Abstract
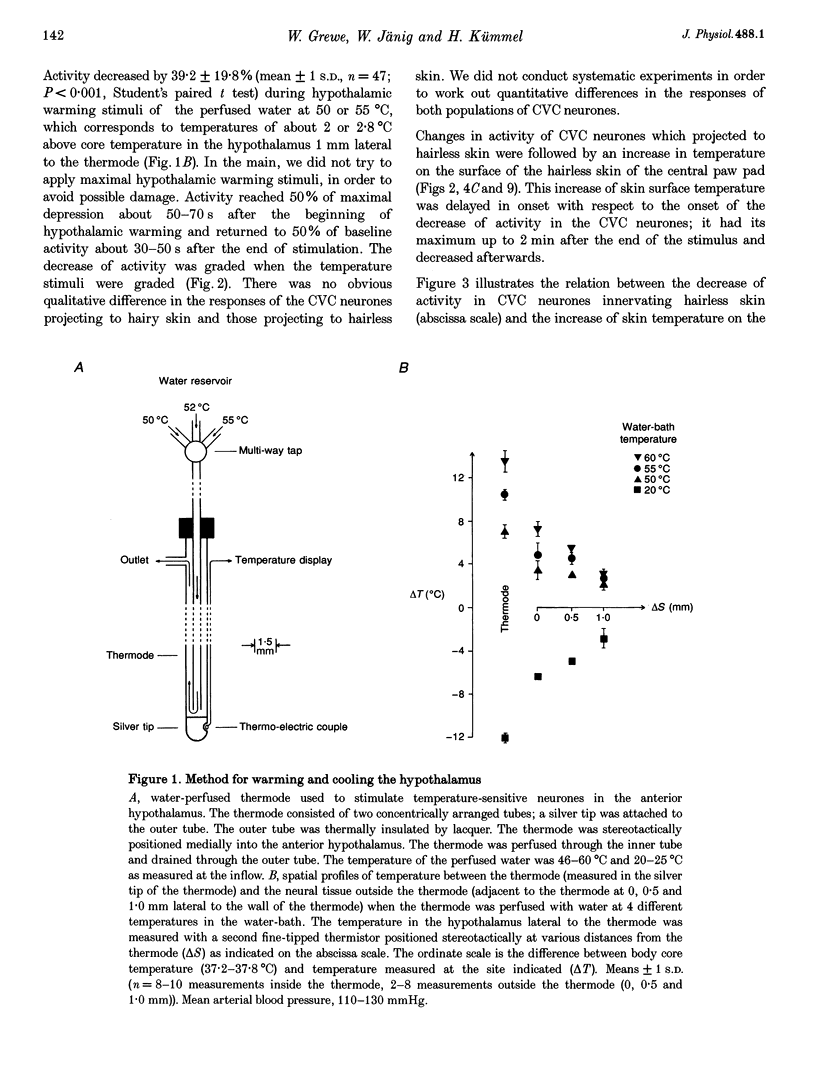
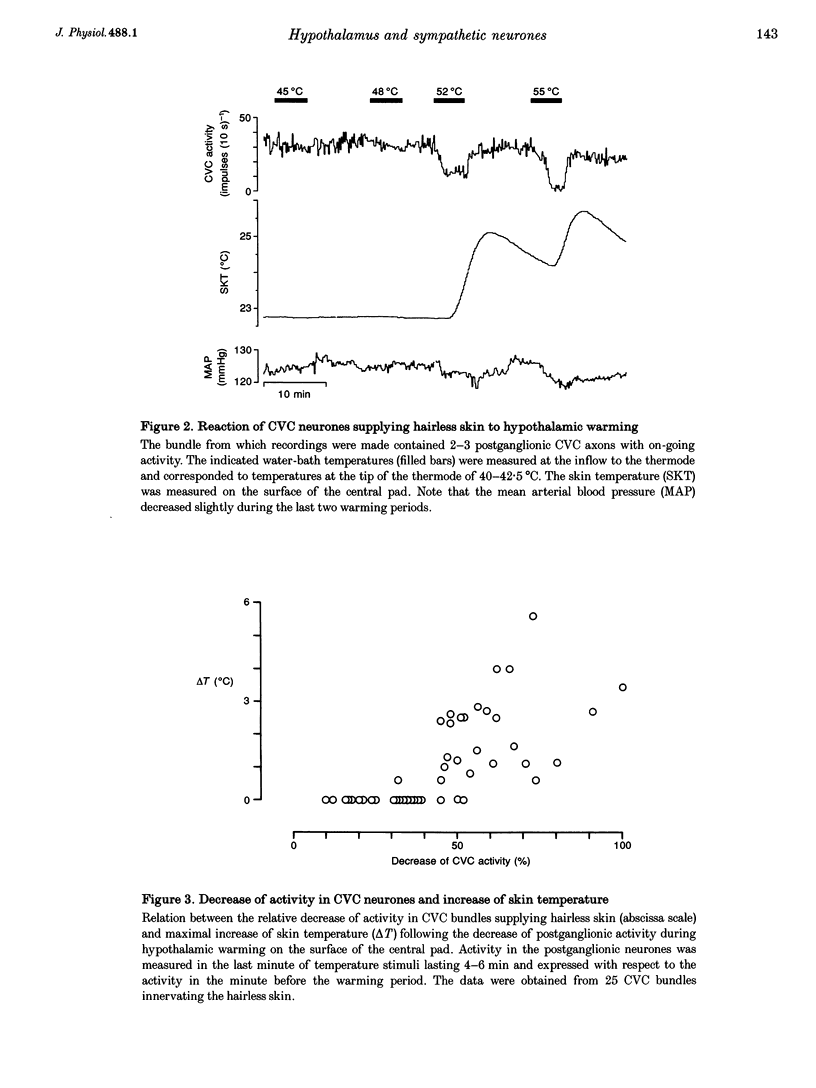
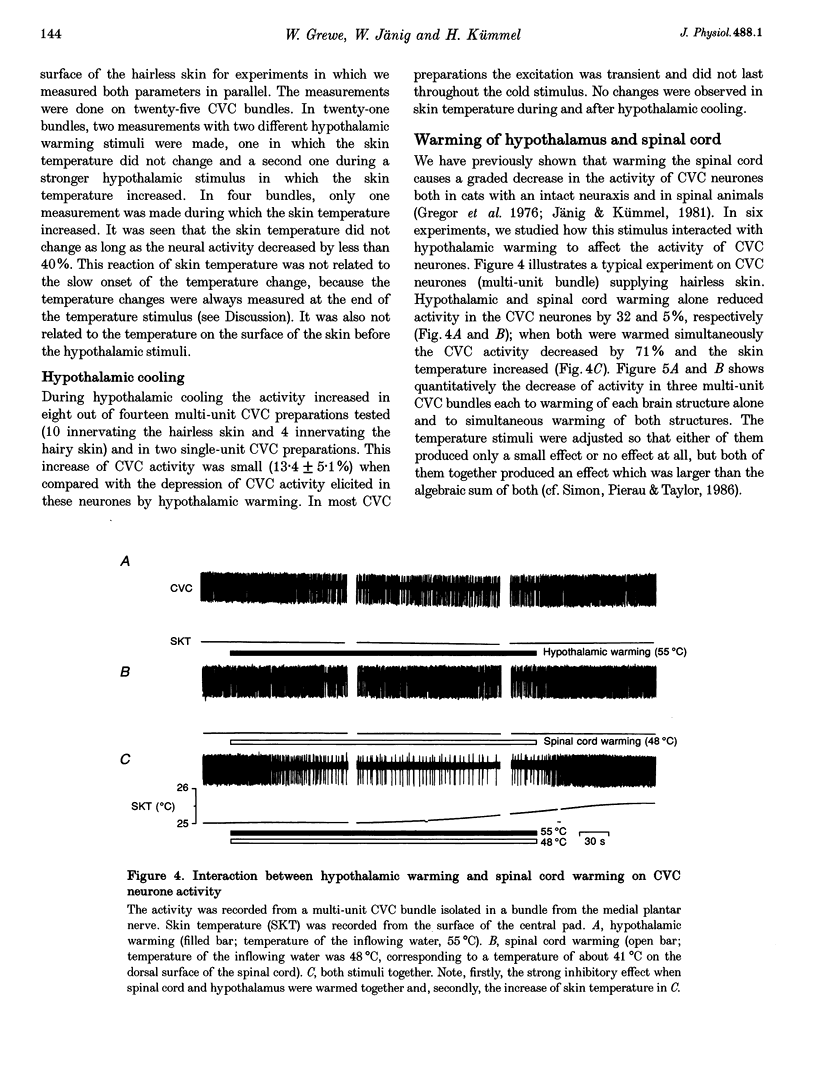
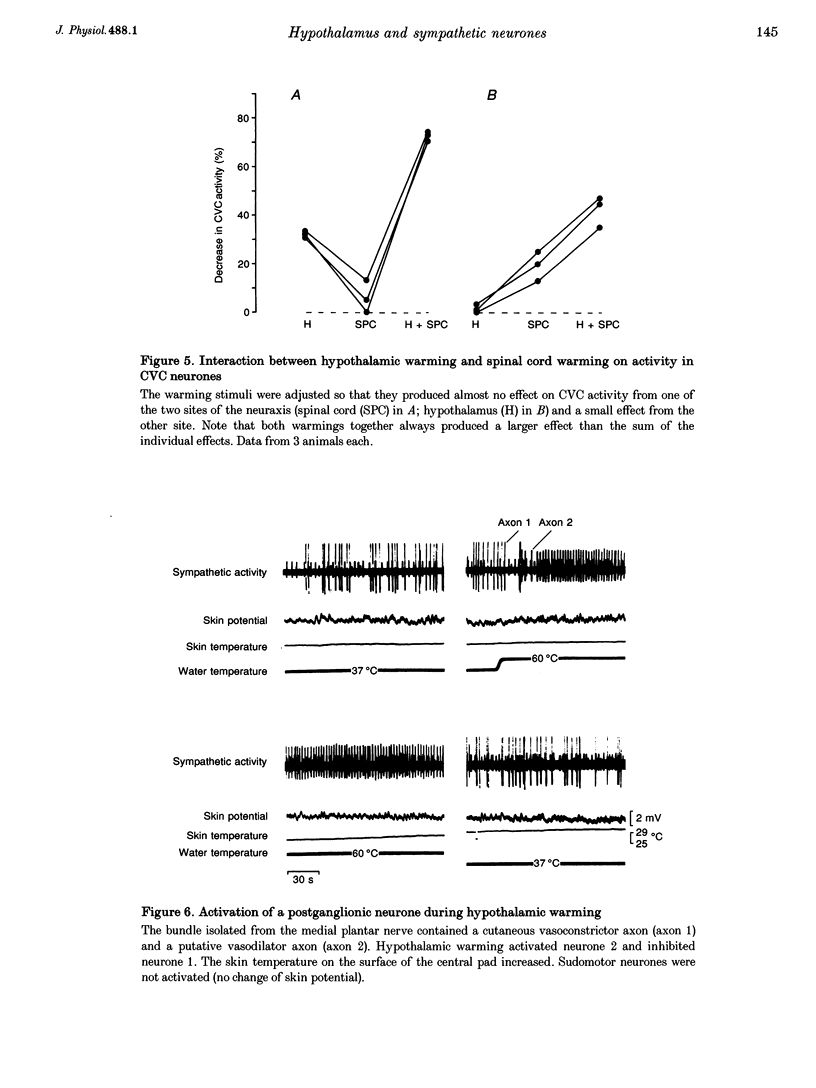
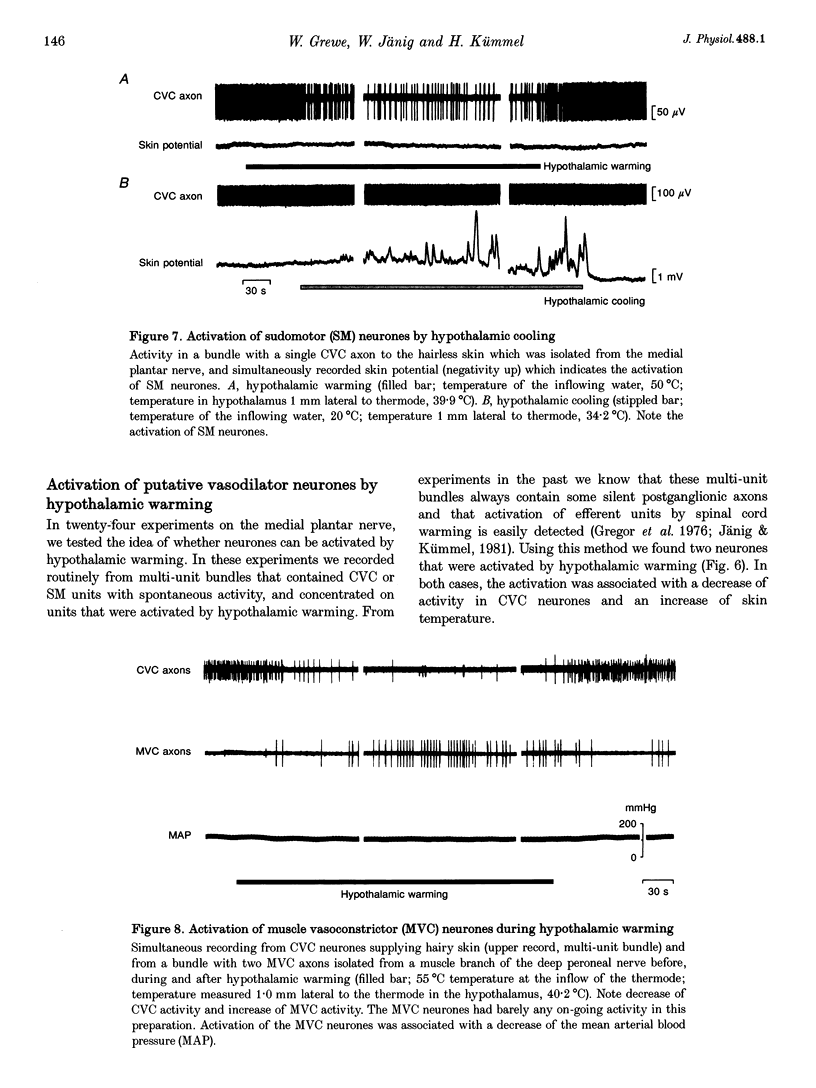
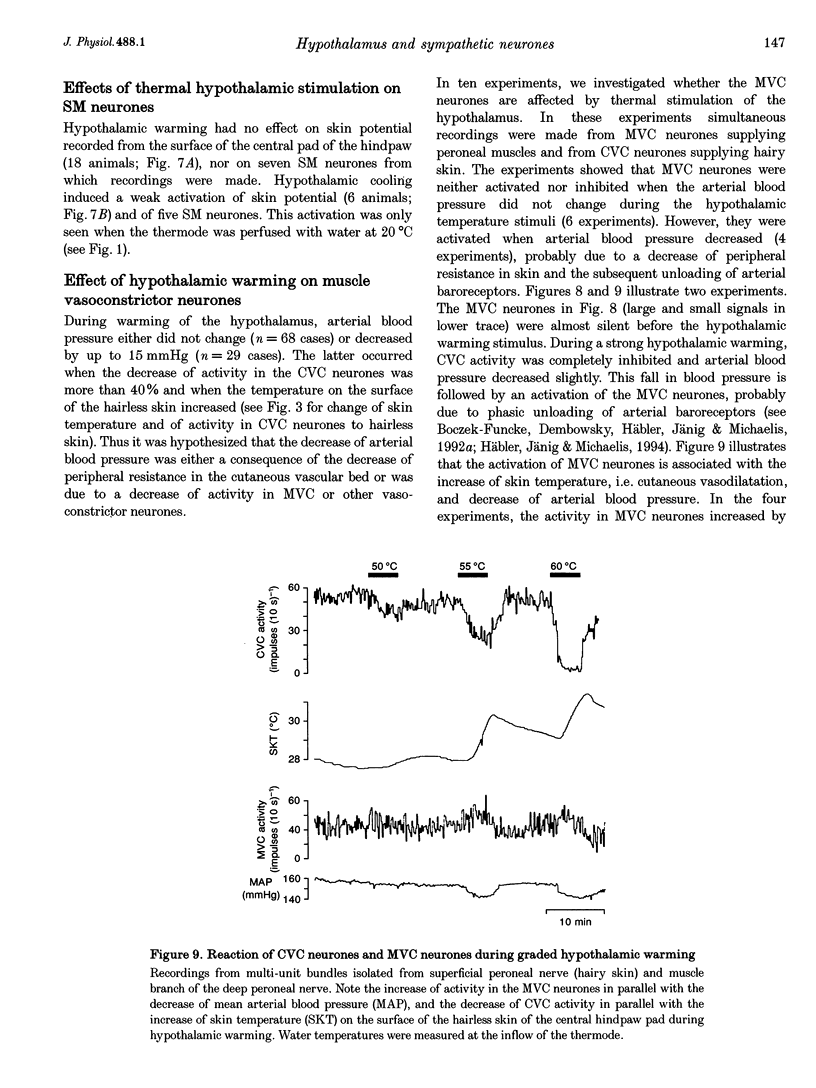
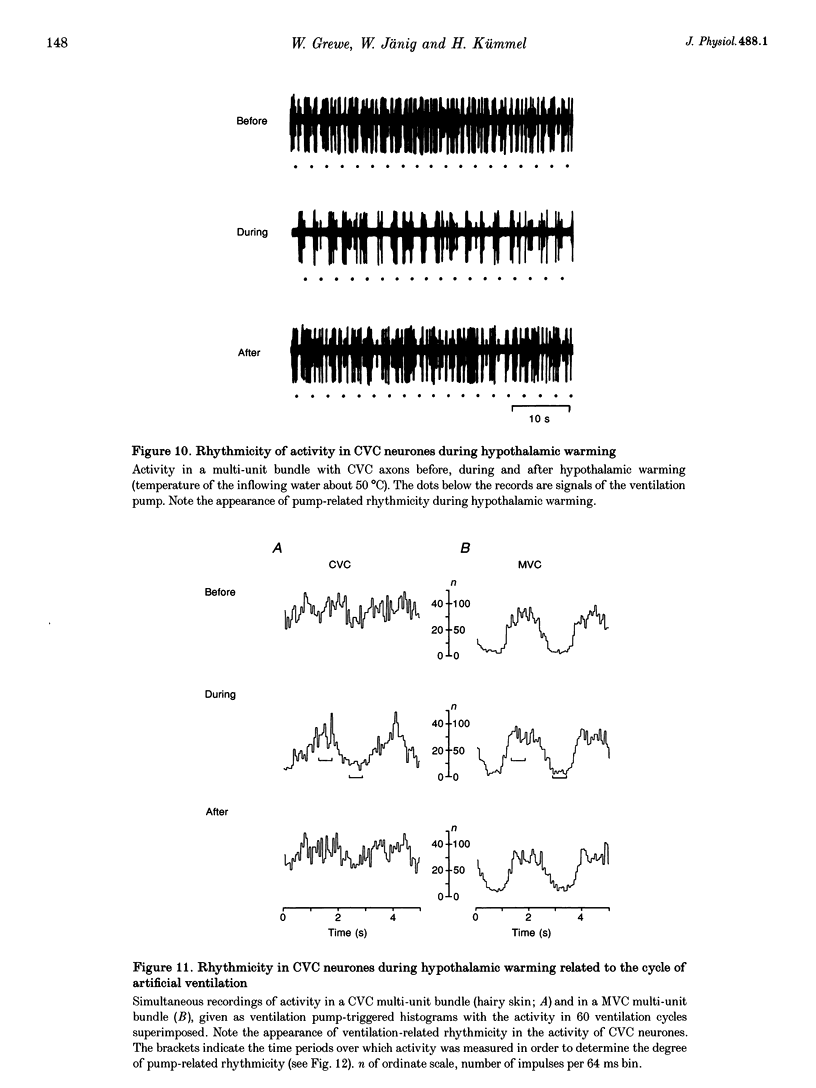
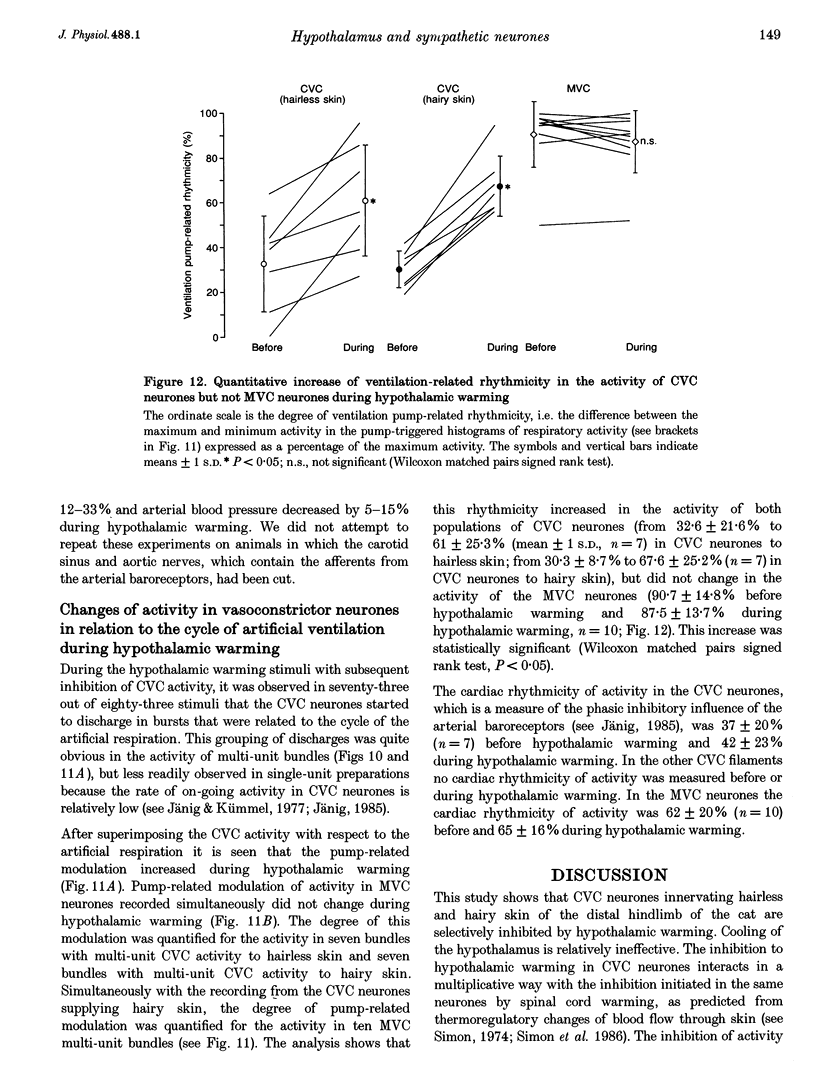
1. Postganglionic neurones supplying hairless and hairy skin of the cat hindlimb were analysed for their responses to thermal stimuli applied to the anterior hypothalamus and spinal cord in anaesthetized and artificially ventilated cats. Activity was recorded from multi- and single-unit bundles which were isolated from peripheral nerves. The neurones were functionally identified as cutaneous vasoconstrictor (CVC) and muscle vasoconstrictor (MVC) neurones. Activity in sudomotor (SM) neurones was either monitored indirectly by recording the phasic negative deflections of the skin potential from the surface of the hairless skin, or in some experiments additionally by recording activity directly from the SM axons. 2. The activity in forty-one out of forty-four multi-unit and six out of six single-unit CVC bundles was inhibited, in a graded manner, by hypothalamic warming. An increase in the temperature of the surface of hairless skin followed the decrease in activity of the CVC neurones supplying it. Large changes in skin temperature only followed decreases in CVC activity of more than 40%. Cooling of the hypothalamus had only weak transient effects on CVC neurones. 3. Simultaneous warming of hypothalamus and spinal cord had multiplicative effects on the activity in CVC neurones. Subthreshold warming of one structure increased the response to warming of the other one and reduced the threshold temperature. 4. SM neurones were not affected by hypothalamic warming, but activated during hypothalamic cooling. 5. MVC neurones were weakly activated during hypothalamic warming only if arterial blood pressure decreased, otherwise they were unaffected. It is likely that this activation was due to secondary unloading of arterial baroreceptors. 6. Two silent postganglionic neurones projecting to skin were activated during hypothalamic warming. These neurones may have had a vasodilatory function. 7. Rhythmicity of the activity in CVC neurones, related to the cycle of artificial ventilation, increased during hypothalamic warming whereas that of MVC neurones was unchanged. 8. The functionally highly specific responses to hypothalamic warming in CVC neurones indicate a pathway from the hypothalamus that is specific for CVC neurones, in contrast to MVC and SM neurones. This central pathway is integrated with other spinal and supraspinal reflex pathways that determine the characteristic reflex pattern of CVC neurones to somatic and visceral stimuli and possibly with pathways that generate other physiological changes during hypothalamic warming (e.g. increase in respiratory drive).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C., Jänig W., Kümmel H., Xu H. Differentiation of vasodilator and sudomotor responses in the cat paw pad to preganglionic sympathetic stimulation. J Physiol. 1985 Jul;364:93–104. doi: 10.1113/jphysiol.1985.sp015732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh J. The thermosensitivity of the hypothalamus and thermoregulation in mammals. Biol Rev Camb Philos Soc. 1966 Aug;41(3):317–368. doi: 10.1111/j.1469-185x.1966.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A., Dembowsky K., Häbler H. J., Jänig W., Michaelis M. Respiratory-related activity patterns in preganglionic neurones projecting into the cat cervical sympathetic trunk. J Physiol. 1992 Nov;457:277–296. doi: 10.1113/jphysiol.1992.sp019378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek-Funcke A., Häbler H. J., Jänig W., Michaelis M. Respiratory modulation of the activity in sympathetic neurones supplying muscle, skin and pelvic organs in the cat. J Physiol. 1992 Apr;449:333–361. doi: 10.1113/jphysiol.1992.sp019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanac M. Temperature regulation. Annu Rev Physiol. 1975;37:415–439. doi: 10.1146/annurev.ph.37.030175.002215. [DOI] [PubMed] [Google Scholar]
- Chai C. Y., Lin M. T. Effects of heating and cooling the spinal cord and medulla oblongata on thermoregulation in monkeys. J Physiol. 1972 Sep;225(2):297–308. doi: 10.1113/jphysiol.1972.sp009941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai C. Y., Lin M. T. Effects of thermal stimulation of medulla oblongata and spinal cord on decerebrate rabbits. J Physiol. 1973 Oct;234(2):409–419. doi: 10.1113/jphysiol.1973.sp010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney R. A., McAllen R. M. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol. 1988 Jan;395:41–56. doi: 10.1113/jphysiol.1988.sp016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J., Heath J. E. Integration and central processing in temperature regulation. Annu Rev Physiol. 1986;48:595–612. doi: 10.1146/annurev.ph.48.030186.003115. [DOI] [PubMed] [Google Scholar]
- Gregor M., Jänig W., Riedel W. Response pattern of cutaneous postganglionic neurones to the hindlimb on spinal cord heating and cooling in the cat. Pflugers Arch. 1976 May 12;363(2):135–140. doi: 10.1007/BF01062281. [DOI] [PubMed] [Google Scholar]
- Guyenet P. G., Darnall R. A., Riley T. A. Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Physiol. 1990 Nov;259(5 Pt 2):R1063–R1074. doi: 10.1152/ajpregu.1990.259.5.R1063. [DOI] [PubMed] [Google Scholar]
- Haselton J. R., Guyenet P. G. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol. 1989 Mar;256(3 Pt 2):R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Häbler H. J., Jänig W., Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol. 1994 Aug;43(6):567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Iriki M., Riedel W., Simon E. Regional differentiation of sympathetic activity during hypothalamic heating and cooling in anesthetized rabbits. Pflugers Arch. 1971;328(4):320–331. doi: 10.1007/BF00586834. [DOI] [PubMed] [Google Scholar]
- Jänig W., Kümmel H. Functional discrimination of postganglionic neurones to the cat's hindpaw with respect to the skin potentials recorded from the hairless skin. Pflugers Arch. 1977 Nov 23;371(3):217–225. doi: 10.1007/BF00586261. [DOI] [PubMed] [Google Scholar]
- Jänig W., Kümmel H. Organization of the sympathetic innervation supplying the hairless skin of the cat's paw. J Auton Nerv Syst. 1981 Apr;3(2-4):215–230. doi: 10.1016/0165-1838(81)90064-3. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Jänig W., Räth B. Electrodermal reflexes in the cat's paws elicited by natural stimulation of skin. Pflugers Arch. 1977 May 6;369(1):27–32. doi: 10.1007/BF00580806. [DOI] [PubMed] [Google Scholar]
- Lipton J. M., Dwyer P. E., Fossler D. E. Effects of brainstem lesions on temperature regulation in hot and cold environments. Am J Physiol. 1974 Jun;226(6):1356–1365. doi: 10.1152/ajplegacy.1974.226.6.1356. [DOI] [PubMed] [Google Scholar]
- Lipton J. M. Thermosensitivity of medulla oblongata in control of body temperature. Am J Physiol. 1973 Apr;224(4):890–897. doi: 10.1152/ajplegacy.1973.224.4.890. [DOI] [PubMed] [Google Scholar]
- McAllen R. M. Central respiratory modulation of subretrofacial bulbospinal neurones in the cat. J Physiol. 1987 Jul;388:533–545. doi: 10.1113/jphysiol.1987.sp016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M., May C. N. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol. 1994 Oct;267(4 Pt 2):R935–R944. doi: 10.1152/ajpregu.1994.267.4.R935. [DOI] [PubMed] [Google Scholar]
- McAllen R. M., May C. N. Effects of preoptic warming on subretrofacial and cutaneous vasoconstrictor neurons in anaesthetized cats. J Physiol. 1994 Dec 15;481(Pt 3):719–730. doi: 10.1113/jphysiol.1994.sp020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Jänig W. The cell bodies of origin of sympathetic and sensory axons in some skin and muscle nerves of the cat hindlimb. J Comp Neurol. 1983 Feb 20;214(2):115–130. doi: 10.1002/cne.902140202. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Boczek-Funcke A., Häbler H. J., Jänig W. Responses of lumbar vasoconstrictor neurons supplying different vascular beds to graded baroreceptor stimuli in the cat. J Auton Nerv Syst. 1993 Mar;42(3):241–249. doi: 10.1016/0165-1838(93)90369-6. [DOI] [PubMed] [Google Scholar]
- Schönung W., Wagner H., Jessen C., Simon E. Differentiation of cutaneous and intestinal blood flow during hypothalamic heating and cooling in anesthetized dogs. Pflugers Arch. 1971;328(2):145–154. doi: 10.1007/BF00592442. [DOI] [PubMed] [Google Scholar]
- Simon E., Iriki M. Sensory transmission of spinal heat and cold sensitivity in ascending spinal neurons. Pflugers Arch. 1971;328(2):103–120. doi: 10.1007/BF00592439. [DOI] [PubMed] [Google Scholar]
- Simon E., Pierau F. K., Taylor D. C. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986 Apr;66(2):235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol. 1974;(71):1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]


