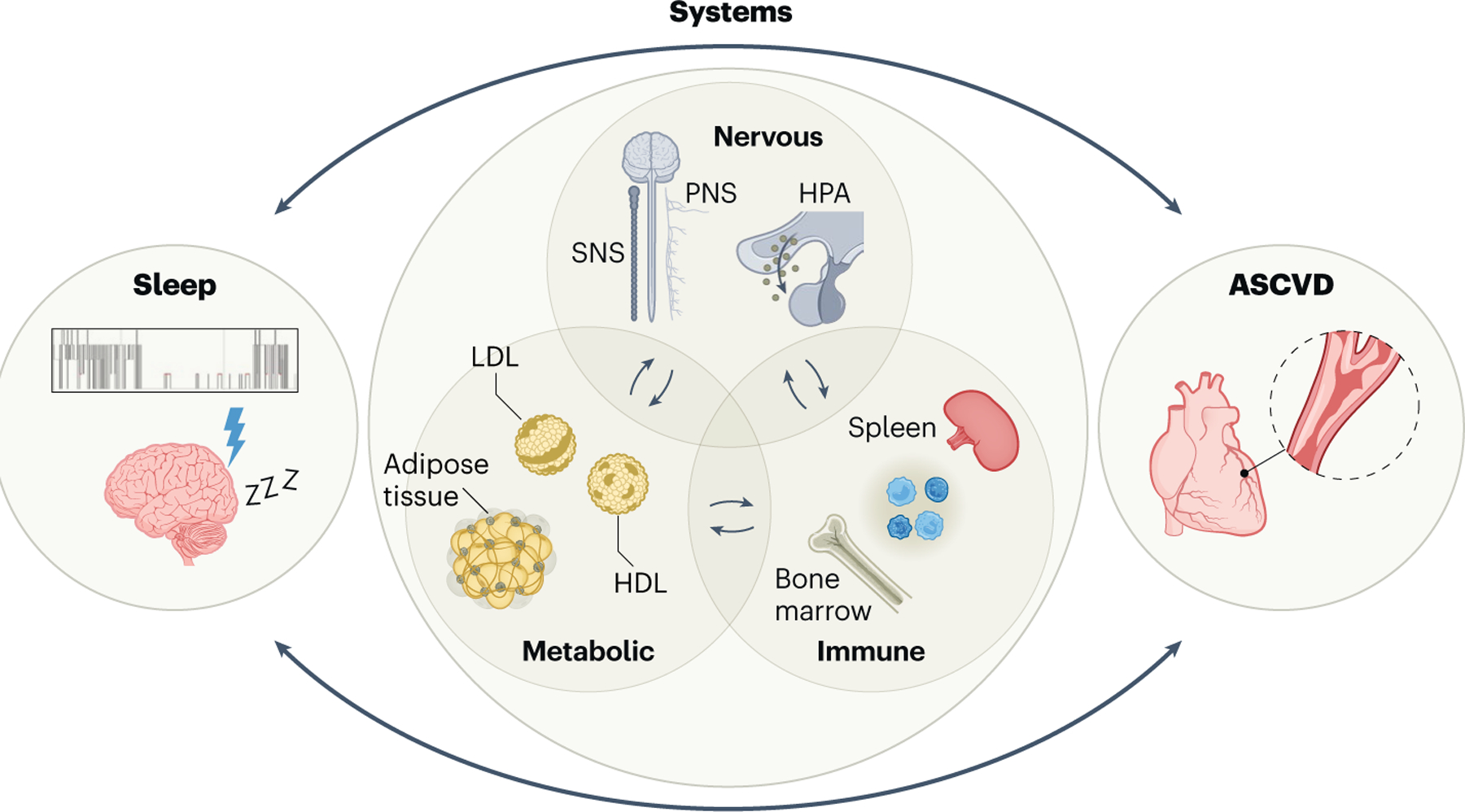
Sleep calibrates ASCVD by modulating multiple core body systems. Interference of adequate sleep including sleep duration, quality and timing adversely affects the function of the nervous, metabolic and immune systems, which predisposes to ASCVD and its complications, including myocardial infarction and stroke. Emerging studies suggest these connections between ASCVD and sleep are bidirectional and peripheral metabolic, nervous and immune imbalance alter sleep.
Clinical and epidemiological evidence associates sleep and ASCVD
Abundant clinical and epidemiological data link sleep and ASCVD in humans and emerging studies support a causal relationship. Sleep is a multidimensional state, and deviations in its abundance, timing and quality, in addition to sleep disorders such as obstructive sleep apnea (OSA), influence ASCVD risk.
Short and long sleep duration associate with ASCVD
Prospective data demonstrate that self-reported short sleep (<6 h per night) associates with higher rates of acute myocardial infarction (AMI) compared to 6–9 h of sleep per night (hazard ratio (HR) 1.20 (confidence interval (CI) 1.07–1.33))13. Applying Mendelian randomization analysis supports a causal effect of short sleep on ASCVD with a 21% increased incidence of AMI (HR 1.21 (CI 1.08–1.37)13. These results were confirmed by other Mendelian randomization analyses, thereby demonstrating links between genetically predicted short sleep and ASCVD (including AMI) and hypertension (HTN)14. Adding to this evidence are findings of a dose–response relationship between decreasing self-reported sleep duration and ASCVD occurrence (in individuals reporting 7 h, 6 h and <5 h of sleep compared with those reporting 8 h of sleep; relative risk 1.06 (CI 0.89–1.26), 1.30 (CI 1.08–1.57) and 1.82 (CI 1.34–2.41), respectively)15. At the other extreme, excessive sleep (>9 h per night) entails elevated risk of ASCVD (relative risk 1.38 (CI 1.03–1.86))15. Similar findings have been acquired in diverse cohorts that include men and women across different racial/ethnic backgrounds16. The associations between short-versus-long sleep and ASCVD are likely disparate, with long sleepers potentially having greater sleep drive in the setting of underlying illness. This difference is captured in studies evaluating self-reported napping. Although those with short sleep (≤6 h per night) are at higher risk of major adverse cardiac events and death, the addition of self-reported napping does not raise their risk17. By contrast, individuals who sleep more than 6 h a night and report requiring daytime naps are at increased risk of composite CVD events and death17, a result that suggests that sleep pressure has an important role in the association between sleep and ASCVD. However, these epidemiological studies are limited in their ability to draw causal relationships. It is unknown whether experimentally extending sleep to adequate levels among short sleepers improves atherosclerotic plaques and ASCVD risk. The feasibility of such experimental models of sleep extension in humans and direct analysis of cardiovascular disease remains a challenge among healthy or at-risk participants.
Poor sleep and daytime symptoms and habits affect ASCVD risk
Another key factor is the interaction between short and poor-quality sleep. Self-reported sleep problems associate with CVD, even after controlling for confounders (odds ratio (OR) 1.75 (CI 1.41–2.16))6. However, individuals reporting both short and restless/disturbed sleep are at highest risk (relative risk 1.55 (CI 1.33–1.81))18. In a prospective observational study using objective measures of sleep duration, insomnia or poor-quality sleep combined with a sleep duration of <6 h per night associates with risk of incident CVD (HR 1.29 (CI 1.00–1.66))19. To achieve rigor and granularity for self-reported sleep data, we can apply advanced statistical methods and machine learning to assess sleep phenotypes and interactions. Latent class analysis, which identifies clusters with variable ASCVD risk, reveals that those with dissatisfactory/inefficient sleep and naps are at high risk of CVD (relative risk 1.29 and 1.38, respectively)20. Latent class analysis also identifies symptom-based OSA-specific subgroups with high CVD risk. Individuals with OSA who have excessive daytime sleepiness are at highest risk of CVD compared to other symptom subgroups and those without OSA21, thus highlighting the importance of sleep drive—in this case measured by symptoms rather than requirement for napping—on cardiovascular risk. These findings demonstrate how combining sleep-related symptoms, even when they are daytime symptoms related to quality of life, with formal sleep diagnostics better assesses health risk. Indeed, the effects of short sleep on CVD mortality are amplified when combined with other daytime unhealthy/sedentary behaviors such as limited vigorous activity, excessive time spent watching television and elevated body mass index (BMI, relative risk 1.90 (CI 1.67–2.17))22.
Impact of sleep timing and variability on ASCVD
Sleep quality, timing, variability and entrainment with circadian rhythm are as influential on ASCVD as sleep duration. Night shift work is associated with increased incidence of ASCVD, and more years working night shifts result in greater CVD risk even after multivariable adjustment (<5-year HR 1.02 (0.97–1.08), 5–9-year HR 1.12 (CI 1.02–1.22), and ≥10-year HR 1.18 (CI 1.10–1.26))23. That these results are largely unchanged after adjusting for sleep duration suggests sleeping out of phase with normal circadian rhythm carries an intrinsic risk of ASCVD. Strikingly, it takes more than 24 years after night shift cessation for ASCVD risk to normalize. Even less extreme forms of out-of-phase sleep, like social jetlag with as little as 2–4-h deviation from individualized chronotype, shows a trend toward elevated ASCVD risk24. Despite no difference in sleep time, evening chronotype, compared to morning chronotype, is associated with a small but statistically significant rise in CVD prevalence (OR 1.07 (CI 1.04–1.10))25. Another consideration intertwined with circadian disorders is variability in sleep schedule, which associates with higher CVD burden, even after adjustment for chronotype, work schedule, average sleep duration and insomnia symptom scores26. Each additional hour in the standard deviation of sleep duration or sleep-onset timing increases the risk of CVD by 39% and 18%, respectively. Individuals with irregular sleep duration and sleep-onset timing are more likely to have high coronary artery calcium burden27 and are at increased risk of cardiometabolic mortality28.
Analysis of multidimensional sleep and ASCVD
Principal component analysis (PCA) has been used to generate a sleep score that combines data from polysomnography (PSG), actigraphy and sleep questionnaires29. Higher scores—indicating better sleep health—associate with reduced all-cause mortality (HR 0.75 (CI 0.65–0.87) per one standard deviation increase in sleep score). The components of this multidimensional tool that contribute the most to mortality are sleep pattern regularity, sleep duration and apnea–hypopnea index (AHI). These features can represent consequences of OSA; alternatively, however, they can represent an intersection of multiple comorbid sleep conditions and symptoms. Another analysis explored whether adding the same multidimensional sleep data to the AHA’s Life’s Simple 7 score provided additional information regarding ASCVD risk. Although the Life’s Simple 7 score alone does not associate with incident CVD, adding sleep health scores does show an association with CVD incidence (HR 0.53 (CI 0.32–0.89))30, a result that prompted the AHA to incorporate sleep into Life’s Essential 8. Multidimensional sleep scores are also useful when combined with weighted genetic risk. Individuals with poor sleep and high genetic risk scores have the highest risk of ASCVD compared to individuals with better sleep scores and/or lower genetic risk, implying the importance of and interaction between predisposing genetic risk and environmental/lifestyle factors31. Advances in machine and deep learning will allow for integration of multidimensional sleep data to capture a myriad of comorbid sleep disease states and thus predict outcomes. For example, a deep neural network trained on 2,500 PSGs from seven studies can estimate mortality from PSG-based age estimation32. There is no one single measure of sleep that encapsulates all CVD risk. Therefore, it stands to reason that novel predictive risk scores that assess multiple parameters of sleep health will provide a better understanding of which individuals are at risk of ASCVD. Going beyond risk prediction, novel forms of heterogeneous treatment effects analyses combined with multiple feature domains will enable better estimation of treatment effects on CVD outcomes.
Obstructive sleep apnea and cardiovascular disease
OSA and CVD are closely connected. Large-scale epidemiological studies demonstrate that OSA positively associates with CVD and, on long-term follow-up, with CVD mortality33–36. These observations were preceded by smaller studies37–39, confirmed by other single-center cohorts40, and replicated in diverse cohorts41,42. Studies have found that adherence to OSA treatment with continuous positive airway pressure (CPAP) therapy benefits CVD43–46. However, randomized controlled trials (RCTs) evaluating CPAP for primary and secondary CVD prevention consistently show neutral findings47. This discrepancy may be due to a ‘healthy user’ bias within observational data or the inherent limitations of RCTs including restrictive inclusion criteria and poor adherence to therapy. OSA affects almost one billion people worldwide48; therefore, it is unlikely that all CVD risk within this group is attributable to OSA. Endotyping and phenotyping OSA can achieve a precision approach to treatment for specific disease outcomes49 including CVD endpoints. Endotypic processes that underlie OSA pathophysiology, including arousal threshold, loop gain, airway muscle compensation and pharyngeal collapsibility, have been suggested as targets for specific and novel treatments50 that would challenge the current one-size-fits-all approach. How treatment algorithms targeting these specific axes impact ASCVD outcomes needs to be fully assessed in future studies.
A critical deficiency in RCTs evaluating OSA treatment on ASCVD is the lack of inclusion of important symptom-based phenotypes. Individuals with OSA who have excessive daytime sleepiness are known to be at increased CVD risk21,51 and, conversely, OSA without excessive daytime sleepiness may have the same CVD risk as no OSA51. Additionally, OSA must be evaluated as part of a holistic, multidimensional approach to sleep. For example, outcomes for individuals with comorbid insomnia and OSA are much worse than for either condition alone52–55. As underlying mechanisms differ between OSA endophenotypes and ASCVD, future work must rigorously collect data to analyze OSA cohorts by their heterogeneous individualized subgroups. Clinicians must be vigilant in obtaining detailed history and objective evidence of comorbid sleep disorders to better understand sleep’s comprehensive impact on ASCVD. Through this lens, we will be able to explore intermediate endpoints that may represent the fundamental autonomic, metabolic and inflammatory pathways through which sleep disruption causes ASCVD.
Effects of sleep on the autonomic nervous system
The autonomic nervous system is composed of two branches, the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS). The SNS and PNS highly innervate organs and arterial adventitia56 to modulate function through neurotransmitters, such as catecholamines and acetylcholine (ACh), respectively. While the SNS is considered a quick response, ‘fight-or-flight’ system, the PNS exerts slowly activated complementary effects to dampen bodily functions. When humans transition from the waking state to progressively deeper stages of sleep, SNS tone decreases and PNS vagal tone increases. Non-rapid eye movement (NREM) sleep, most notably slow-wave sleep, can be considered a phase that allows autonomic stability and metabolic recovery due to parasympathetic dominance57. This is in contrast to rapid eye movement (REM) sleep where SNS tone and neurotransmitter release rises, enhancing heart rate variability and elevating systolic and diastolic blood pressure58. These changes in SNS and PNS parallel the activity of the hypothalamic–pituitary–adrenal (HPA) axis, which is a vital neuroendocrine system connecting perceived stress to physiological reactions via glucocorticoids59. Individuals with OSA60, narcolepsy61 and chronic insomnia62 have higher sympathetic nerve activity, during both sleep and wakefulness, which may be caused by disturbed chemoreflex and baroreflex activity, altered cardiac electrophysiology, endothelial dysfunction or increased neural cardiovascular responsiveness. A persistent increase in SNS activity can favor the emergence of permitted local inflammatory zones. In chronic inflammatory settings and sleep-related hyperarousal states, this has detrimental effects that lead to comorbidities such as high blood pressure, cardiovascular mortality or insulin resistance63–65. These observations suggest interplay between the autonomic nervous system and sleep in the regulation of inflammation, which may be able to be harnessed therapeutically (Box 2). In addition, sleep disruption may modulate changes in adrenal and gonadal androgen levels that in turn promote a pro-inflammatory state, thereby perpetuating sleep disturbance66. Moreover, signals through gonadal steroids together with inputs from the paraventricular hypothalamic nucleus can be integrated in the preoptic area of the hypothalamus, where activation of glutamatergic neurons elicits microarousals and subsequent sleep fragmentation67. Heightened autonomic activity together with sleep disruption may instigate the underlying causes of ASCVD by simultaneously affecting the interconnections between cardiac physiology, local inflammation and hormonal balance (Fig. 2).
BOX 2.
Interplay between sleep and the autonomic system in modulating inflammation
Chronic activation of the SNS mediates inflammatory responses in CVD through various neurotransmitters, including norepinephrine, ATP, neuropeptide Y and nitric oxide226. This sympathetic control can be direct, through pro-inflammatory cytokine production and increased adhesion to endothelial cells, or indirect, by regulating leukocyte distribution, production or recruitment. However, causal mechanistic data connecting sleep-mediated SNS activity and inflammatory CVD remain limited. In mice, frequent sleep fragmentation (every 30 s) elevates systemic corticosterone217 and SNS abrogation attenuates inflammation in multiple models of CVD227. Acute and chronic sleep fragmentation expand cytokine production in cardiovascular tissue and elevate systemic norepinephrine. Chemical sympathectomy blunts these responses and sleep recovery normalizes inflammation but not norepinephrine levels228. Sleep fragmentation-induced expression of inflammatory cytokines in heart and spleen is abated by blockade of alpha-adrenergic or beta-adrenergic receptors229. These findings suggest pharmacological manipulation of autonomic responses can reduce inflammation resulting from sleep disruption. In humans, increased sympathetic activation has been observed due to sleep fragmentation in the setting of OSA-related respiratory events68. Increased pulse rate response to OSA events represents a noninvasive measure of these sympathetic surges and is associated with worse CVD outcomes95. Stabilization of breathing and sleep fragmentation through OSA treatment among individuals with an elevated pulse rate response results in lower rates of major adverse CVD events96.
The PNS has a key role in the regulation of inflammation via the ‘cholinergic anti-inflammatory pathway’ that is mediated via the vagus nerve and its major neurotransmitter, ACh230. The vagus nerve collects information on the inflammatory status of multiple organs via its sensory fibers and conveys signals back to the brain; a process known as the inflammatory reflex. Importantly, vagus nerve stimulation can modulate local inflammation by ACh-mediated suppression of cytokine production by macrophages231. Accordingly, manipulation of the inflammatory reflex shows promising therapeutic potential in many inflammatory diseases, including ASCVD232.
In the heart, ACh mediates anti-inflammatory effects233 through systemic and local attenuation of inflammation and decreased leukocyte infiltrates. Whether sleep affects the vagus inflammatory reflex is poorly understood. In mice, vagal afferents affect sleep via TNF234 and, in a rat model, vagal nerve stimulation attenuated insufficient sleep-induced pro-inflammatory cytokine levels in the blood235. The spleen is a key organ in orchestrating the vagus nerve control over inflammation, where activated catecholaminergic fibers release norepinephrine, which promotes ACh production by local β2-adrenergic receptor-expressing cells, including B cells and T cells236. Using a lipopolysaccharide (LPS)-induced sepsis model, a recent study described a microbiota–vagus nerve–spleen axis in modulating systemic inflammation upon sleep deprivation237. Further studies are warranted to understand whether persistent sleep manipulation influences cholinergic anti-inflammatory pathways by suppressing vagal tone and local ACh production.
Fig. 2 |. Sleep and autonomic nervous balance.
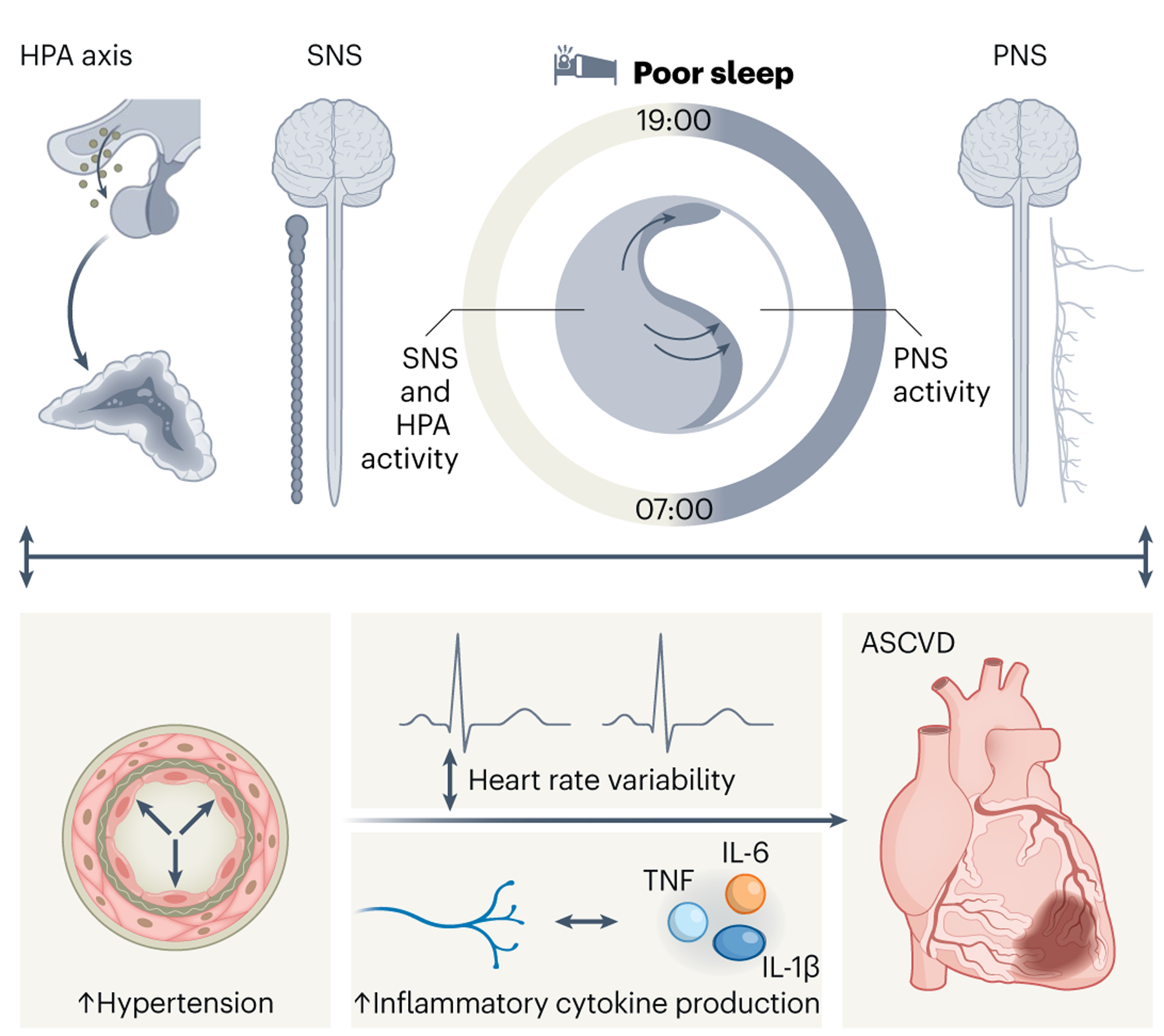
Sleep exerts a modulatory effect on the activity of the autonomic nervous system. Persistent sleep disturbances lead to elevated SNS and HPA activity during sleep and wakefulness and modulate the tone of the PNS. Consequently, desynchronization of the HPA axis compromises heart electrophysiology leading to extremities in heart rate variability, while aberrant SNS and PNS activation triggers nocturnal non-dipping of blood pressure (BP) and daytime HTN. In addition, sleep manipulates SNS/PNS equilibrium instigating adverse immune responses in the heart including accentuated inflammatory cytokine production. Altogether, sleep deficiencies promote heart rate alterations, HTN and inflammation via compromising the autonomic nervous system, thus exacerbating ASCVD.
Sleep and hypertension
SNS activation is a predominant contributor to HTN. OSA-related respiratory events increase sympathetic nerve activity68, drive nocturnal non-dipping of blood pressure69 and lead to daytime HTN70, while CPAP treatment for OSA reduces nocturnal71 and daytime72,73 blood pressure. The influence of sleep on HTN can be characterized more effectively when multiple dimensions of sleep are combined. For example, systolic blood pressure is highest among those with moderate-to-severe OSA and a morning chronotype, as compared with intermediate OSA or evening chronotypes74. Short sleep and insomnia also associate with HTN. Compared to those sleeping >6 h a night, individuals with insomnia and <5 h of nightly sleep have elevated risk of HTN (OR 5.1 (CI 2.2–11.8))75. Short sleep (<6 h versus 7–8 h) raises the risk of HTN (OR 1.66 (CI 1.35–2.04)), an effect that persists even after adjusting for confounding variables76. These dynamics are not limited to short sleepers; those reporting >9 h of sleep per night also have increased HTN risk (OR 1.30 (CI 1.04–1.62))76. These findings have been validated in prospective cohorts, in which reporting ≤5 h of sleep per night associates with greater risk of developing HTN compared to sleeping 7 h per night (HR 1.20 (CI 1.09–1.31)), an effect that obesity partially mediates77. Although they do not prove causation, prospective incidence data provide stronger evidence that sleep modulates autonomic and vascular function. For example, studies using actigraphy to assess objective sleep duration and consolidation demonstrate higher rates of both HTN and incident HTN over 5 years (OR 1.37 (CI 1.05–1.78))78. Experimental studies strengthen this association by revealing a causal relationship between sleep restriction (that is, 4-h time-in-bed restriction for 9 days) and 24-h blood pressure, increased morning plasma norepinephrine and attenuated vasodilatory capacity79.
Sleep and circadian regulation also have central roles in modulating diurnal blood pressure80. Evening chronotypes have a 1.3-fold OR for arterial HTN than morning types81. Derangements in blood pressure are regulated by wake–sleep states with significantly elevated systolic, diastolic and mean blood pressure during wakefulness compared to NREM sleep and these oscillations are accentuated in obesity-prone leptin-deficient (ob/ob) mice compared to wild-type controls82. In experimental manipulations, 30 days of sleep fragmentation impairs neurovascular coupling and decreases whisker-stimulated cerebral blood flow in normotensive and hypertensive mice83. Furthermore, sleep-fragmented mice have expanded vascular network density but unaltered arteriole vascular remodeling83. In genetic models of insufficient sleep, hypocretin-ataxin3 transgenic and hypocretin-deficient mice (both are models of human narcolepsy) display elevated blood pressure during NREM and REM sleep and blunted sleep–wake blood pressure changes84,85.
Sleep and heart rate
Many aspects of cardiovascular physiology, including heart rate variability, are orchestrated by circadian rhythmicity. Accordingly, circadian misalignment leads to internal desynchronization of the HPA axis and can subsequently result in altered cardiac electrophysiology in mice86 and humans87. Perturbations in sleep-dependent cardiovascular sympathetic control also occur in narcoleptic hypocretin-deficient mice88. Mistimed sleep and unsynchronized behavior disrupt sinoatrial node rhythms and atrioventricular node activity in humans and mice89. In perfused heart preparations, susceptibility to ventricular tachycardia is increased at ZT12, compared to ZT0, and mice prone to catecholamine-induced arrhythmogenesis elicit bidirectional ventricular tachycardia at ZT12, but not ZT0, upon caffeine or adrenaline administration89. These findings align with increased incidence of early morning sudden cardiac deaths in humans90. It is not yet fully understood whether mistimed or irregular sleep can predispose humans to arrhythmia or other cardiac events. However, a recent large-scale, longitudinal sleep study using wearable devices found inverse associations between REM and deep sleep and the odds of atrial fibrillation incidence91. Moreover, irregular shift workers showed prolonged correlated QT (QTc) interval and more frequent conduction or repolarization disorders compared to regular shift workers92,93.
Emerging metrics of sympathetic activation in sleep
Given early evidence demonstrating sympathetic activation in OSA68 and its ill effects on CVD94, a noninvasive measure of autonomic surges using PSG would help to improve risk stratification of patients. One such metric is the relationship between the pulse-rate response and OSA-related respiratory events (that is, apneas and hypopneas). Individuals with OSA who have high pulse-rate response are at increased risk of both CVD mortality and all-cause mortality (HR 1.68 (CI 1.22–2.30) and 1.29 (1.07–1.55), respectively), as compared to those with a pulse-rate response within the 25th–75th percentiles95. A clinical trial of CPAP for secondary CVD prevention shows CPAP has a protective effect among individuals with OSA who have an increased pulse-rate response96. While this novel approach is encouraging, evaluating the pulse-rate response represents more complex physiology than just autonomic function as it requires healthy cardiac conduction system responses and is limited by medications that interfere with chronotropy (for example, beta blockers). Another OSA-related metric is the respiratory event duration. Individuals with OSA who have shorter average respiratory event duration have an increased risk of all-cause mortality (1.31 (CI 1.11–1.54))97. While this measure likely represents a combination of factors including arousal threshold, it may suggest higher autonomic tone. Short sleep duration, insomnia and poor sleep quality also contribute to lower high-frequency heart rate variability, another marker of imbalance between sympathetic and parasympathetic tone98.
The role of sleep in obesity and lipid metabolism
Sleep and its disorders shape metabolic function, including effects on obesity, diabetes and insulin sensitivity99,100, and lipid metabolism101. These metabolic syndrome components are important contributors to the development and progression of ASCVD (Fig. 3). Here, we give particular attention to obesity and lipid metabolism.
Fig. 3 |. Sleep and the metabolic system.
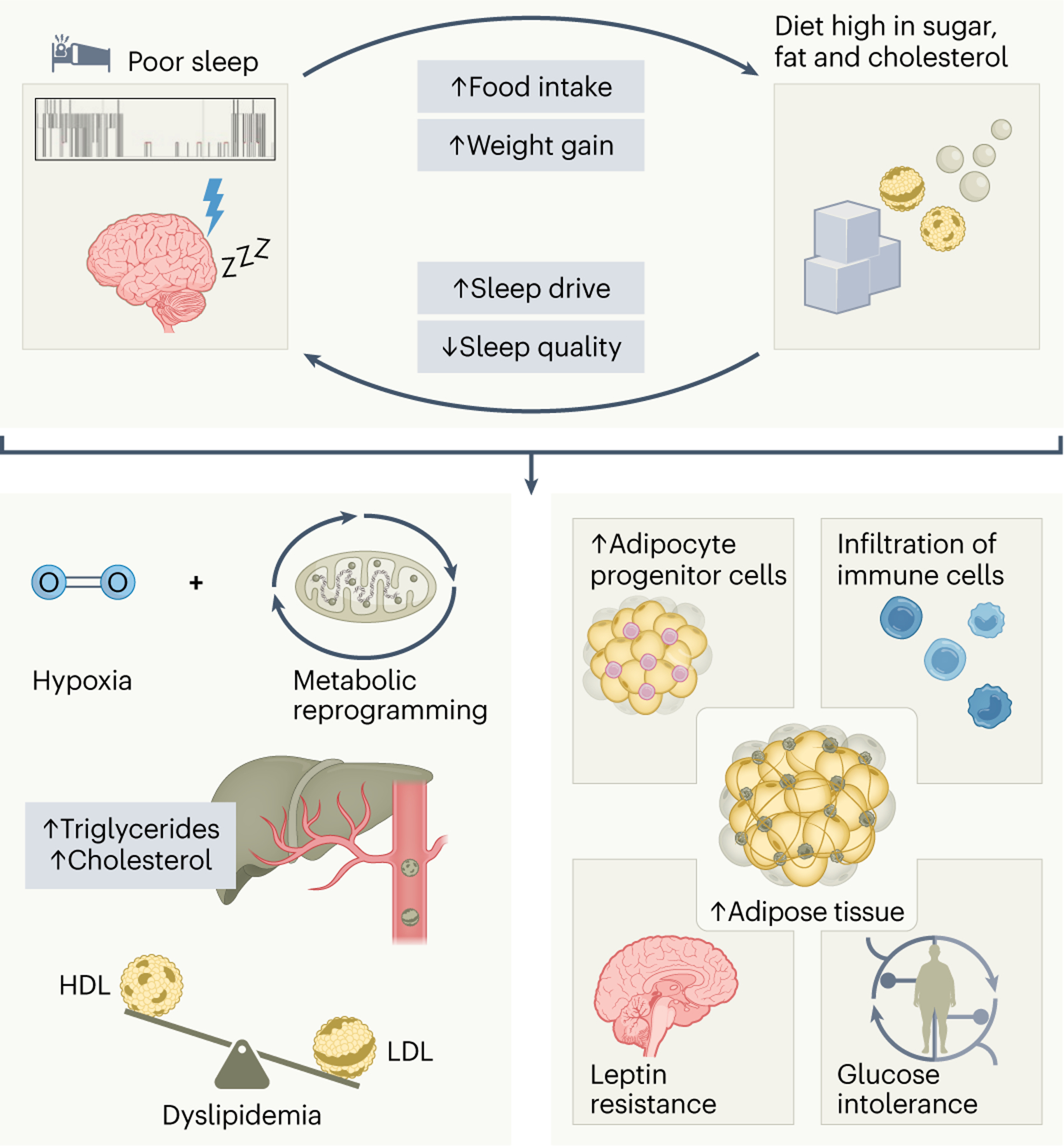
Sleep and the metabolic system share a bidirectional relationship. While sleep disturbances promote food intake and can cause subsequent weight gain, excess consumption of a sugar-rich and cholesterol-rich diet increases sleep drive with curtailed sleep quality. On the one hand, insufficient or irregular sleep can fuel metabolic syndrome by affecting hepatic triglyceride and cholesterol output via the modulation of hypoxia-dependent metabolic genes in the liver. Consequently, persistent sleep disruption may result in an atherogenic lipid profile with heightened LDL-c and triglyceride and lower HDL-c levels. On the other hand, suboptimal sleep hygiene propagates fat mass accumulation, adipocyte progenitor cell production and adipose tissue inflammation. Prolonged metabolic reprogramming of the adipose tissue contributes to leptin resistance as well as glucose intolerance and insulin resistance, thereby predisposing to cardiometabolic complications.
Suboptimal sleep drives obesity and emergence of the metabolic syndrome
The associations between sleep, food consumption, obesity and energy balance in humans are numerous and have been reviewed in detail102. Sleep insufficiency both increases food consumption via alterations in the appetite hormones leptin and ghrelin, and alters meal timing and food choices, which can lead to obesity103–105. Sleep duration is shortest and most variable among obese children and associates with changes in insulin, low-density lipoprotein cholesterol (LDL-c), and C-reactive protein (CRP)106. These phenomena highlight the importance of early-life sleep habits for future metabolic and ASCVD health. Night-to-night variability in sleep duration and daytime napping are independently associated with obesity in older men and women107. Other studies have validated these findings and further shown that irregular sleep–wake timing correlates with increases in obesity, fasting glucose and diabetes, 10-year CVD risk and comorbid metabolic abnormalities108. Recent human intervention experiments indicate that sleep restriction increases caloric intake without a commensurate rise in energy expenditure, thereby resulting in weight gain and added subcutaneous and visceral fat109. Importantly, among overweight adults who sleep fewer than 6.5 h per night, a randomized control trial of sleep extension reverses excess energy intake and reduces weight110. Weight and BMI cannot be viewed in isolation, and more granular measures such as lipid accumulation product, which accounts for both waist circumference and triglyceride levels, strongly associate with sleep disorders111. For example, lipid accumulation product was helpful in predicting risk of diabetes in individuals with OSA and, when combined with high-density lipoprotein cholesterol (HDL-c), heart disease.
Sleep and obesity in experimental studies
Sleep and obesity have a bidirectional relationship. A high-calorie diet enhances sleep pressure, as obese mice have reduced wakefulness in both the dark and light cycles and spend more time in NREM sleep with higher slow-wave activity112. Wake episode duration decreases following high-fat diet feeding, and body weight positively correlates with NREM sleep and negatively associates with wakefulness113,114. Sleep disruption also worsens in diet-induced obesity and leptin-deficient mice115,116, leading to sleep-disordered breathing characterized by increased flow-limited breathing and hypoxemia117 and more circadian variation in respiratory rate and diaphragmatic bursts during REM sleep118. However, obese female mice are less susceptible than males to sleep-disordered breathing and sleep fragmentation119. Importantly, dietary change and weight loss reverse obesity-dependent sleep alterations120. Regarding the effect sleep has on obesity onset and progression, 10 days of sleep deprivation in mice heightens food intake and elevates weight gain, with the effects mitigated by melatonin121. When fed a high-fat diet, mice with a history of persistent sleep deprivation experience weight gain, insulin resistance, expanded heightened fat mass and more adipose tissue inflammation122, whereas prolonged sleep fragmentation leads to increased food intake, higher body weight gain123 and glucose intolerance124. Sleep-fragmented animals also develop larger subcutaneous and visceral fat depots, have more adipocyte progenitor cells in the visceral white adipose tissue and greater adipose tissue inflammation125. In a mouse model of shift work, timed sleep restriction leads to prolonged metabolic reprogramming of white adipose tissue and early leptin resistance in a Per1- and Per2-dependent manner126. Recurrent circadian disruption in mice fed a high-fat diet results in increased weight gain, higher body fat mass, impaired glucose tolerance and insulin sensitivity127. In genetic models, ablation of wake-promoting hypocretin neurons in hypocretin/ataxin3 mice combined with high-fat diet promotes weight gain128,129. Consistent with this, daily light-phase, but not dark-phase, administration of suvorexant, a hypocretin antagonist, for 2–4 weeks ameliorates glucose tolerance in leptin-deficient mice and improves hepatic glucose metabolism130. In summary, the collective empirical evidence derived from human and animal studies underscores the detrimental effects insufficient and irregular sleep has on obesity development and progression.
Sleep and lipids
Poor sleep leads to dysfunctional lipid metabolism. Large epidemiological studies report increases in hepatic triglyceride content among the shortest sleepers (<5th percentile, average 5 hour per night) and elevated serum triglyceride among those with poor sleep quality. These associations are tempered after adjustment for BMI and OSA, suggesting more complex dynamics between sleep time, obesity and comorbid sleep disorders131. Poor sleep hygiene during transition from childhood to adolescence associates with an atherogenic lipid profile including lower HDL-c and increased triglyceride levels, an effect more prominent in adolescent girls132. Sleep architecture also affects dyslipidemia, as the ratio of slow-wave sleep to total sleep time correlates independently with triglyceride and total cholesterol levels, and microarousal index also independently associates with HDL-c and LDL-c133. Despite the highlighted studies, a systematic meta-analysis of 13 prospective studies found no significant relationship between sleep duration or quality and the emergence of dyslipidemia134.
A meta-analysis assessing lipid profiles of individuals with OSA found higher total cholesterol, LDL-c and triglyceride as well as lower HDL-c than in individuals without OSA135. Moreover, there is a dose–response relationship between OSA severity and total cholesterol, LDL-c and triglycerideTG, such that oxygen desaturation index associates with total cholesterol while AHI better predicts LDL-c and triglyceride136. These findings indicate mechanisms involved in lipid metabolism may depend on the underlying sleep abnormality. In contrast to these large epidemiological studies, an experimental study found that a 5-h time-in-bed restriction for 4 nights suppresses postprandial triglyceride, which returned to baseline after one night of recovery sleep137. Interestingly, sleep-restriction participants in this study also reported feeling less full or satiated after meals.
In mice, chronic intermittent hypoxia, a feature of OSA, modulates sterol regulatory element binding protein 1 and stearoyl-coenzyme A desaturase 1 to increase total cholesterol, phospholipids, LDL-c, triglyceride and atherosclerosis138. In humans, however, oxidized LDL-c associates with OSA and overall dyslipidemia139. Among individuals with severe OSA, CPAP therapy to alleviate apnea events improves the clearance rate of radiolabeled lipids140. This study also presents an inverse correlation between cholesteryl ester clearance rate and hypoxemia (that is, total sleep time with oxygen saturation < 90% (T90%)), and carotid intima–media thickness. Some studies have validated these findings, showing CPAP therapy reduces total cholesterol141 and LDL-c142, but other studies have observed no meaningful changes143. Another interesting consideration is that disruption in different sleep stages results in different effects on lipids. A clinical cohort study reveals that AHI in NREM specifically associates with elevated total cholesterol, LDL-c and apolipoprotein B, while REM AHI does not144. Sleep stage-specific impacts on metabolism are apparent throughout the metabolome. For example, while fatty acid oxidation rises during stage N3 sleep and drops during wakefulness, the tricarboxylic acid cycle and glycolysis seem to be more active during REM sleep145.
The effects of sleep manipulation on lipids in murine models
Mouse sleep disruption increases serum cholesterol and LDL-c, decreases HDL-c and very low-density lipoprotein (VLDL)146, and mediates liver cholesterol through the circadian rhythm genes NR1D1 and CYP7A1 (ref. 147). Additionally, short-term (5 days) sleep deprivation suppresses the circadian expression of lipid metabolism genes in the liver and lowers daily peaks in serum cholesterol and triglyceride148. In long-term models, chronically shifting the light–dark cycle exacerbates atherosclerosis in female, but not male, apolipoprotein E-deficient (Apoe−/−) mice, a change that associates with heightened total serum cholesterol and atherogenic VLDL/LDL particles, independently of food intake149. In APOE*3-Leiden mice, however, the same method produced no obvious changes in lipid levels, although these mice exhibit lower total cholesterol levels than Apoe−/− mice150. Long-term sleep fragmentation does not impact body weight, total cholesterol or triglyceride in western-diet-fed Apoe−/− and LDL receptor-deficient (Ldlr−/−) mice12. Together, these results indicate that, in mice, while acute sleep disruption may engender short-term fluctuations in lipid levels, chronic poor sleep does not seem to modulate plasma cholesterol levels.
Sleep modulates inflammation and the immune system
Inflammation contributes to ASCVD. Following retention in the arterial wall, apolipoprotein B-containing lipoproteins undergo structural modifications and become immunogenic, thus inciting inflammation and the recruitment of circulating monocytes to the growing atheroma. Hematopoiesis is enhanced and contributes to the circulating monocyte pool, exacerbating vascular inflammation and atherogenesis. Inflammatory mediators like cytokines and growth factors perpetuate inflammation leading to atheroma growth and instability. Although our knowledge of the influence of sleep at each stage of plaque growth is incomplete, studies have begun to identify important mechanistic links (Fig. 4).
Fig. 4 |. Sleep protects from ASCVD by modulating inflammation.
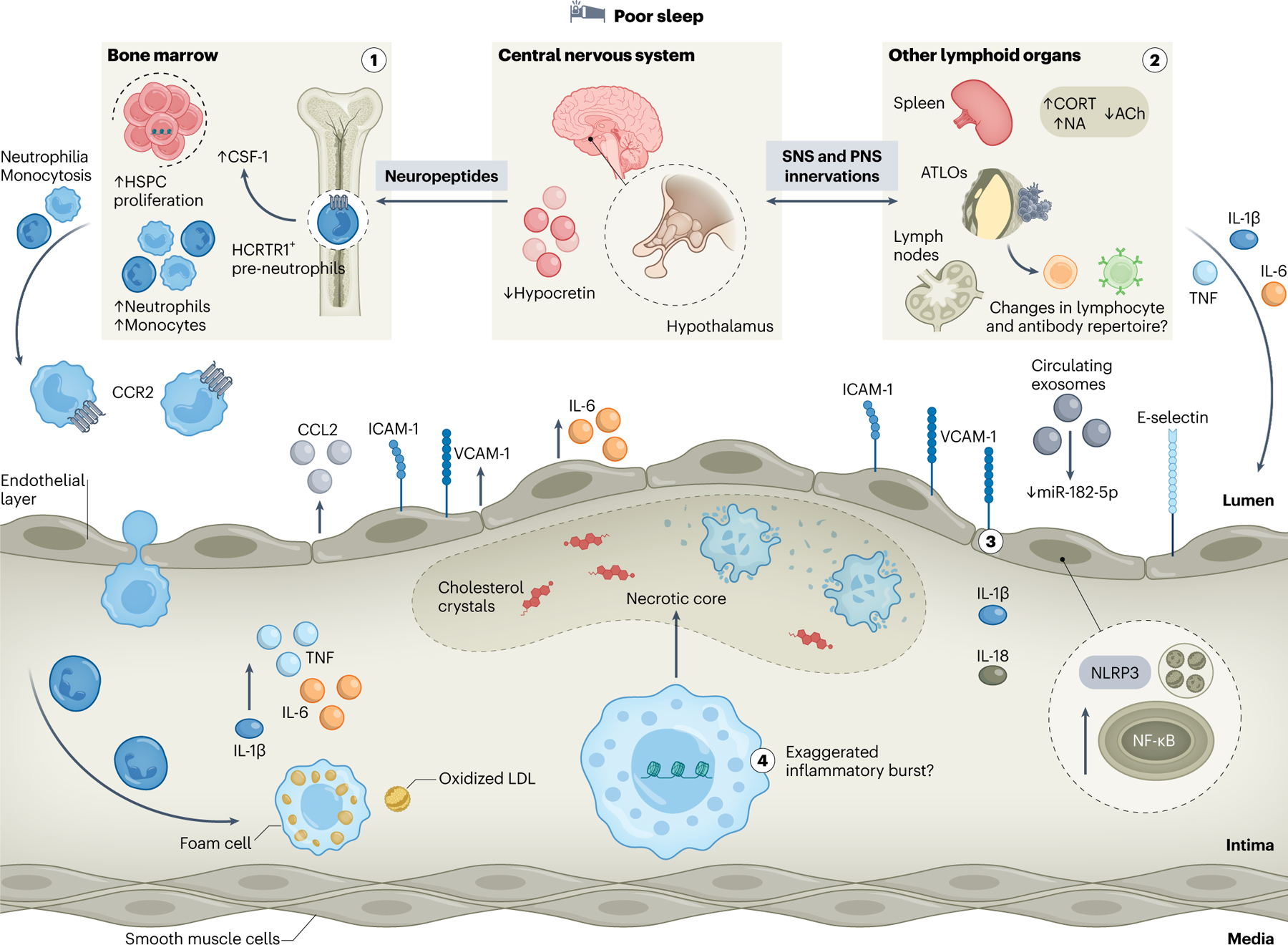
The beneficial impacts of proper sleep on the immune system are manifold; thus, insufficient or poor-quality sleep exerts various adverse effects on inflammation that underlies atherosclerosis. Prolonged sleep disruption compromises neuroimmune communication axes that modulate leukocyte numbers and function via neuropeptide, SNS or PNS innervations. (1) Blunted hypocretin signaling from the lateral hypothalamus to bone marrow pre-neutrophils following sleep fragmentation heightens colony-stimulating factor-1 (CSF-1)-mediated medullary hematopoiesis and results in subsequent monocytosis and neutrophilia. Consequently, increased aortic immune cell infiltration drives exacerbated atherosclerotic lesion formation. (2) Enhanced sympathetic and compromised anti-inflammatory parasympathetic inputs to secondary lymphoid organs and neuroimmune cardiovascular interfaces at the adventitia — that is, artery tertiary lymphoid organs (ATLOs) — augment the production of pro-inflammatory cytokines including IL-6, TNF and IL-1β, further inciting an inflammatory milieu. Whether this affects local lymphocyte function and repertoire remains unknown. (3) Sleep disturbances promote endothelial cell dysfunction characterized by enhanced chemoattractant activity, including CCL2 release and increased expression of adhesion molecules, such as ICAM-1, VCAM-1 or E-selectin. Circulating exosomes promote endothelial cell injury via increased miR-182-5p-dependent NF-κB and NLRP3 signaling, thus licensing IL-1β and IL-18 production. These effects compromise endothelial cell integrity and further facilitate immune cell entry into the atheroma. (4) Persistent sleep fragmentation poses profound and lasting changes on hematopoietic stem cells via epigenetic restructuring, leading to exaggerated myeloid-biased inflammatory bursts. Consequently, aortic macrophages derived from recruited monocytes may be strongly pro-inflammatory, defective of rate-limiting processes, such as lipid handling or efferocytosis and more prone to necrotic death, further fueling plaque growth and instability. ACh, acetylcholine; CORT, corticosterone; HCRTR1, hypocretin receptor 1; NA, noradrenaline.
The effect of sleep on cytokines
Cytokines orchestrate many inflammatory processes in ASCVD including activating endothelial cells, recruiting and programming leukocytes in atheroma, and destabilizing plaque. Persistent sleep disruption, such as chronic insomnia151 or circadian misalignment152, promotes prolonged pro-inflammatory cytokine responses that instigate chronic health complications such as ASCVD.
Although the contributions of interleukin-6 (IL-6) and tumor necrosis factor (TNF) to atherosclerosis are complex, several human and animal studies have documented increased plasma IL-6 and TNF after prolonged sleep deprivation. Sleep inconsistency measured over seven nights associates with heightened IL-6 and CRP153. Furthermore, increased levels of circulating IL-6 and TNF correlate with sleep irregularity in older adults154. Importantly, individuals experiencing an acute cardiovascular event combined with preexisting OSA have higher serum levels of IL-6 than individuals without OSA155. Persistent sleep fragmentation in mice raises plasma IL-6, which normalizes with recovery sleep11. By contrast, sleep fragmentation does not affect plasma TNF levels in humans156,157 or rodents158. In a murine model, complete sleep deprivation induces a cytokine storm before death159. Increased wakefulness after sleep onset, a measure of insomnia, associates with increased IL-6 and CRP160. After a single night of forced prolonged (4 h) wakefulness after sleep onset in healthy individuals, monocytes produce more IL-6 and TNF161. Additionally, inflammatory responses to sleep disruption vary by sex as women experiencing wakefulness after sleep onset have greater immune and cytokine activation162. A large epidemiological study of short sleepers confirmed these sex differences in cytokine levels163. Serial 24-h evaluation of plasma IL-6 and TNF among individuals with chronic insomnia found that although average 24-h levels of these fatigue-inducing cytokines are consistent, their major peaks shift significantly151, a result that suggests dysregulated circadian rhythm and highlights the importance of sample collection timing in epidemiological and experimental studies. Interestingly, as with ASCVD risk, habitual sleep time of more than 7–8 h per night also associates with higher levels of IL-6 and CRP164. However, the association between sleeping more than 7–8 h per night and inflammation remains to be fully determined, is experimentally difficult to test and is likely influenced by comorbidities, which themselves associate with ASCVD.
The IL-1 cytokine family is influential to immune responses. IL-1 is either increased165 or unchanged157 upon acute sleep deprivation, whereas IL-1Ra, an atheroprotective natural inhibitor of IL-1, is increased after sleep disruption165,166, which may be a homeostatic response to higher IL-1 levels. It has been shown that five nights of sleep restriction increases peripheral blood mononuclear cell production of IL-6 and IL-1β167. IL-1β maturation and production are controlled by the NLR family pyrin domain containing 3 (NLRP3) inflammasome, which can be promoted by cholesterol crystals in atherosclerotic lesions168. While information is scarce regarding how prolonged sleep disruption impacts NLRP3 activation in innate immune cells, a recent report showed that plasma exosomes isolated from sleep-fragmentated animals trigger NLRP3 activation in endothelial cells, resulting in more deposition of IL-1β and IL-18 in atherosclerotic lesions169.
Obstructive sleep apnea and pathways of inflammation
The underlying pathobiology by which the sequelae of obstructive respiratory events lead to ASCVD is unclear, although many theories exist. Many studies have demonstrated an association between OSA and elevated systemic inflammatory biomarkers, including CRP, IL-6 and TNF, among others170,171, and levels of these cytokines are highest after an acute cardiovascular event among those with OSA, relative to those without it155. To assess how treating OSA affects systemic inflammation, a study evaluated composite serum proteomic inflammatory scores based on 92 inflammatory proteins before and 3 months after CPAP initiation. Among participants with OSA who have elevated baseline inflammatory scores, there is significantly decreased inflammatory protein expression after OSA treatment172. Studies with more limited panels of systemic inflammatory markers173,174 or oxidative stress markers175 do not show similar benefits from CPAP. Monocytes from individuals with severe OSA display higher NLRP3 inflammasome activity, which correlates with hypoxemia indices and is mediated by hypoxia-inducible factor-1α (HIF-1α)176. This increase in HIF-1α among individuals with OSA has been validated177, although others have found that intermittent hypoxemia selectively activates nuclear factor kappa B (NF-κB), rather than HIF-1α178.
Another hypothesis is that immune priming results from local pharyngeal inflammation due to mechanical trauma and strain related to OSA airway collapse. Among individuals with OSA, 18F-fluorodeoxyglucose uptake on positron emission tomography with magnetic resonance imaging (PET/MRI) of the pharyngeal mucosa correlates with a composite proteomic inflammatory score from nasal lavage samples179. In a cluster of individuals with high baseline inflammation, application of CPAP—which stabilizes the upper airway—results in significant reductions in monocyte chemoattractant protein 4 (MCP-4), transforming growth factor beta 1 (TGFβ1) and vascular endothelial growth factor-α (VEGFα). Others have also shown decreases in pharyngeal lavage cytokines (that is, IL-6 and IL-8) and leukocytes one year after initiation of CPAP180. This local inflammatory response and immune cell priming may perpetuate systemic inflammation and ASCVD progression.
Sleep-dependent changes in leukocyte abundance and generation
Emerging data have revealed that sleep modulates leukocyte abundance and production. Short-term sleep restriction results in changes in tissular and circulating immune cell abundance, likely due to redistribution of cells between organs and peripheral blood rather than alterations in generation181. For example, in animals, 1–3 days of sleep deprivation leads to a stress-induced increase in splenic and bone marrow B cells without impacting cell production182. Similarly, short paradoxical sleep deprivation redistributes leukocytes by releasing cells from the bone marrow, resulting in decreased bone marrow cellularity and elevated blood monocytes and neutrophils183. In a skin allograft model, both CD4+ and CD8+ T cells exited the spleen and lymph nodes of mice undergoing short sleep restriction184. However, most of these short-term studies have not directly profiled hematopoietic stem and progenitor cells (HSPCs) or their proliferation and differentiation. More work is needed to understand how short-term insufficient or disrupted sleep manipulates HSPC programming, proliferation, differentiation and lineage commitment.
Growing evidence suggests that long-term sleep disruption or insufficiency modifies immune cell production, a process known as hematopoiesis. Long-term (16 weeks) sleep fragmentation in atherogenic mice compromises hypothalamic control over medullary hematopoiesis, resulting in heightened hematopoietic stem cell proliferation in the bone marrow12. The underlying mechanism involves a sleep fragmentation-induced decrease in hypocretin production in the lateral hypothalamus, a change that dampens hypocretin signaling to pre-neutrophils in the bone marrow, thereby leading to elevated colony-stimulating factor 1 production and myeloid-biased hematopoiesis. Consequently, sleep-fragmented mice display monocytosis that leads to expanded aortic immune cell infiltration and augmented atherosclerotic lesion formation in western-type diet-fed, atherosclerosis-prone Apoe−/− mice; these findings are phenocopied in hypocretin-deficient Apoe−/− mice. There are conflicting data on how extended sleep restriction influences lymphocyte generation167,185,186. Whether sleep recovery can reverse the adverse effects of persistent sleep disruption on hematopoiesis and inflammatory recall is understudied and warrants further investigation (Box 3).
BOX 3.
Sleep recovery and the resolution of inflammation
Can sleep recovery revert the effects of chronic suboptimal sleep on hematopoiesis? In mice, the number and proliferation of bone marrow hematopoietic progenitors remains raised after 4 weeks of sleep recovery following 16 weeks of sleep fragmentation11. However, a longer sleep recovery opportunity (10 weeks) normalizes hematopoiesis. These findings indicate that sleep persistently impacts the hematopoietic system. Indeed, sleep fragmentation exerts long-lasting effects on HSPCs by restructuring their epigenome11. Accordingly, a secondary immunological challenge after sleep fragmentation and recovery results in immunological recall responses that lead to an exaggerated, myeloid-biased inflammatory burst and worse outcomes11. Similarly, in human experimental sleep manipulation studies, 3 to 5 days of sleep restriction increases circulating blood monocytes and neutrophils187,188, while subsequent recovery sleep lowers monocytes and lymphocytes, but not neutrophils188. The effects of prolonged repetitive patterns of sleep loss and recovery have also been investigated in the context of inflammation. Healthy individuals exposed to three sleep disturbance/recovery cycles—each consisting of three nights of various sleep interruptions (including delayed sleep onset, frequent awakenings and advanced sleep offset times) followed by one night of recovery sleep—showed an increase in plasma IL-6 levels238. Men, but not women, displayed increased circulating numbers of CD8+ T cells and monocytosis, and the latter remained elevated even after recovery sleep238. Similar sex-specific effects of repetitive sleep loss with intermittent recovery were reported with regards to plasma IL-8 levels and progressively increased vasodilatory changes of small vessels239. In a slightly different experimental setting, a repetitive five-night sleep restriction/two-night recovery protocol induced increased IL-6 expression by monocytes, which remained heightened following recovery240. While these sleep intervention studies were not perceived as stressful by the participants, a dysregulated rhythm of serum cortisol and altered glucocorticoid sensitivity in monocytes was reported, indicating a compromised interplay between stress and the immune system that may contribute to the inflammatory sequelae. Moreover, prolonged sleep interruption may affect the resolution of inflammation, which is in part mediated by specialized pro-resolving mediators that are dysregulated in CVD241. Indeed, plasma levels of a prominent class of specialized pro-resolving mediators, called resolvins, were decreased following repeated exposure to sleep loss and remained low after a recovery sleep period242. Taken together, these findings suggest that recovery or ‘catch-up’ sleep might be insufficient in restoring inflammatory alterations arising from prolonged sleep disruption.
In humans, chronically restricting sleep by 1.5 h every night for 6 weeks increases the numbers of circulating CD14+CD16− classical monocytes, CD14−CD16+ non-classical monocytes, and Lin−CD34+ HSPCs, changes that suggest enhanced hematopoietic activity11. HSPCs retrieved from participants after 6 weeks of sleep restriction display altered epigenetic programming and myeloid-biased differentiation cues11. Other studies similarly report that one week of sleep restriction is sufficient to expand circulating blood monocytes and neutrophils187,188. Indeed, in humans, healthful and sufficient sleep curtails hematopoiesis and reduces circulating monocytes189, neutrophils190, B cells and T cells157,191, the precursors of mature dendritic cells192 and natural killer cells187, while basophil and eosinophil numbers are unaffected. Accordingly, self-reported insufficient sleep boosts circulating monocytes, neutrophils, and total and memory CD4+ T cells193. Similar findings were reported in a study of young adults, in whom irregular sleep patterns (incorporating sleep duration and sleep onset) correlate with increased generation of circulating immune cells194. A study of white blood cell counts in a large cross-sectional analysis reported that sleep duration relates to higher all-cause mortality195. Investigating monocyte subsets in individuals with OSA shows elevated circulating CD16+ monocytes, increased programmed cell death ligand 1 expression and heightened formation of monocyte–T cell complexes196, observations that normalize after initiation of CPAP197. These studies support the idea that more than a week of chronic insufficient sleep expands hematopoiesis and immune cell generation leading to circulating leukocytosis, an important ASCVD risk factor in humans198. Finally, there are important associations between sleep, aging and the immune system. For example, sleep abundance declines in the elderly which may influence the rate of ‘inflammaging’ in ASCVD (Box 4).
BOX 4.
Sleep’s influence on ‘inflamm-aging’ in ASCVD
As we age, consolidated nightly sleep decreases, incidence of sleep disorders increases, our immune system shifts toward a more inflammatory and myeloid-dominant profile, and our risk for ASCVD rises dramatically. These changes raise the hypothesis that age-related alterations in sleep compromise immunological function and thus contribute to ASCVD. In older adults, the hematopoietic system becomes less heterogeneous and a myeloid bias emerges. In murine clonal tracking experiments, chronic sleep disruption expedites hematopoietic neutral drift, thereby accelerating the decline of hematopoietic heterogeneity and skewing the system toward a myeloid fate11. Clonal hematopoiesis, a premalignant expansion of often deleterious hematopoietic stem cell clones, results from somatic mutations common in epigenetic modifiers, such as Tet2 or Dmnt3a, and increased risk of CVD twofold243. Murine data suggest poor-quality sleep expedites mutant HSPC clonal expansion244. In mixed chimeric mice, sleep fragmentation accelerates the proliferation and expansion of Tet2-mutant HSPC clones, leading to more frequent mutant myeloid cells in the blood. In support of the hypothesis that healthy sleep restricts HSPC epigenetic dysregulation and promotes diversity, human data demonstrate that sleep duration and fragmentation in adolescents correlate with leukocyte DNA methylation patterns and epigenetic age245. Symptoms of insomnia, including restlessness, difficulties falling asleep and frequent wake bouts, associate with increased epigenetic aging, as determined by DNA methylation levels, and immune senescence, including elevated counts of late differentiated T cells246,247. Additionally, individuals with severe OSA have shorter leukocyte telomers, and among these individuals, higher arousal index is associated with leukocyte telomere attrition over the prior decade248. Using PSG and sleep electroencephalography, estimations of ‘brain age’ can by derived that incorporate sleep fragmentation and changes in sleep architecture; greater age estimations associate with increased all-cause mortality and CVD32. Altogether, these observations support the hypothesis that poor-quality sleep contributes to systemic and cellular immunological aging and thus increased risk of age-related inflammatory diseases like ASCVD.
Sleep-related modulation of endothelial cell function
In addition to its effects on immune cell production and biology, sleep impacts endothelial cell inflammation. Circulating levels of soluble adhesion molecules, such as intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) or E-selectin, are increased following short-term sleep deprivation in humans199. OSA also associates with complement system activation and higher levels of C3a leading to vascular endothelial cell inflammation and dysfunction200,201. Moreover, OSA increases microRNAs miR-92a and miR-630, which are implicated in endothelial inflammation202. T cells in individuals with OSA have expanded cytotoxicity and specifically target vascular endothelial cells, parameters that CPAP improves203, while VCAM-1 (but not ICAM-1) abundance is independently associated with higher incidence of coronary artery disease in individuals with moderate-to-severe OSA204. Exposing human coronary artery endothelial cells to serum retrieved from individuals with OSA results in chemoattractant activity and augmented VCAM1, ICAM1 and IL8 mRNA expression, whereas these effects are less potent with serum retrieved individuals with OSA treated with CPAP205,206. Mechanistic studies suggest endothelial dysfunction in individuals with OSA may involve sterol regulatory element binding protein 2, miR-210 and mitochondrial alterations207.
Proper vasodynamics are important to vascular health. Moderate sleep restriction for 8 days lowers flow-mediated brachial artery vasodilation208, while short nightly sleep duration associates with nitric oxide-dependent vasodilator dysfunction209. In line with this, individuals sleeping 6 h or fewer show increased occurrence of pathological vascular inflammation, while carotid wall thickness positively associates with sleep fragmentation210. In mice, sleep fragmentation induces vascular endothelial dysfunction and instigates morphological changes in vessels, changes that include the emergence of elastic fiber disruptions and elevated markers of senescence211. In accordance, a recent report demonstrated that circulating exosomes from mice undergoing persistent sleep fragmentation promote endothelial cell injury and exacerbate atherosclerosis through exosomal miR-182–5p-dependent upregulation of endothelial NF-κB and NLRP3 signaling169. Fluctuations in clock genes may also contribute to endothelial dysfunction upon sleep loss, as overexpression of a circadian regulator, cryptochrome 1, in vascular endothelial cells mitigates pro-inflammatory cytokines, adhesion factors and NF-κB activity caused by sleep deprivation212. Taken together, these findings show that sleep additionally benefits ASCVD by preserving endothelial cell function.
Conclusion
Healthy sleep is essential for cardiovascular health. A growing body of data from humans and animal models causally connect poor sleep and increased ASCVD and have begun to uncover the underlying mechanisms. While further work is needed to better understand this association, the autonomic nervous, metabolic and immune systems clearly link sleep to ASCVD. Sleep is a body-wide phenomenon; therefore, insufficient or poor-quality sleep elevates ASCVID risk by dysregulating intercommunication among multiple systems. Furthermore, the relationship between sleep and CVD is bidirectional as emerging data suggest cardiovascular complications perpetuate underlying sleep disturbances213,214.
While epidemiological studies clearly associate insufficient or poor sleep with increased risk for ASCVD, causal evidence remains limited. Thorough and expanded assessments of specific sleep interventions, in humans and mice, are needed to demonstrate the direct influence of sleep on ASCVD and its molecular and cellular drivers. Most of the data summarized here illustrate the impact of inadequate sleep and sleep disorders on ASCVD. However, we cannot assume that reversing sleep deficiencies will translate to favorable ASCVD outcomes in clinical populations without rigorous empirical evidence. Indeed, as outlined in this Review, reversal of OSA symptoms with CPAP has generated mix results on ASCVD risk. Behavior-change therapy to increase sleep represents a feasible intervention110, with low implementation burden and cost, that might meaningfully lower ASCVD risk. Additionally, personalized treatment of sleep disorders may also have ASCVD benefit. However, there is a critical need to test behavioral sleep intervention programs, explore new pharmacological and device-based options and refine treatment plans. Future studies in clinical populations will need to causally demonstrate that improving sleep among short or poor sleepers mitigates ASCVD and its underlying autonomic, metabolic and inflammatory drivers. Such data would not only uncover novel biological connections between sleep and ASCVD but also influence public health policy, lifestyle guidelines and clinical management.
Acknowledgements
This work was funded by a Stony World-Herbert Fund Fellowship and a National Institutes of Health (NIH) award T32HL160511 (to O.C.); an Erwin Schrödinger Postdoctoral Fellowship J4645 from the Austrian Science Fund (to M.G.K.); NIH R01HL158534, R01AG082185, R00HL151750, the Cure Alzheimer’s Fund, and an ISMMS Karen Strauss Cook Research Scholar Award (to C.S.M.); NIH P01HL142494, P01HL131478 and the Leducq Foundation (to F.K.S.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Adamantidis AR & de Lecea L Sleep and the hypothalamus. Science 282, 405–412 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Lewis LD The interconnected causes and consequences of sleep in the brain. Science 374, 564–568 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girardeau G & Lopes-Dos-Santos V Brain neural patterns and the memory function of sleep. Science 374, 560–564 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dom¡nguez F et al. Association of sleep duration and quality with subclinical atherosclerosis. J. Am. Coll. Cardiol 73, 134–144 (2019). [DOI] [PubMed] [Google Scholar]
- 5.McAlpine CS & Swirski FK Circadian influence on metabolism and inflammation in atherosclerosis. Circ. Res 119, 131–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadier K et al. Association of sleep-related disorders with cardiovascular disease among adults in the United States: a cross-sectional study based on national health and nutrition examination survey 2005–2008. Front. Cardiovasc. Med 9, 954238 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pankowska MM et al. Prevalence and geographic patterns of self-reported short sleep duration among US adults, 2020. Prev. Chronic Dis 20, E53 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y et al. Prevalence of healthy sleep duration among adults—United States, 2014. MMWR Morb. Mortal Wkly Rep 65, 137–141 (2019). [DOI] [PubMed] [Google Scholar]
- 9.St-Onge M-P et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation 134, e367–e386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnett DK et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140, e596–e646 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAlpine CS et al. Sleep exerts lasting effects on hematopoietic stem cell function and diversity. J. Exp. Med 219, e20220081 (2022). This study discovered that sleep modifies the epigenetic profile of hematopoietic stem cells, heterogeneity of the immune system, and in humans demonstrated that experimental sleep restriction augments monocytosis and hematopoietic activity.
- 12. McAlpine CS et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019). This mouse study causally demonstrates that disrupted sleep accelerates atherosclerosis through a brain–bone marrow neuroimmune axis.
- 13. Daghlas I et al. Sleep duration and myocardial infarction. J. Am. Coll. Cardiol 74, 1304–1314 (2019). This study shows that poor sleep increases the risk of myocardial infarction even after adjusting to genetic predisposition for cardiovascular disease.
- 14. Ai S et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear Mendelian randomization analyses in UK Biobank. Eur. Heart J 42, 3349–3357 (2021). An extensive study demonstrating a U-shaped correlation between sleep time at CVD risk.
- 15.Ayas NT et al. A prospective study of sleep duration and coronary heart disease in women. Arch. Intern. Med 163, 205–209 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Kim Y et al. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: the Multiethnic Cohort Study. Prev. Med 57, 377–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116,632 people from 21 countries. Eur. Heart J 40, 1620–1629 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandola T, Ferrie JE, Perski A, Akbaraly T & Marmot MG The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep 33, 739–744 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertisch SM et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 41, zsy047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S et al. Cardiovascular risks and sociodemographic correlates of multidimensional sleep phenotypes in two samples of US adults. Sleep Adv 3, zpac005 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazzotti DR et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am. J. Respir. Crit. Care Med 200, 493–506 (2019). Using latent class analysis, this study found that a subgroup of individuals with OSA and excessive sleepiness were at the greatest risk of CVD.
- 22.Xiao Q, Keadle SK, Hollenbeck AR & Matthews CE Sleep duration and total and cause-specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am. J. Epidemiol 180, 997–1006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vetter C et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315, 1726–1734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamboa Madeira S et al. Social jetlag, a novel predictor for high cardiovascular risk in blue-collar workers following permanent atypical work schedules. J. Sleep Res 30, e13380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson KL & von Schantz M Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol. Int 35, 1045–1053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang T, Mariani S & Redline S Sleep irregularity and risk of cardiovascular events: The Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol 75, 991–999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Full KM et al. Sleep irregularity and subclinical markers of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc 12, e027361 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Windred DP et al. Sleep regularity is a stronger predictor of mortality risk than sleep duration: a prospective cohort study. Sleep 47, zsad253 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung J et al. Multi-dimensional sleep and mortality: The Multi-Ethnic Study of Atherosclerosis. Sleep 46, zsad048 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makarem N et al. Redefining cardiovascular health to include sleep: prospective associations with cardiovascular disease in the MESA Sleep Study. J. Am. Heart Assoc 11, e025252 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan M et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385,292 UK biobank participants. Eur. Heart J 41, 1182–1189 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brink-Kjaer A et al. Age estimation from sleep studies using deep learning predicts life expectancy. NPJ Digit. Med 5, 103 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahar E et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med 163, 19–25 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb DJ et al. A prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation 122, 352–360 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Punjabi NM et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6, e1000132 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young T et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep 31, 1071–1078 (2008). [PMC free article] [PubMed] [Google Scholar]
- 37.Hung J, Whitford EG, Hillman DR & Parsons RW Association of sleep apnoea with myocardial infarction in men. Lancet 336, 261–264 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Mooe T, Rabben T, Wiklund U, Franklin KA & Eriksson P Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am. J. Med 101, 251–256 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Peker Y et al. An independent association between obstructive sleep apnoea and coronary artery disease. Eur. Respir. J 14, 179–184 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Shah NA, Yaggi HK, Concato J & Mohsenin V Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 14, 131–136 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Fülöp T et al. Sleep-disordered breathing symptoms among African-Americans in the Jackson Heart Study. Sleep Med 13, 1039–1049 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeboah J et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis 219, 963–968 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos-Rodriguez F et al. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am. J. Respir. Crit. Care Med 189, 1544–1550 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Campos-Rodriguez F et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann. Intern. Med 156, 15–122 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Marin JM, Carrizo SJ, Vicente E & Agusti AGN Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365, 1046–1053 (2005). [DOI] [PubMed] [Google Scholar]
- 46. Pépin JL et al. Relationship between CPAP termination and all-cause mortality: a french nationwide database analysis. Chest 161, 1657–1665 (2022). Real-world evidence that individuals with OSA who discontinue CPAP therapy are at a higher risk of incident heart failure and all-cause mortality.
- 47. Barbé F et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 307, 2161–2168 (2012). One of several randomized controlled trials demonstrating no CVD benefit of CPAP therapy among individuals with OSA without excessive daytime sleepiness.
- 48.Benjafield AV et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med 7, 687–698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards BA, Redline S, Sands SA & Owens RL More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am. J. Respir. Crit. Care Med 200, 691–703 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sands SA et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med 197, 1187–1197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie J et al. Excessive daytime sleepiness independently predicts increased cardiovascular risk after myocardial infarction. J. Am. Heart Assoc 7, e007221 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sweetman A et al. Association of co-morbid insomnia and sleep apnoea symptoms with all-cause mortality: analysis of the NHANES 2005–2008 data. Sleep Epidemiol 2, 100043 (2022). [Google Scholar]
- 53.Lechat B et al. Comorbid insomnia and sleep apnoea is associated with all-cause mortality. Eur. Respir. J 60, 2101958 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Lechat B et al. All-cause mortality in people with co-occurring insomnia symptoms and sleep apnea: analysis of the Wisconsin Sleep Cohort. Nat. Sci. Sleep 14, 1817–1828 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho YW et al. Comorbid insomnia with obstructive sleep apnea: clinical characteristics and risk factors. J. Clin. Sleep Med 14, 409–417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohanta SK et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Kim H, Jung HR, Kim JB & Kim DJ Autonomic dysfunction in sleep disorders: from neurobiological basis to potential therapeutic approaches. J. Clin. Neurol 18, 140–151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somers VK, Dyken ME, Mark AL & Abboud FM Sympathetic-nerve activity during sleep in normal subjects. New Engl. J. Med 328, 303–307 (1993). [DOI] [PubMed] [Google Scholar]
- 59.Irwin MR Sleep and inflammation: partners in sickness and in health. Nat. Rev. Immunol 19, 702–715 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Narkiewicz K & Somers VK Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand 177, 385–390 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Berteotti C & Silvani A The link between narcolepsy and autonomic cardiovascular dysfunction: a translational perspective. Clin. Autonom. Res 28, 545–555 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Kalmbach DA et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat. Sci. Sleep 10, 193–201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pongratz G & Straub RH The sympathetic nervous response in inflammation. Arthritis Res. Ther 16, 504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pepper GS & Lee RW Sympathetic activation in heart failure and its treatment with β-blockade. Arch. Intern. Med 159, 225–234 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Irwin MR, Straub RH & Smith MT Heat of the night: sleep disturbance activates inflammatory mechanisms and induces pain in rheumatoid arthritis. Nat. Rev. Rheumatol 19, 545–559 (2023). [DOI] [PubMed] [Google Scholar]
- 66.Morssinkhof MWL et al. Associations between sex hormones, sleep problems and depression: a systematic review. Neurosci. Biobehav. Rev 118, 669–680 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Smith J et al. Regulation of stress-induced sleep fragmentation by preoptic glutamatergic neurons. Curr. Biol 34, 12–23 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Somers VK, Dyken ME, Clary MP & Abboud FM Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest 96, 1897–1904 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas SJ et al. Association of obstructive sleep apnea with nighttime blood pressure in African Americans: The Jackson Heart Study. Am. J. Hypertens 33, 949–957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson DA et al. Association between sleep apnea and blood pressure control among blacks. Circulation 139, 1275–1284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crinion SJ et al. Non-dipping nocturnal blood pressure correlates with obstructive sleep apnoea severity in normotensive subjects and may reverse with therapy. ERJ Open Res 7, 00338–02021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Z et al. Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial. Am. J. Hypertens 28, 300–306 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Gottlieb DJ et al. CPAP versus oxygen in obstructive sleep apnea. New Engl. J. Med 370, 2276–2285 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sansom K et al. Cross-sectional interrelationships between chronotype, obstructive sleep apnea and blood pressure in a middle-aged community cohort. J. Sleep Res 32, e13778 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vgontzas AN, Liao D, Bixler EO, Chrousos GP & Vela-Bueno A Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 32, 491–497 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottlieb DJ et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep 29, 1009–1014 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Gangwisch JE, Feskanich D, Malaspina D, Shen S & Forman JP Sleep duration and risk for hypertension in women: results from the nurses’ health study. Am. J. Hypertens 26, 903–911 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knutson KL et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch. Intern. Med 169, 1055–1061 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Covassin N et al. Effects of experimental sleep restriction on ambulatory and sleep blood pressure in healthy young adults: a randomized crossover study. Hypertension 78, 859–870 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rhoads MK, Balagee V & Thomas SJ Circadian regulation of blood pressure: of mice and men. Curr. Hypertens. Rep 22, 40 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merikanto I et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int 30, 470–477 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Silvani A et al. Sleep modulates hypertension in leptin-deficient obese mice. Hypertension 53, 251–255 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Xue Y et al. Durative sleep fragmentation with or without hypertension suppress rapid eye movement sleep and generate cerebrovascular dysfunction. Neurobiol. Dis 184, 106222 (2023). [DOI] [PubMed] [Google Scholar]
- 84.Bastianini S et al. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep 34, 213–218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silvani A et al. Sleep and cardiovascular phenotype in middle-aged hypocretin-deficient narcoleptic mice. J. Sleep Res 23, 98–106 (2014). [DOI] [PubMed] [Google Scholar]
- 86.West AC et al. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun 8, 417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skornyakov E et al. Cardiac autonomic activity during simulated shift work. Ind. Health 57, 118–132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alvente S et al. Autonomic mechanisms of blood pressure alterations during sleep in orexin/hypocretin-deficient narcoleptic mice. Sleep 44, zsab022 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Hayter EA et al. Distinct circadian mechanisms govern cardiac rhythms and susceptibility to arrhythmia. Nat. Commun 12, 2472 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muller JE et al. Circadian variation in the frequency of sudden cardiac death. Circulation 75, 131–138 (1987). [DOI] [PubMed] [Google Scholar]
- 91. Zheng NS et al. Sleep patterns and risk of chronic disease as measured by long-term monitoring with commercial wearable devices in the All of Us Research Program. Nat. Med 30, 2648–2656 (2024). This study used electronic health record data from over 6,500 participants to identify an inverse association for REM sleep and deep sleep with the incidence of atrial fibrillation, as well as an increased risk of obesity upon heightened sleep irregularity.
- 92.Murata K, Yano E & Shinozaki T Impact of shift work on cardiovascular functions in a 10-year follow-up study. Scand. J. Work Environ. Health 25, 272–277 (1999). [DOI] [PubMed] [Google Scholar]
- 93.Meloni M, Setzu D, Del Rio A, Campagna M & Cocco P QTc interval and electrocardiographic changes by type of shift work. Am. J. Ind. Med 56, 1174–1179 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Yeghiazarians Y et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 144, E56–E67 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Azarbarzin A et al. The sleep apnea-specific pulse-rate response predicts cardiovascular morbidity and mortality. Am. J. Respir. Crit. Care Med 203, 1546–1555 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Azarbarzin A et al. Cardiovascular benefit of continuous positive airway pressure in adults with coronary artery disease and obstructive sleep apnea without excessive sleepiness. Am. J. Respir. Crit. Care Med 206, 767–774 (2022). Individuals with OSA without excessive daytime sleepiness and an increased pulse-rate response to sleep apnea-related respiratory events sustained CVD benefit from CPAP treatment.
- 97.Butler MP et al. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med 199, 903–912 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castro-Diehl C et al. Sleep duration and quality in relation to autonomic nervous system measures: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 39, 1927–1940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sondrup N et al. Effects of sleep manipulation on markers of insulin sensitivity: a systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev 62, 101594 (2022). [DOI] [PubMed] [Google Scholar]
- 100.Zuraikat FM et al. Chronic insufficient sleep in women impairs insulin sensitivity independent of adiposity changes: results of a randomized trial. Diabetes Care 47, 117–125 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meszaros M & Bikov A Obstructive sleep apnoea and lipid metabolism: the summary of evidence and future perspectives in the pathophysiology of OSA-associated dyslipidaemia. Biomedicines 10, 2754 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.St-Onge MP Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes. Rev 18, 34–39 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Barragán R, Zuraikat FM, Tam V, RoyChoudhury A & St-Onge MP Changes in eating patterns in response to chronic insufficient sleep and their associations with diet quality: a randomized trial. J. Clin. Sleep Med 19, 1867–1875 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gallegos JV, Boege HL, Zuraikat FM & St-Onge MP Does sex influence the effects of experimental sleep curtailment and circadian misalignment on regulation of appetite? Curr. Opin. Endocr. Metab. Res 17, 20–25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.St-Onge MP, Pizinger T, Kovtun K & RoyChoudhury A Sleep and meal timing influence food intake and its hormonal regulation in healthy adults with overweight/obesity. Eur. J. Clin. Nutr 72, 76–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spruyt K, Molfese DL & Gozal D Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics 127, e345–e352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel SR et al. The association between sleep patterns and obesity in older adults. Int. J. Obes 38, 1159–1164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lunsford-Avery JR, Engelhard MM, Navar AM & Kollins SH Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci. Rep 8, 14158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Covassin N et al. Effects of experimental sleep restriction on energy intake, energy expenditure, and visceral obesity. J. Am. Coll. Cardiol 79, 1254–1265 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tasali E, Wroblewski K, Kahn E, Kilkus J & Schoeller DA Effect of sleep extension on objectively assessed energy intake among adults with overweight in real-life settings: a randomized clinical trial. JAMA Intern. Med 182, 365–374 (2022). This study demonstrates that extending sleep among short sleepers benefits obesity.
- 111.Bikov A et al. Comparison of composite lipid indices in patients with obstructive sleep apnoea. Nat. Sci. Sleep 14, 1333–1340 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jenkins JB et al. Sleep is increased in mice with obesity induced by high-fat food. Physiol. Behav 87, 255–262 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Panagiotou M, Meijer JH & Deboer T Chronic high-caloric diet modifies sleep homeostasis in mice. Eur. J. Neurosci 47, 1339–1352 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Panagiotou M & Deboer T Chronic high-caloric diet accentuates age-induced sleep alterations in mice. Behav. Brain Res 362, 131–139 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Laposky AD, Bradley MA, Williams DL, Bass J & Turek FW Sleep–wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, 2059–2066 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laposky AD et al. Altered sleep regulation in leptin-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 290, 894–903 (2006). [DOI] [PubMed] [Google Scholar]
- 117.Fleury Curado T et al. Sleep-disordered breathing in C57BL/6J mice with diet-induced obesity. Sleep 41, zsy089 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davis EM, Locke LW, McDowell AL, Strollo PJ & O’Donnell CP Obesity accentuates circadian variability in breathing during sleep in mice but does not predispose to apnea. J. Appl. Physiol 115, 474–482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim LJ et al. The effect of diet-induced obesity on sleep and breathing in female mice. Sleep 46, zsad158 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guan Z, Vgontzas AN, Bixler EO & Fang J Sleep is increased by weight gain and decreased by weight loss in mice. Sleep 31, 627–633 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu S, Liu X, Wang Y, Zhang R & Wei S Melatonin protects against body weight gain induced by sleep deprivation in mice. Physiol. Behav 257, 113975 (2022). [DOI] [PubMed] [Google Scholar]
- 122.De Oliveira EM et al. Late effects of sleep restriction: potentiating weight gain and insulin resistance arising from a high-fat diet in mice. Obesity 23, 391–398 (2015). [DOI] [PubMed] [Google Scholar]
- 123. Wang Y et al. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity 22, 758–762 (2014). This mouse study illustrates that prolonged sleep fragmentation induces obesity via promoting obesogenic behaviors and leptin resistance.
- 124.Baud MO, Magistretti PJ & Petit JM Sustained sleep fragmentation affects brain temperature, food intake and glucose tolerance in mice. J. Sleep Res 22, 3–12 (2013). [DOI] [PubMed] [Google Scholar]
- 125.Gozal D et al. Protein-tyrosine phosphatase-1b mediates sleep fragmentation-induced insulin resistance and visceral adipose tissue inflammation in mice. Sleep 40, 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Husse J, Hintze SC, Eichele G, Lehnert H & Oster H Circadian clock genes Per1 and Per2 regulate the response of metabolism-associated transcripts to sleep disruption. PLoS ONE 7, e52983 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zitting KM et al. Chronic circadian disruption on a high-fat diet impairs glucose tolerance. Metabolism 130, 155158 (2022). [DOI] [PubMed] [Google Scholar]
- 128.Hara J, Yanagisawa M & Sakurai T Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett 380, 239–242 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Hara J et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Tsuneki H et al. Timed inhibition of orexin system by suvorexant improved sleep and glucose metabolism in type 2 diabetic db/db mice. Endocrinology 157, 4146–4157 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Bos MM et al. Associations of sleep duration and quality with serum and hepatic lipids: The Netherlands Epidemiology of Obesity Study. J. Sleep Res 28, e12776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuula L et al. Sleep and lipid profile during transition from childhood to adolescence. J. Pediatrics 177, 173–178 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Feng N et al. The associations between sleep architecture and metabolic parameters in patients with obstructive sleep apnea: a hospital-based cohort study. Front. Neurol 12, 606031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kruisbrink M et al. Association of sleep duration and quality with blood lipids: a systematic review and meta-analysis of prospective studies. BMJ Open 7, e018585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nadeem R et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J. Clin. Sleep Med 10, 475–489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gündüz C et al. Obstructive sleep apnoea independently predicts lipid levels: data from the European Sleep Apnea Database. Respirology 23, 1180–1189 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Ness KM et al. Four nights of sleep restriction suppress the postprandial lipemic response and decrease satiety. J. Lipid Res 60, 1935–1945 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ. Res 97, 698–706 (2005). [DOI] [PubMed] [Google Scholar]
- 139.Feres MC et al. An assessment of oxidized LDL in the lipid profiles of patients with obstructive sleep apnea and its association with both hypertension and dyslipidemia, and the impact of treatment with CPAP. Atherosclerosis 241, 342–349 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Drager LF et al. Obstructive sleep apnea and effects of continuous positive airway pressure on triglyceride-rich lipoprotein metabolism. J. Lipid Res 59, 1027–1033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen B et al. Effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. J. Clin. Med 11, 596 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.e Silva LO et al. The effects of continuous positive airway pressure and mandibular advancement therapy on metabolic outcomes of patients with mild obstructive sleep apnea: a randomized controlled study. Sleep Breath 25, 797–805 (2021). [DOI] [PubMed] [Google Scholar]
- 143.Uniken Venema JAM et al. Cardiovascular and metabolic effects of a mandibular advancement device and continuous positive airway pressure in moderate obstructive sleep apnea: a randomized controlled trial. J. Clin. Sleep Med 18, 1547–1555 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu H et al. Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. J. Clin. Sleep Med 16, 475–482 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nowak N, Gaisl T, Sinues P, Brown SA & Kohler Correspondence M Rapid and reversible control of human metabolism by individual sleep states. Cell Rep 37, 109903 (2021). [DOI] [PubMed] [Google Scholar]
- 146.da Silva BRD, Nunes PIG, Santos FA, de Bruin PFC & de Bruin VMS Exercise modifies lipid and glucose metabolism alterations induced by sleep deprivation in mice. Sleep Sci 15, 347–350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xing C et al. Sleep disturbance induces increased cholesterol level by NR1D1 mediated CYP7A1 inhibition. Front. Genet 11, 610496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ferrell JM & Chiang JYL Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell Mol. Gastroenterol. Hepatol 1, 664–677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chalfant JM et al. Chronic environmental circadian disruption increases atherosclerosis and dyslipidemia in female, but not male, ApolipoproteinE-deficient mice. Front. Physiol 14, 1167858 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Schilperoort M et al. Disruption of circadian rhythm by alternating light-dark cycles aggravates atherosclerosis development in APOE*3-Leiden.CETP mice. J. Pineal Res 68, e12614 (2020). This study showed that circadian disruption by alternating light–dark cycles directly exacerbates atherosclerosis lesion formation in hyperlipidemic mice.
- 151.Vgontzas AN et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 51, 887–892 (2002). [DOI] [PubMed] [Google Scholar]
- 152.Wright KP et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun 47, 24–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dzierzewski JM, Donovan EK, Kay DB, Sannes TS & Bradbrook KE Sleep inconsistency and markers of inflammation. Front. Neurol 11, 1042 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Okun ML et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom. Med 73, 142–150 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Testelmans D et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine 62, 210–216 (2013). [DOI] [PubMed] [Google Scholar]
- 156.Lekander M et al. Subjective health perception in healthy young men changes in response to experimentally restricted sleep and subsequent recovery sleep. Brain Behav. Immun 34, 43–46 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Ruiz FS et al. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immun 18, 44–54 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Hurtado-Alvarado G et al. The yin/yang of inflammatory status: blood–brain barrier regulation during sleep. Brain Behav. Immun 69, 154–166 (2018). [DOI] [PubMed] [Google Scholar]
- 159.Sang D et al. Prolonged sleep deprivation induces a cytokinestorm-like syndrome in mammals. Cell 186, 5500–5516 (2023). [DOI] [PubMed] [Google Scholar]
- 160.Smagula SF et al. Actigraphy and polysomnography measured sleep disturbances, inflammation, and mortality among older men. Psychosom. Med 78, 686–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A & Cole S Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med 166, 1756–1762 (2006). [DOI] [PubMed] [Google Scholar]
- 162.Irwin MR, Carrillo C & Olmstead R Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav. Immun 24, 54–57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller MA et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II Study. Sleep 32, 857–864 (2009). [PMC free article] [PubMed] [Google Scholar]
- 164.Patel SR et al. Sleep duration and biomarkers of inflammation. Sleep 32, 200–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Frey DJ, Fleshner M & Wright KP The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav. Immun 21, 1050–1057 (2007). [DOI] [PubMed] [Google Scholar]
- 166.Hu J et al. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav. Immun 17, 498–504 (2003). [DOI] [PubMed] [Google Scholar]
- 167.van Leeuwen WMA et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 4, e4589 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Duewell P et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li X et al. Sleep deprivation promotes endothelial inflammation and atherogenesis by reducing exosomal miR-182-5p. Arterioscler. Thromb. Vasc. Biol 43, 995–1014 (2023). [DOI] [PubMed] [Google Scholar]
- 170.Bouloukaki I et al. Evaluation of inflammatory markers in a large sample of obstructive sleep apnea patients. Eur. Resp. J 50, PA2319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thunström et al. Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apnea. Sleep 38, 463–471 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kundel V et al. Advanced proteomics and cluster analysis for identifying novel obstructive sleep apnea subtypes before and after continuous positive airway pressure therapy. Ann. Am. Thorac. Soc 20, 1038–1047 (2023). Hierarchical clustering of human plasma proteomic panels revealed three inflammatory subgroups of patients with OSA. Those with high composite inflammatory protein expression at baseline experienced reductions in plasma protein levels after initiating CPAP therapy.
- 173.Campos-Rodriguez F et al. Effect of continuous positive airway pressure on inflammatory, antioxidant, and depression biomarkers in women with obstructive sleep apnea: a randomized controlled trial. Sleep 42, zsz145 (2019). [DOI] [PubMed] [Google Scholar]
- 174.Thunström E et al. CPAP does not reduce inflammatory biomarkers in patients with coronary artery disease and nonsleepy obstructive sleep apnea: a randomized controlled trial. Sleep 40, 11 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Paz y Mar HL et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the Sleep Apnea Stress Randomized Controlled Trial. Chest 150, 80–90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Díaz-García E et al. Inflammasome activation: a keystone of proinflammatory response in obstructive sleep apnea. Am. J. Respir. Crit. Care Med 205, 1337–1348 (2022). [DOI] [PubMed] [Google Scholar]
- 177.Gabryelska A et al. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J. Clin. Sleep Med 16, 1761–1768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ryan S, Taylor CT & McNicholas WT Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112, 2660–2667 (2005). [DOI] [PubMed] [Google Scholar]
- 179. Cohen O et al. Pharyngeal inflammation on positron emission tomography/magnetic resonance imaging before and after obstructive sleep apnea treatment. Ann. Am. Thorac. Soc 20, 574–583 (2023). This study used PET/MRI to evaluate metabolic activity within the upper airway of patients with OSA. Not only did baseline PET uptake correlate with inflammatory protein expression from nasal lavage samples, but 3 months of CPAP was shown to reduce inflammation in the most-diseased regions.
- 180.Vicente E et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur. Respir. J 48, 1108–1117 (2016). [DOI] [PubMed] [Google Scholar]
- 181.Ingram LA, Simpson RJ, Malone E & Florida-James GD Sleep disruption and its effect on lymphocyte redeployment following an acute bout of exercise. Brain Behav. Immun 47, 100–108 (2015). [DOI] [PubMed] [Google Scholar]
- 182.Lungato L et al. Effects of sleep deprivation on mice bone marrow and spleen B lymphopoiesis. J. Cell. Physiol 231, 1313–1320 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Guariniello LD, Vicari P, Lee KS, de Oliveira AC & Tufik S Bone marrow and peripheral white blood cells number is affected by sleep deprivation in a murine experimental model. J. Cell. Physiol 227, 361–366 (2012). [DOI] [PubMed] [Google Scholar]
- 184.Ruiz FS et al. Sleep influences the immune response and the rejection process alters sleep pattern: evidence from a skin allograft model in mice. Brain Behav. Immun 61, 274–288 (2017). [DOI] [PubMed] [Google Scholar]
- 185.Everson CA, Bergmann BM & Rechtschaffen A Sleep deprivation in the rat: III. Total sleep deprivation. Sleep 12, 13–21 (1989). [DOI] [PubMed] [Google Scholar]
- 186.Sá-Nunes A et al. The dual effect of paradoxical sleep deprivation on murine immune functions. J. Neuroimmunol 290, 9–14 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Born J, Lange T, Hansen K, Mölle M & Fehm HL Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol 158, 4454–4464 (1997). [PubMed] [Google Scholar]
- 188.Lasselin J, Rehman JU, Åkerstedt T, Lekander M & Axelsson J Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav. Immun 47, 93–99 (2015). [DOI] [PubMed] [Google Scholar]
- 189.Dimitrov S, Besedovsky L, Born J & Lange T Differential acute effects of sleep on spontaneous and stimulated production of tumor necrosis factor in men. Brain Behav. Immun 47, 201–210 (2015). [DOI] [PubMed] [Google Scholar]
- 190.Christoffersson G et al. Acute sleep deprivation in healthy young men: impact on population diversity and function of circulating neutrophils. Brain Behav. Immun 41, 162–172 (2014). [DOI] [PubMed] [Google Scholar]
- 191.Besedovsky L, Dimitrov S, Born J & Lange T Nocturnal sleep uniformly reduces numbers of different T-cell subsets in the blood of healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol 311, R637–R642 (2016). [DOI] [PubMed] [Google Scholar]
- 192.Dimitrov S, Lange T, Nohroudi K & Born J Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 30, 401–411 (2007). [DOI] [PubMed] [Google Scholar]
- 193.Pérez de Heredia F et al. Self-reported sleep duration, white blood cell counts and cytokine profiles in European adolescents: The HELENA study. Sleep Med 15, 1251–1258 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Hoopes EK et al. Consistency where it counts: Sleep regularity is associated with circulating white blood cell count in young adults. Brain Behav. Immun. Health 13, 100233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shattuck EC & Sparks CS Sleep duration is related to increased mortality risk through white blood cell counts in a large national sample. Am. J. Hum. Biol 34, e23574 (2022). [DOI] [PubMed] [Google Scholar]
- 196.Polasky C et al. Redistribution of monocyte subsets in obstructive sleep apnea syndrome patients leads to an imbalanced PD-1/PD-L1 cross-talk with CD4/CD8 T cells. J. Immunol 206, 51–58 (2021). [DOI] [PubMed] [Google Scholar]
- 197.Polasky C et al. Reconstitution of monocyte subsets and PD-L1 expression but not T cell PD-1 expression in obstructive sleep apnea patients upon PAP therapy. Int. J. Mol. Sci 22, 11375 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rogacev KS et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol 60, 1512–1520 (2012). [DOI] [PubMed] [Google Scholar]
- 199.Ohga E et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J. Appl. Physiol 87, 10–14 (1999). [DOI] [PubMed] [Google Scholar]
- 200.Gao S et al. Complement promotes endothelial von Willebrand factor and angiopoietin-2 release in obstructive sleep apnea. Sleep 44, zsaa286 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Horvath P et al. Complement system activation in obstructive sleep apnea. J. Sleep Res 27, e12674 (2018). [DOI] [PubMed] [Google Scholar]
- 202.Gongol B et al. Serum miR-92a is elevated in children and adults with obstructive sleep apnea. J. Mol. Biomark. Diagn 11, 4 (2020). [PMC free article] [PubMed] [Google Scholar]
- 203.Dyugovskaya L, Lavie P, Hirsh M & Lavie L Activated CD8+ T-lymphocytes in obstructive sleep apnoea. Eur. Respir. J 25, 820–828 (2005). [DOI] [PubMed] [Google Scholar]
- 204.Lv Q et al. Increased levels of VCAM-1 is associated with higher occurrence of coronary artery disease in adults with moderate to severe obstructive sleep apnea. Sleep Med 85, 131–137 (2021). [DOI] [PubMed] [Google Scholar]
- 205.Hoffmann M et al. Serum of obstructive sleep apnea patients impairs human coronary endothelial cell migration. Arch. Med. Sci 13, 223–227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zychowski KE et al. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis 254, 59–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shang F et al. Obstructive sleep apnea-induced endothelial dysfunction is mediated by miR-210. Am. J. Respir. Crit. Care Med 207, 323–335 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Calvin AD et al. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J. Am. Heart Assoc 3, e001143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bain AR et al. Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Atherosclerosis 265, 41–46 (2017). [DOI] [PubMed] [Google Scholar]
- 210.Kundel V et al. Sleep duration and vascular inflammation using hybrid positron emission tomography/magnetic resonance imaging: results from the multi-ethnic study of atherosclerosis. J. Clin. Sleep Med 17, 2009–2018 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Carreras A et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 37, 1817–1824 (2014). This study describes that long-term sleep fragmentation promotes vascular endothelial dysfunction, structural vessel changes and mild blood pressure alterations in mice.
- 212.Qin B & Deng Y Overexpression of circadian clock protein cryptochrome (CRY) 1 alleviates sleep deprivation-induced vascular inflammation in a mouse model. Immunol. Lett 163, 76–83 (2015). [DOI] [PubMed] [Google Scholar]
- 213.Sharma R et al. Ischemic stroke disrupts sleep homeostasis in middle-aged mice. Cells 11, 2818 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Ziegler KA et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 381, 285–290 (2023). This report demonstrated both in mice and humans that cardiac disease promotes immune-mediated sympathetic denervation and dysfunction of the pineal gland resulting in sleep disruption.
- 215.Colavito V et al. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front. Syst. Neurosci 7, 106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bian WJ & de Lecea L Automated sleep deprivation setup using a shaking platform in mice. Bio Protoc 13, e4620 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Parhizkar S et al. Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice. Sci. Transl. Med 15, eade6285 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kam K et al. Effect of aging and a dual orexin receptor antagonist on sleep architecture and non-REM oscillations including an REM behavior disorder phenotype in the PS19 mouse model of tauopathy. J. Neurosci 43, 4738–4749 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Duncan MJ et al. Effects of the dual orexin receptor antagonist DORA-22 on sleep in 5XFAD mice. Alzheimers Dement 5, 70–80 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Honda T et al. A single phosphorylation site of SIK3 regulates daily sleep amounts and sleep need in mice. Proc. Natl Acad. Sci. USA 115, 10458–10463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Funato H et al. Forward-genetics analysis of sleep in randomly mutagenized mice. Nature 539, 378–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chung S et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 545, 477–481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rolls A et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc. Natl Acad. Sci. USA 108, 13305–13310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Savransky V et al. Chronic intermittent hypoxia induces atherosclerosis. Am. J. Respir. Crit. Care Med 175, 1290–1297 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Song D et al. Chronic intermittent hypoxia induces atherosclerosis by NF-κB-dependent mechanisms. Biochim. Biophys. Acta 1822, 1650–1659 (2012). [DOI] [PubMed] [Google Scholar]
- 226.Hadaya J & Ardell JL Autonomic modulation for cardiovascular disease. Front. Physiol 11, 617459 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ensminger DC, Wheeler ND, Al Makki R, Eads KN & Ashley NT Contrasting effects of sleep fragmentation and angiotensin-II treatment upon pro-inflammatory responses of mice. Sci. Rep 12, 14763 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mishra I et al. Chemical sympathectomy reduces peripheral inflammatory responses to acute and chronic sleep fragmentation. Am. J. Physiol. Regul. Integr. Comp. Physiol 318, R781–R789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wheeler ND, Ensminger DC, Rowe MM, Wriedt ZS & Ashley NT Alpha- and beta- adrenergic receptors regulate inflammatory responses to acute and chronic sleep fragmentation in mice. PeerJ 10.7717/peerj.11616 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pavlov VA, Wang H, Czura CJ, Friedman SG & Tracey KJ The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med 9, 125–134 (2003). [PMC free article] [PubMed] [Google Scholar]
- 231.Borovikova LV et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). [DOI] [PubMed] [Google Scholar]
- 232.Kelly MJ, Breathnach C, Tracey KJ & Donnelly SC Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med 3, 100696 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rocha-Resende C, da Silva AM, Prado MAM & Guatimosim S Protective and anti-inflammatory effects of acetylcholine in the heart. Am. J. Physiol. Cell. Physiol 320, C155–C161 (2021). [DOI] [PubMed] [Google Scholar]
- 234.Zielinski MR, Dunbrasky DL, Taishi P, Souza G & Krueger JM Vagotomy attenuates brain cytokines and sleep induced by peripherally administered tumor necrosis factor-α and lipopolysaccharide in mice. Sleep 36, 1227–1238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ma SN, Liu XH & Cai WS Preventive noninvasive vagal nerve stimulation reduces insufficient sleep-induced depression by improving the autonomic nervous system. Biomed. Pharmacother 173, 116344 (2024). [DOI] [PubMed] [Google Scholar]
- 236.Rosas-Ballina M et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang Y, Xie B, Chen X, Zhang J & Yuan S A key role of gut microbiota-vagus nerve/spleen axis in sleep deprivation-mediated aggravation of systemic inflammation after LPS administration. Life Sci 265, 118736 (2021). [DOI] [PubMed] [Google Scholar]
- 238.Besedovsky L et al. Differential effects of an experimental model of prolonged sleep disturbance on inflammation in healthy females and males. PNAS Nexus 1, pgac004 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang H et al. Macro- and microvascular reactivity during repetitive exposure to shortened sleep: sex differences. Sleep 44, zsaa257 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Simpson NS et al. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav. Immun 58, 142–151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bazan HA et al. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot. Essent. Fatty Acids 125, 43–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Engert LC, Mullington JM & Haack M Prolonged experimental sleep disturbance affects the inflammatory resolution pathways in healthy humans. Brain Behav. Immun 113, 12–20 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jaiswal S et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New Engl. J. Med 377, 111–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Heyde A et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 184, 1348–1361 (2021). This study shows that in mice and humans, modeling accelerating hematopoietic stem cell proliferation, as in sleep fragmentation, quickens the homogenization of the immune system and the emergence of clonal hematopoiesis.
- 245.Jansen EC et al. Sleep duration and fragmentation in relation to leukocyte DNA methylation in adolescents. Sleep 42, zsz121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Carroll JE et al. Postpartum sleep loss and accelerated epigenetic aging. Sleep Health 7, 362–367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Carskadon MA et al. A pilot prospective study of sleep patterns and DNA methylation-characterized epigenetic aging in young adults. BMC Res. Notes 12, 583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Carroll JE et al. Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Sleep 42, zsz089 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


