Summary
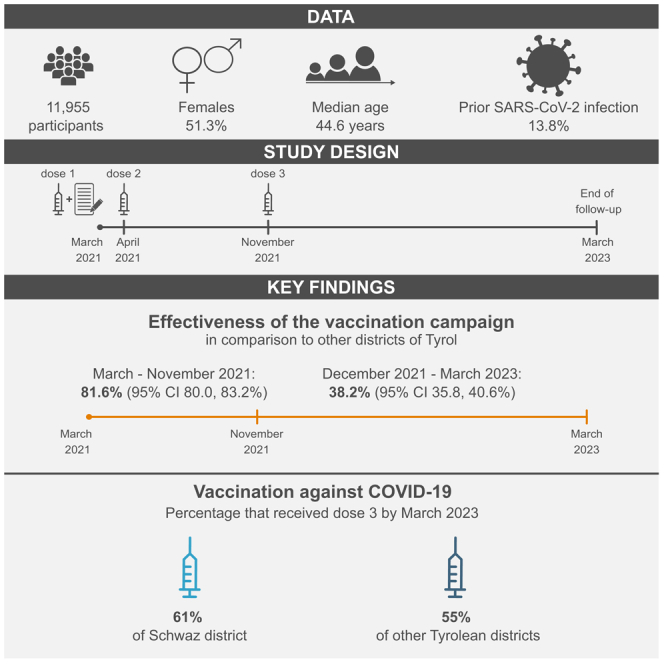
In 2021, an ultra-rapid rollout vaccination campaign in the Schwaz district, Tyrol, Austria, delivered the COVID-19 vaccine BNT162b2 to 66.9% of eligible residents (dose 1: March 11–16, dose 2: April 8–13). Alongside the campaign, we recruited 11,955 residents into the prospective study REDUCE, of whom 3,859 participated in a booster vaccination initiative (November 20–28, 2021). Over a 24-month follow-up, 1,672 participants had incident RT-PCR-confirmed SARS-CoV-2. Compared to other Tyrolean districts, effectiveness in reducing SARS-CoV-2 infection at months 1–9 versus months 10–24 was 81.6% (95% CI 80.0–83.2%; hazard ratio 0.18 [0.17–0.20]) versus 38.2% (35.8–40.6%; 0.62 [0.59–0.64]) among REDUCE participants, and 22.5% (20.5–24.4%; 0.78 [0.76–0.80]) versus 17.0% (16.2–17.8%; 0.83 [0.82–0.84]) in the entire Schwaz district, with substantial variability during follow-up. By March 2023, 61% of Schwaz residents had received booster vaccination versus 55% in other Tyrolean districts. Consequently, vaccinating individuals at high pace effectively reduced SARS-CoV-2 infections and achieved higher vaccination coverage.
Subject areas: Health sciences, Virology, Public health
Graphical abstract
Highlights
-
•
We report 2-year follow-up findings of an ultra-rapid rollout vaccination campaign
-
•
11,955 Schwaz district residents, Tyrol, Austria were enrolled in the REDUCE study
-
•
Effectiveness in reducing incident SARS-CoV-2 infection was 45.5% over two years
-
•
After two years, 61% of Schwaz district residents had received booster vaccination
Health sciences; Virology; Public health
Introduction
The outbreak of the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) and the resulting coronavirus disease 2019 (COVID-19) pandemic in 2020 had a major impact on public health and economy. By May 2023, when the World Health Organization no longer constituted COVID-19 as “a public health emergency of international concern”,1 over 760 million cases of SARS-CoV-2 had been documented worldwide.2 Consequently, the majority of individuals developed an immune response against SARS-CoV-2 either acquired through infection, vaccination, or both. For instance, in the Federal State of Tyrol in Austria, 95.8% of blood donors were seropositive for Spike receptor-binding domain IgG antibodies against SARS-CoV-2 (anti-S IgG) by September 2022, albeit at differing concentrations.3
Vaccines have been shown to effectively reduce the risk of SARS-CoV-2 infection and COVID-19.4,5,6 For instance, between July and November 2020, i.e., during a time when the SARS-CoV-2 wild type variant was dominant, two doses of BNT162b2 were 95% (95% credible interval 90.3–97.6%) efficacious in preventing COVID-19 over a median follow-up of two months.4 Because protection against SARS-CoV-2 infection wanes over time, especially with newly emerging viral variants,7,8 additional booster doses have been recommended, and a third dose of BNT162b2 has been shown to be 95.3% (95% confidence interval [CI] 89.5–98.3%) effective in reducing the risk of COVID-19 during a median follow-up of 2.5 months9 and was 50% (95% CI 4–74%) effective in reducing documented COVID-19 at 16–20 weeks after vaccine administration.10 Long-term effectiveness of a booster dose of BNT162b2 versus a two-dose regimen during a time, when mainly Omicron sub-variants of SARS-CoV-2 were dominant, was 30.4% (95% CI 27.5–33.3%) over a median follow-up of 203 days, although it waned over time.11
As of September 2023, 13.4 billion vaccine doses had been administered worldwide.2 In order to provide vaccination to the public, vaccination campaigns have been conducted around the globe.12,13,14,15,16,17 In March 2021, an ultra-rapid rollout vaccination campaign was initiated in the district of Schwaz in the Federal State of Tyrol in Austria in order to counteract the large number of SARS-CoV-2 infections with the Beta and Alpha + E484K sub-variants.17 This initiative stands out by providing vaccination to over 40,000 people within only one week, corresponding to 66.9% of the eligible population.17 We have previously shown in the accompanied REDUCE study, including 11,955 individuals, that the vaccination campaign was 91.1% (95% CI 89.6–92.3%) effective in reducing incident SARS-CoV-2 infections over six months upon receipt of two BNT162b2 doses when comparing participants from the REDUCE study to individuals from other districts of Tyrol.17 The extent to which vaccination with booster doses also translates into reduced incidence of SARS-CoV-2 infections over a long-term follow-up is, however, unclear.
In November 2021, residents of the district of Schwaz were offered to receive booster doses of BNT162b2 as part of the previously conducted vaccination campaign.17 We recruited REDUCE study participants into a two-year follow-up prospective cohort study to assess the long-lasting impact of the vaccination campaign. Our specific aims were 3-fold: (1) to estimate the effectiveness of the vaccination campaign in reducing incidence of SARS-CoV-2 in REDUCE study participants and the district of Schwaz compared to other districts in the Federal State of Tyrol, (2) to assess vaccination coverages achieved in the district of Schwaz compared to other districts in Tyrol, and (3) to identify factors associated with a two-year risk of breakthrough infection, i.e., acquiring a SARS-CoV-2 infection.
Results
Study population, participant characteristics, and SARS-CoV-2 incidence
Our analyses included 11,955 REDUCE study participants. During the initial six-month follow-up period, four participants died from causes unrelated to COVID-19, two individuals withdrew from the study, and 215 participants received the second dose of BNT162b2 outside 19–42 days after the first dose. Of the 11,734 participants that completed the six-month follow-up, we were able to recruit 3,862 during the booster vaccination campaign in the district of Schwaz. Of those, we censored three participants after six months, as they did not receive the third dose of BNT162b2 during the vaccination initiative after aligning their vaccination dates with information from the national electronic vaccination registry. Moreover, five additional participants died during the two-year follow-up period. Consequently, 3,854 participants completed the two-year follow-up.
An overview of the participant characteristics at time of enrollment is provided in Table S1. Median age was 44.6 (IQR 32.2–55.8) years, 51.3% were female, and 13.8% had a prior SARS-CoV-2 infection. Of the individuals with long-term follow-up data, 56.8% were living in households of three or more persons as compared to 53.2% of those without long-term follow-up data (p < 0.001). They also differed by occupation and by highest education (both p < 0.001). Also, individuals who did not participate in the long-term follow-up were less likely to have chronic lung disease (p = 0.002).
Vaccination coverage with at least three doses was higher in the district of Schwaz
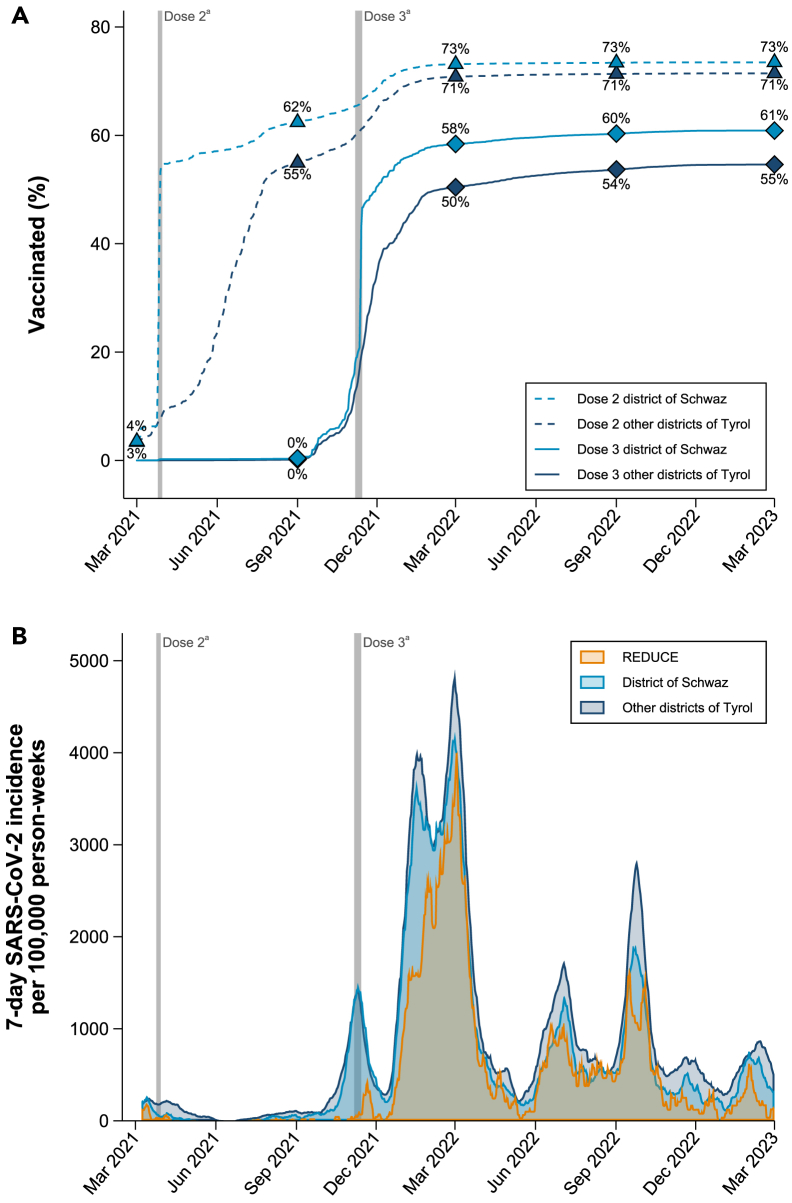
As demonstrated in Figure 1A, over the entire follow-up period, the proportion of individuals vaccinated with at least three doses was persistently higher in the district of Schwaz compared to all other districts of Tyrol combined (see Figure S1 for district-specific vaccination coverage). While the proportion of individuals vaccinated with at least three doses was comparable before the booster vaccination campaign in November 2021, after the campaign, the proportion was substantially higher in the district of Schwaz than in other districts of Tyrol. For instance, in March 2022, 58% of residents of the district of Schwaz were vaccinated with at least three doses versus 50% in other districts of Tyrol. This trend lasted until the end of the two-year follow-up, when 61% had received at least three doses of vaccination in the district of Schwaz as compared to 55% in the other districts of Tyrol. Percentages of individuals vaccinated with four doses are provided in Figure S2. By the end of follow-up, 14.1% of the participants of the REDUCE study, 13.2% of individuals living in the district of Schwaz, and 14.4% of individuals living in other districts of Tyrol had received four doses of vaccination against COVID-19.
Figure 1.
Percentage vaccinated against COVID-19 and incidence rate of SARS-CoV-2 infections
(A) This panel shows the percentage of people that had received two doses vs. three doses of vaccination against COVID-19 in the district of Schwaz and other districts of Tyrol.
(B) This panel shows the 7-day SARS-CoV-2 incidence per 100,000 person-weeks in the REDUCE study and the district of Schwaz compared to other districts of Tyrol. aPeriod in which BNT162b2 dose 2 (April 8-13, 2021) and dose 3 (November 20-28, 2021) were administered within the REDUCE study. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
The vaccination campaign was effective in the REDUCE study and the district of Schwaz
Over 532,005 person-weeks of follow-up, an incident SARS-CoV-2 infection was recorded in 1,672 individuals (Table S2). The majority (90.2%) of incident infections were symptomatic with 83.7% of infections being accompanied by respiratory symptoms. Over the entire follow-up, the incidence rate was 314.3 (95% CI 299.6–329.7) per 100,000 person-weeks and was the lowest between June and September 2021 and the highest between December 2021 and March 2022. SARS-CoV-2 incidence rates in the REDUCE study, the district of Schwaz, and other districts of Tyrol are depicted in Figure 1B.
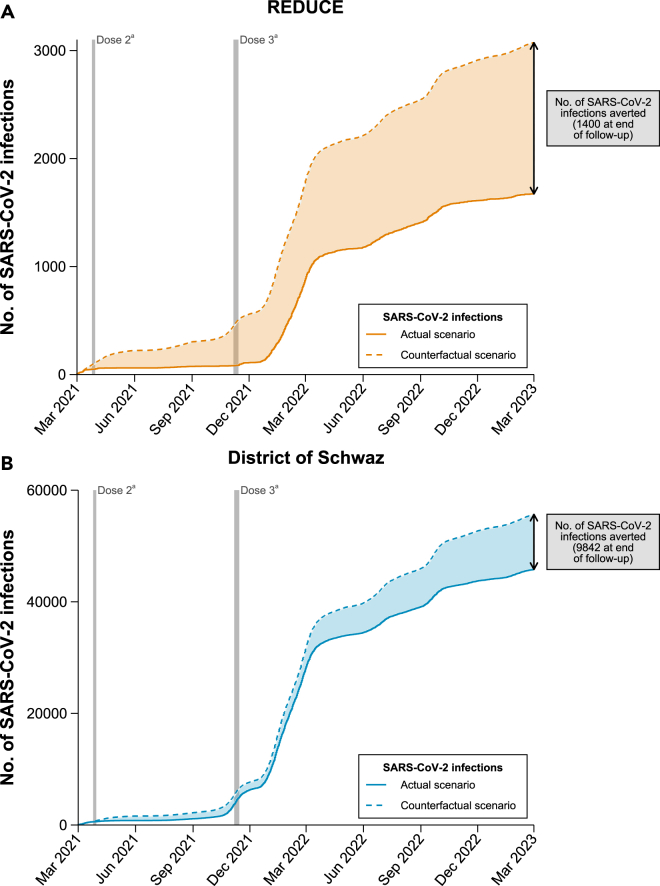
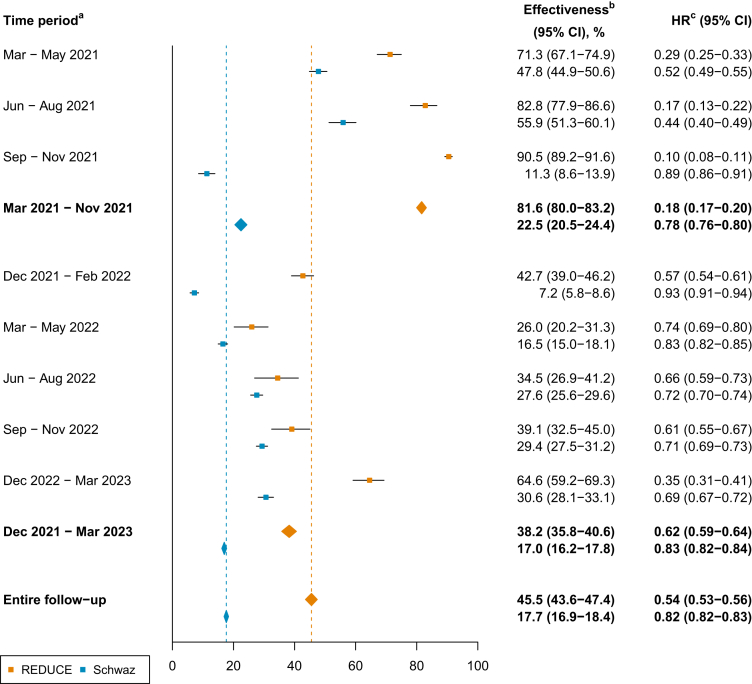
Cumulative incidence plots for the REDUCE study, the district of Schwaz, and all other districts of Tyrol combined are depicted in Figure S3. District-specific cumulative incidences are provided in Figure S4. As depicted in Figure 2, we estimated that after the implementation of the vaccination initiative, 1,400 SARS-CoV-2 infections had been averted in the REDUCE study population and 9,842 SARS-CoV-2 infections in the whole district of Schwaz by the end of the study period, in case the daily incidence rate would have behaved similarly to other districts of Tyrol. In addition, as shown in Figure 3, compared to other districts of Tyrol, the booster vaccination campaign was significantly effective in reducing the risk of incident SARS-CoV-2 infections. Over the entire follow-up period, effectiveness of the vaccination campaign was 45.5% (95% CI 43.6–47.4%) in the REDUCE study and 17.7% (16.9–18.4%) in the district of Schwaz, corresponding to hazard ratios of 0.54 (95% CI 0.53–0.56) and 0.82 (0.82–0.83), respectively. Effectiveness estimates varied over time and were 81.6% (80.0–83.2%; hazard ratio 0.18 [95% CI 0.17–0.20]) in the REDUCE study and 22.5% (20.5–24.4%; 0.78 [0.76–0.80]) in the district of Schwaz between March 2021 and November 2021, before the booster initiative took place. After the booster initiative, between December 2021 and March 2023, effectiveness estimates were 38.2% (35.8–40.6%; 0.62 [0.59–0.64]) and 17.0% (16.2–17.8%; 0.83 [0.82–0.84]) in the REDUCE study and the district of Schwaz, respectively. Results remained broadly similar when including recurrent SARS-CoV-2 infections as shown in Figures S5 and S6.
Figure 2.
Estimated number of SARS-CoV-2 infections averted
(A) This panel shows the estimated number of SARS-CoV-2 infections averted among participants of the REDUCE study.
(B) This panel shows the estimated number of SARS-CoV-2 infections averted in the district of Schwaz. The counterfactual scenario assumes that numbers of SARS-CoV-2 infections would have been the same as in other districts of Tyrol. aPeriod in which BNT162b2 dose 2 (April 8-13, 2021) and dose 3 (November 20-28, 2021) were administered within the REDUCE study. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Figure 3.
Effectiveness of the booster vaccination campaign in the REDUCE study and the district of Schwaz compared to other districts of Tyrol
Data are represented as effectiveness, defined as one minus the corresponding hazard ratio, and 95% confidence interval. aIn general, time periods start at the first day of the month first mentioned and last until the last day of the month second mentioned, except for the first period, March–May 2021, which started on March 15, and the last period, December 2022 – March 2023, which ended on March 15. bEffectiveness of the vaccination campaign as compared to other districts of Tyrol. cHazard ratio for incident SARS-CoV-2 infection as compared to other districts of Tyrol. Abbreviations: CI, confidence interval; HR, hazard ratio.
Several participant characteristics were related to the risk for incident SARS-CoV-2 infections
Hazard ratios for incidence of SARS-CoV-2 across several participant characteristics are provided in Table 1. Individuals who were female, never/former smokers, currently employed, and those without a previous SARS-CoV-2 infection had a statistically significantly higher risk of incident SARS-CoV-2 over the entire follow-up period. In addition, between baseline and May 2022, individuals at younger age were at higher risk for incident SARS-CoV-2. When splitting the follow-up period at December 1, 2021 (after all participants received the booster dose), we found a significantly higher risk for incident SARS-CoV-2 infections in individuals living in larger households and in those with chronic lung disease during the first period. Additionally, during the second period, individuals who were female, never/former smokers, currently employed, and those without a previous SARS-CoV-2 infection were at higher risk for incident SARS-CoV-2. Also, between December 2021 and May 2022 individuals at younger age were at higher risk for incident SARS-CoV-2. We did not find a statistically significant association between receipt of a fourth dose during follow-up and the risk to develop incident SARS-CoV-2 (hazard ratio 1.21 [95% CI 0.79–1.85]; p = 0.389).
Table 1.
Hazard ratios for incidence of SARS-CoV-2 across participant characteristics including 11,154 participants of the REDUCE study with complete data on all characteristics
| Characteristic | Entire follow-upa |
Period 1: until November 2021b |
Period 2: from December 2021b |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P- value | HR (95% CI) | P- value | HR (95% CI) | P- value | |
| Age - per 10 years younger | ||||||
| March 2021 – May 2022c | 1.20 (1.15–1.26) | <0.001 | 1.08 (0.91–1.28) | 0.357 | 1.21 (1.16–1.27) | <0.001 |
| June 2022 – March 2023d | 0.91 (0.85–0.97) | 0.006 | – | – | 0.92 (0.86–0.98) | 0.012 |
| Female sex | 1.20 (1.08–1.33) | <0.001 | 0.95 (0.61–1.46) | 0.808 | 1.22 (1.10–1.35) | <0.001 |
| ≥3 persons in household | 1.13 (1.02–1.26) | 0.017 | 2.69 (1.62–4.46) | <0.001 | 1.08 (0.97–1.20) | 0.148 |
| Overweight/obese | 1.03 (0.93–1.14) | 0.561 | 1.31 (0.84–2.03) | 0.227 | 1.02 (0.91–1.13) | 0.784 |
| Current smoker | 0.73 (0.65–0.83) | <0.001 | 0.78 (0.46–1.31) | 0.345 | 0.73 (0.65–0.83) | <0.001 |
| Currently employed | 1.22 (1.08–1.38) | 0.002 | 1.32 (0.77–2.27) | 0.309 | 1.21 (1.07–1.37) | 0.003 |
| High educatione | 1.14 (1.03–1.26) | 0.014 | 1.39 (0.89–2.15) | 0.144 | 1.12 (1.01–1.25) | 0.032 |
| Prior SARS-CoV-2 infection | 0.43 (0.35–0.51) | <0.001 | 0.19 (0.06–0.61) | 0.005 | 0.44 (0.36–0.53) | <0.001 |
| Other pre-existing conditions | ||||||
| Cardiovascular disease | 1.20 (1.01–1.42) | 0.036 | 0.82 (0.37–1.82) | 0.630 | 1.22 (1.03–1.46) | 0.024 |
| Diabetes mellitus | 0.79 (0.53–1.18) | 0.253 | 3.40 (1.29–8.92) | 0.013 | 0.67 (0.43–1.03) | 0.069 |
| Chronic lung disease | 1.26 (0.98–1.63) | 0.072 | 3.21 (1.60–6.47) | 0.001 | 1.14 (0.87–1.50) | 0.346 |
| Cancer | 0.93 (0.69–1.25) | 0.649 | 0.82 (0.20–3.37) | 0.778 | 0.95 (0.70–1.28) | 0.716 |
| Renal disease | 1.22 (0.78–1.91) | 0.392 | 0.97 (0.13–7.23) | 0.979 | 1.25 (0.79–1.98) | 0.345 |
| Liver disease | 1.26 (0.71–2.25) | 0.429 | 1.76 (0.24–12.90) | 0.579 | 1.25 (0.68–2.28) | 0.476 |
| Intake of immunosuppressants | 1.10 (0.68–1.77) | 0.706 | 1.04 (0.14–7.57) | 0.966 | 1.09 (0.67–1.79) | 0.732 |
Analyses included participants with complete data on all characteristics (for more details see Table S1).
The Cox regression model was adjusted for all characteristics mentioned in the table and an interaction term of age with time split at June 1, 2022.
The Cox regression model was adjusted for all characteristics mentioned in the table, an interaction term of age with time split at June 1, 2022, and interaction terms of all of these variables with time split at December 1, 2021.
This period starts at March 15, 2021 and ends at May 31, 2022.
This period starts at June 1, 2022 and ends at March 15, 2023.
High education was defined as vocational school, Advanced level, or university diploma. Abbreviations: CI, confidence interval; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2. After correcting for multiple testing, p-values<0.003 (0.05/16 covariates) are deemed statistically significant, which are marked in bold.
Discussion
In this study, we demonstrate that an ultra-rapid rollout vaccination campaign conducted in March 2021, in the district of Schwaz in the Federal State of Tyrol, Austria, was effective in reducing the general risk to develop SARS-CoV-2 infections over a long-term follow-up of two years. In addition, the vaccination campaign resulted in a sustained higher vaccination coverage in the Schwaz district compared to other districts of Tyrol.
Several previously conducted vaccination campaigns have been demonstrated to effectively lower incidence of SARS-CoV-2.13,18,19,20 Mass vaccination campaigns were conducted at different pace across countries, with an average of 64, 54, and 51 days to reach 5 doses per 100 people in Gulf Cooperation Council, the G7 countries, and Organization for Economic Cooperation and Development countries, respectively.21 Accelerated vaccination rollout was suggested to have a beneficial effect on SARS-CoV-2 incidence and on the management of the pandemic. For instance, an investigation of the COVID-19 vaccination program in Brazil demonstrated that providing vaccination faster could have prevented more than 100,000 hospitalisations and almost 50,000 deaths.22 The mass vaccination in Israel was particularly fast, reaching 50 doses per 100 people in only 39 days,21 and its first nationwide vaccination program with BNT162b2, initiated in late 2020, was largely effective in reducing SARS-CoV-2 infections.13,15 Israel was also the first country that implemented a nationwide booster vaccination campaign,23 which decreased the number of SARS-CoV-2 infections by an estimated 80% between July and November 2021.24 The vaccination initiative in the district of Schwaz, in which the REDUCE study was embedded, stands out by administering vaccination at a highly accelerated pace (during the initiative, dose 1 and 2 were each administered within seven days and dose 3 within ten days). As we have shown previously, this led to an effectiveness in reducing incident SARS-CoV-2 infections by >90% in the REDUCE study population and >60% in the whole district of Schwaz during the initial six months after receiving the first dose of BNT162b2, when compared to other districts of Tyrol.17 In the present study, we now show that accelerated vaccination rollout had a sustained effect on the incidence of SARS-CoV-2 infections. By the end of the two-year follow-up, between December 2022 and March 2023, the effectiveness of the vaccination campaign was still significant, with 65% in the REDUCE study and 31% in the district of Schwaz compared to other districts of Tyrol.
The underlying reasons for a long-term effectiveness could be multifaceted. The fact that the percentage of individuals vaccinated with three doses was substantially higher in the district of Schwaz compared to other districts of Tyrol throughout the follow-up period may play a major role. By March 2023, vaccination coverage with at least three doses was 6% higher in the district of Schwaz compared to other districts of Tyrol. Among participants of the BNT162b2 phase II/III clinical trial, a third dose of BNT262b2 resulted in an efficacy of 95.3% (95% CI 89.5–98.3%) over 2.5 months in reducing the risk of incident SARS-CoV-2 compared to two doses of BNT162b2.9 Vaccine effectiveness against documented SARS-CoV-2 infections has also been shown to be high in real-world studies but is prone to waning over time.10 Notably, a feature of our study distinguishing it from previous effectiveness studies is that it does not compare individuals with a third dose of BNT162b2 to individuals with less or without vaccination, but compares individuals embedded into the vaccination campaign and in the district of Schwaz to individuals living in other districts of Tyrol who have a different vaccination behavior (including individuals without vaccination and with different numbers of vaccination doses). However, we cannot certainly conclude that the booster vaccination alone was responsible for the effectiveness of the vaccination campaign. Although the percentage of individuals vaccinated with at least three doses was higher in the district of Schwaz as compared to other districts of Tyrol, the difference in these percentages decreased over time while effectiveness of the vaccination initiative tended to increase over time. Furthermore, it is unlikely that an additional booster dose contributed to the long-term effectiveness of the vaccination initiative, as the percentage of individuals vaccinated with four doses was similar in the REDUCE study, the district of Schwaz, and the other districts of Tyrol. Therefore, the specific reason for a higher effectiveness of the vaccination campaign toward the end of the study is not entirely clear and could be multifaceted. First, between study baseline and February 2022, numerous non-pharmaceutical interventions against the spread of SARS-CoV-2 were implemented in Austria. It has been demonstrated that non-pharmaceutical interventions alone and in combination with vaccination have an important effect on reducing SARS-CoV-2 transmission.25 Moreover, seasonal trends have been demonstrated for SARS-CoV-2 with accelerated activity during winter months in Europe and the United States.26 In Austria, the majority of non-pharmaceutical interventions against SARS-CoV-2 were lifted by March 2022. Consequently, the winter months in 2022/2023 were the first ones without active non-pharmaceutical interventions against SARS-CoV-2. A combination of higher SARS-CoV-2 activity, absence of non-pharmaceutical interventions, and lower vaccination coverage by the end of the two-year follow-up period could have introduced higher incidence of SARS-CoV-2 infections in other districts of Tyrol. Second, although effectiveness of a third dose of BNT162b2 wanes over time,10 waning is slower after three doses as compared to two doses,27 and a third dose resulted in a higher antibody response.28,29 For instance, among blood donors from the Federal State of Tyrol, in September-December 2022, geometric mean levels of anti-S IgG antibodies were 2,196 Binding Antibody Units (BAU)/mL (95% CI 2,133–2,261) in individuals who were vaccinated with a booster dose and 1,063 BAU/mL (1,007–1,121) in vaccinated individuals without a booster dose.30 In addition, between March 2021 and December 2022 (i.e., during a time that is similar to our study period), twice the level of anti-S IgG has been shown to be related to a 12.5% lower risk of incident SARS-CoV-2 infection, and this inverse association remained even after >21 months of follow-up.31 Third, during the two-year follow-up period, the COVID-19 pandemic was characterized by different dominant SARS-CoV-2 variants. While Alpha was the predominant variant in Austria until June 2021, Delta dominated between June and December 2021,32 and different Omicron sub-variants dominated since December 2021.33 As the effectiveness of BNT162b2 has been demonstrated to vary across variants,34,35 this could have introduced time varying effectiveness of the vaccination campaign. Fourth, it is possible that a specific group of individuals were recruited into the REDUCE study, whose awareness for SARS-CoV-2 infections could be higher as compared to individuals that did not participate in the study. Consequently, study participants may have a more careful behavior and protect themselves more cautiously against SARS-CoV-2 infections. Conversely, participating in the REDUCE study may have affected the behavior of individuals, which is known as the so-called Hawthorn effect.36 Finally, the situation regarding vaccine availability changed during the two-year follow-up period. While the first phase of the REDUCE study was conducted during a time of vaccine scarcity, vaccines were broadly available during the second phase of the study. This may have influenced the composition of our study sample. For instance, individuals who had received a third vaccine dose before the start of the booster vaccination campaign would not qualify for inclusion into the long-term follow-up study.
Our study did not show a significant association with a fourth dose of vaccination against COVID-19 and the risk of SARS-CoV-2 infections. A second booster dose has shown to effectively lower risk for hospitalization and death due to COVID-19 in individuals ≥60 years of age.37 However, in a study among health care workers, vaccine effectiveness of four versus three doses of BNT162b2 has been reported to wane over time with no statistically significant effectiveness 15–26 weeks after receipt of the fourth dose against SARS-CoV-2 infection (−2% [95% CI −27 to 17%]).38 Moreover, a nationwide study conducted in Austria including approximately four million previously infected individuals compared individuals with four vaccine doses against COVID-19 with those with three vaccine doses (>80% of all vaccine doses are from BNT162b2).39 The study found a significantly lower risk for incident SARS-CoV-2 infections from November 1 until December 1, 2022 (hazard ratio 0.83 [95% CI 0.81, 0.86]) but a significantly higher risk for incident SARS-CoV-2 infections from January 1 until June 30, 2023 (hazard ratio 1.17 [95% CI 1.15, 1.19]).39 This study’s and our results could be affected by several, possibly biasing, factors including a potential higher willingness of testing for SARS-CoV-2 infections of individuals deciding upon taking a fourth dose of vaccination against COVID-19.
In the present study, the risk to develop incident SARS-CoV-2 infections was higher in females as compared to males. Results from the literature regarding sex differences in risk of incident SARS-CoV-2 infections point toward the same direction.40,41 In a study including over 60,000 health care workers from Europe who had received a booster dose of a COVID-19 vaccine, the odds for a SARS-CoV-2 breakthrough infection were significantly higher in females as compared to males.40 Moreover, in a prospective, population-based study in the UK including >10,000 participants who had received a booster dose, the hazard ratio for SARS-CoV-2 infection was 0.86 (95% CI 0.74–1.00) in males as compared to females.41 In addition, in line with previous findings, our study yielded a reduced risk of incident SARS-CoV-2 infection in individuals with a previous infection.40,41 We also found a lower risk for SARS-CoV-2 infections in current versus former or never smokers. This paradox finding has been observed previously.42,43 However, it has been discussed that the negative association between current smoking and risk for SARS-CoV-2 infection is likely to be biased.44,45 A Mendelian randomization analysis, which allows estimating associations independent of confounding, has shown that smoking initiation was causally related to a higher risk of SARS-CoV-2 infection.46 In general, it has to be noted that our study had an observational design, which does not allow drawing any conclusions about causality. Furthermore, participation in our study was voluntary, which may have introduced specific forms of selection bias such as collider bias.45
Our study has several strengths. These include the large sample size and the long-term follow-up of two years. In addition, the successful implementation of this vaccination initiative was only possible because of an intensive and tight collaboration of different stakeholders from universities (e.g., designing and conducting the scientific study) and politics and public health institutions (e.g., organizing the vaccine doses in a situation of high demand, setting up the vaccination centers). As we were able to set this study up with immense speed (within only a week), we believe that such an initiative may also feasible within other settings, given that various stakeholders are acting in concert. Moreover, our study stands out by participants being vaccinated over a very short time frame and according to the same vaccination regimen. Consequently, study participants were exposed to the same occurrence of infection waves and to the same SARS-CoV-2 variants of concern during follow-up. Furthermore, we obtained data on a range of covariates by questionnaire, which allowed assessing the risk of incident SARS-CoV-2 infections across several participant characteristics and adjusting hazard ratios for potential confounders.
Conclusion
This study demonstrates that an ultra-rapid rollout vaccination campaign was effective in reducing risk of incident SARS-CoV-2 infections over a long-term follow-up of two years in the study itself and in the whole district of Schwaz, in which it was conducted. Moreover, vaccination coverage was higher in the district of Schwaz compared to the other districts of Tyrol throughout the follow-up period.
Limitations of the study
Our study also has limitations. Data on SARS-CoV-2 infections were obtained from the central database of the Austrian Agency for Health and Food Safety and is strongly dependent on the testing behavior of the participants. Consequently, incidence of SARS-CoV-2 may have been underestimated. However, we compared SARS-CoV-2 incidence with district-level data, which also depend on the testing behavior of individuals, and still found significant effectiveness in the REDUCE phase 2 study as well as the district of Schwaz compared to other districts of Tyrol. In addition, the majority (90.2%) of SARS-CoV-2 infections in REDUCE study participants were symptomatic. It is likely that asymptomatic SARS-CoV-2 infections were under-ascertained specifically toward the end of the follow-up period of our study. Also, the proportion of asymptomatic infections might have been higher in the district of Schwaz as compared to other districts of Tyrol, as vaccination coverage with three doses was higher. Unfortunately, data on symptomatic infections were only available for REDUCE study participants but not for the whole district of Schwaz or for other districts of Tyrol. This prevented us from obtaining effectiveness estimates for symptomatic SARS-CoV-2 infections. Performing serological analyses would have enabled to assess asymptomatic infections. However, due to the rapid set up and large sample size of our study it was not feasible to perform serological tests.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Prof Peter Willeit (peter.willeit@i-med.ac.at).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
This paper analyzes existing, publicly available data. The websites where these datasets can be accessed are listed in the key resources table. In addition, the paper analyzes participant-level data, which cannot be deposited in a public repository because of data protection regulations.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
The first phase of the study was supported by a grant of the Federal State of Tyrol. We are indebted to the teams of ELGA GmbH and ITSV GmbH for their invaluable work on matching and curating the study participants’ data on COVID-19 vaccination from the national electronic vaccination database.
Author contributions
L.T. and P.W. had unrestricted access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. C.L.F., F.K., S.E.A., H.T., G.W., F.A., and P.W. designed the study. P.W. secured funding. L.T., L.S., S.E.A., and P.W. assisted supervision of participant recruitment. L.T., H.I., L.S., L.W., and P.W. were responsible for data management and cleaning. L.T. and P.W. performed the statistical analysis. L.R. provided information on incident infections. L.F. and S.S. designed the web-based entry of the questionnaire. L.T. and P.W. prepared the first draft of the manuscript and all other authors provided critical input on the manuscript. All authors agreed to submit the manuscript, read and approved the final draft, and take full responsibility of its content, including the accuracy of the data and its statistical analysis.
Declaration of interests
P.W. reports consulting fees from Novartis Pharmaceuticals; outside the submitted work. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines influenza virus vaccines and influenza virus therapeutics which list F.K. as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, CastleVax, to develop SARS-CoV-2 vaccines. F.K.is co-founder and scientific advisory board member of CastleVax. F.K. has consulted for Merck, Curevac, Seqirus, and Pfizer and is currently consulting for 3rd Rock Ventures, GSK, Gritstone, and Avimex. The Krammer laboratory is collaborating with Dynavax on influenza vaccine development.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Open-source data on SARS-CoV-2 infections at district-level. Accessed June 30, 2023. | https://www.data.gv.at/katalog/dataset/covid-19-zeitliche-darstellung-von-daten-zu-covid19-fallen-je-bezirk | N/A |
| Software and algorithms | ||
| Stata | https://www.stata.com/ | Version 15.1 |
| Other | ||
| Data obtained by questionnaire. | This paper. | N/A |
| Data on incident SARS-CoV-2 infections of study participants. | This paper. Obtained from Austrian Agency for Health and Food Safety. | N/A |
| Data on vaccination against COVID-19. | This paper. Obtained from the National Electronic Vaccination database. | N/A |
Experimental model and subject details
Study oversight and reporting
The study was approved by the Ethics Committee of the Medical University of Innsbruck (no 1095/2021). All participants provided written informed consent. Results of this study are reported according to the strengthening the reporting of observational studies in epidemiology (STROBE) statement. The STROBE checklist is provided in Table S3.
Study design and participants
The design of this study has been published previously.17 Briefly, the REDUCE study is an observational prospective cohort study that accompanied the ultra-rapid rollout vaccination campaign in the district of Schwaz in the Federal State of Tyrol, located in the Western part of Austria. Between March 12 and 15, 2021, 11,955 individuals were recruited into the REDUCE study across nine vaccination centers. Individuals were eligible to be included if they (1) received vaccination as part of the vaccination campaign, (2) were 18 years of age or older, (3) were legally competent, and (4) fulfilled the vaccination criteria according to European Medicines Agency approval.47 As part of the study, the participant’s sex was requested by questionnaire and was taken into account in the statistical analyses whenever possible.
Method details
Study setting
During the recruitment into the vaccination campaign, participants received their first dose (30 μg) of the BNT162b2 vaccine by intramuscular injection. According to the vaccination regimen, individuals received a second dose of BNT162b2 between April 8 and 13, 2021 in any of the vaccination centers. During the resting period after the first dose, individuals completed a questionnaire on date of birth, sex, previous SARS-CoV-2 infection, medical history, socioeconomic status, and lifestyle.
Eight months later, the vaccination campaign was extended to offer booster vaccination with BNT162b2 to residents of the district of Schwaz during November 13-28, 2021. REDUCE study participants were recruited at nine centers and received vaccination during November 20-21, 2021 (one center) and November 26-28, 2021 (eight centers). During the resting period after receiving vaccination, individuals affirming that they participated in the first phase of the REDUCE study were asked for written consent to consult their information on incident SARS-CoV-2 infections and vaccination against COVID-19 for two years after their first dose of BNT162b2.
Ascertainment of vaccination and outcomes
We obtained information on vaccination against COVID-19 from the national electronic vaccination database. We verified that participants received the third dose of BNT162b2 during the extension of the REDUCE study. Furthermore, data on additional booster doses were extracted. Incident SARS-CoV-2 infections were obtained from the central database of the Austrian Agency for Health and Food Safety and were based on a confirmed RT-PCR test. SARS-CoV-2 infections were considered symptomatic if individuals reported at least one of the following symptoms: respiratory symptoms, disturbance of taste or smell, diarrhea, pneumonia, or other symptoms.
Quantification and statistical analysis
Summary statistics are reported as numbers and percentages for categorical variables, means and standard deviations (SDs) for normally distributed continuous variables, and medians and interquartile ranges (IQRs) for other continuous variables. We compared participant characteristics between REDUCE study participants who did versus did not participate in the long-term follow-up using a t-test for continuous normally distributed variables, a Mann-Whitney-U-test for continuous non-normally distributed variables, and a Χ2-test for categorical variables.
For all statistical analyses, we define the baseline as March 15, 2021 when all participants had received a first dose of BNT162b2. We split the follow-up time into eight equally spaced periods: Baseline to May 31, 2021; June 1, to September 30, 2021; October 1, to November 30, 2021; December 1, 2021 to February 28, 2022; March 1, to May 31, 2022; June 1, to September 30, 2022; October 1, to November 30, 2022; and December 1, 2022 to March 15, 2023. Moreover, we combined some of these periods to study the time before (baseline to November 30, 2021) and after (December 1, 2021 to March 15, 2023) the booster vaccination initiative took place.
For all analyses, if not stated otherwise, time to event was defined as time from baseline to first SARS-CoV-2 infection, death, withdrawal from the study, or end of follow-up, whichever occurred first. End of follow-up was defined as two years after study entry (March 12-15, 2023) for individuals who consented to participate in the long-term follow-up of the REDUCE study and as six months after study entry, otherwise. We also censored individuals after five weeks of follow-up in case they did not receive a second dose of BNT162b2 within 19–42 days after the first dose.
We calculated incidence rates for SARS-CoV-2 per 100,000 person-weeks. We assessed incidence of SARS-CoV-2 infections at district-level and estimated the effectiveness of the booster vaccination campaign using data from the Austrian epidemiological reporting system (EMS), which includes daily incident SARS-CoV-2 infections starting at February 26, 2020.48 We compared cumulative incidence curves between the REDUCE study, the district of Schwaz, and other districts of Tyrol. Moreover, we estimated SARS-CoV-2 infections averted in the REDUCE study population and the district of Schwaz. This was achieved by using data on the number of SARS-CoV-2 infections that occurred on each day. First, we obtained the percentage of individuals infected on each day within other districts of Tyrol. In a next step, we estimated the number of SARS-CoV-2 infections in the REDUCE study and the district of Schwaz if the daily percentage of individuals infected would have been the same as within other districts of Tyrol (counterfactual scenario). We then obtained a cumulative sum of the daily number of SARS-CoV-2 infections in the counterfactual scenario and compared them to the recorded daily number of SARS-CoV-2 infections (actual scenario). In addition, we obtained hazard ratios for incident SARS-CoV-2 infections in the REDUCE study and the district of Schwaz compared to other districts of Tyrol as described previously.49 Effectiveness was defined as one minus the corresponding hazard ratio. In a sensitivity analysis, we obtained cumulative incidence curves and estimated effectiveness allowing for recurrent events, considering individuals to be at risk for a SARS-CoV-2 re-infection at 91 days after they had experienced an infection, as suggested previously.50
Based on data from the national electronic vaccination database, we compared cumulative numbers of vaccination doses administered in the district of Schwaz to those administered in other districts of Tyrol. We calculated the percentage of individuals vaccinated with two and three doses in the district of Schwaz and in other districts of Tyrol. Moreover, we calculated the percentage of individuals vaccinated with four doses in the district of Schwaz, in other districts of Tyrol, and in the REDUCE study (restricting the REDUCE study population to individuals that participated in the long-term follow-up).
Using Cox regression analysis, we obtained hazard ratios for incident SARS-CoV-2 infections across several participant characteristics. The proportional hazards assumption was checked using Schoenfeld residuals and by inspecting log-log plots of survival. The proportional hazards assumption was violated for age. Therefore, we included age and an interaction of age with time (split at June 1, 2022) into our models. We assessed the risk for incident SARS-CoV-2 for participants with versus without a fourth dose of vaccination against COVID-19. In this analysis, the receipt of a fourth dose was treated as a time-varying variable and we additionally adjusted for sex, baseline age, an interaction of age with time (split at June 1, 2022), and previous SARS-CoV-2 infection at baseline. Moreover, we estimated hazard ratios for incident SARS-CoV-2 infection across the variables age (per 10 years younger), sex (female versus male), previous SARS-CoV-2 infection (yes versus no), household size (≥3 versus fewer persons), obesity (body mass index ≥25 versus <25 kg/m2), smoking (current versus former/never), occupation (employed versus not employed), education (Advanced level [A-level] or higher versus other), pre-existing conditions (yes versus no) including cardiovascular disease, diabetes mellitus, chronic lung disease, cancer, kidney disease, and liver disease, and intake of immunosuppressants (yes versus no). These analyses were adjusted for sex, age, an interaction of age with time (split at June 1, 2022), previous SARS-CoV-2 infection, household size, obesity, smoking, occupation, education, pre-existing cardiovascular disease, diabetes mellitus, chronic lung disease, cancer, kidney disease, liver disease, and intake of immunosuppressants, as appropriate. The analyses were restricted to participants with complete information on all covariates. To study the risk for incident SARS-CoV-2 infections across participant characteristics over time, we split the follow-up period at December 1, 2021 and estimated hazard ratios during the first period, i.e., until November 2021, and during the second period, i.e., from December 2021. For this analysis, we created a variable indicating the time period and included interaction terms with this variable and all covariates in the Cox regression model described above.
Statistical analyses were conducted with Stata 15.1 (StataCorp LLC, Lakeway Drive, Texas: USA). All statistical tests were two-sided. In general, p-values ≤0.05 were deemed as statistically significant and p-values were Bonferroni-corrected for multiple testing, if appropriate.
Published: October 10, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111117.
Supplemental information
References
- 1.World Health Organization Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. 2023. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
- 2.Mathieu E., Ritchie H., Rodés-Guirao L., Appel C., Giattino C., Hasell J., Macdonald B., Dattani S., Beltekian D., Ortiz-Ospina E., et al. Coronavirus Pandemic (COVID-19) 2020. https://ourworldindata.org/coronavirus
- 3.Seekircher L., Siller A., Astl M., Tschiderer L., Wachter G.A., Pfeifer B., Huber A., Gaber M., Schennach H., Willeit P. Seroprevalence of Anti-SARS-CoV-2 IgG Antibodies in Tyrol, Austria: Updated Analysis Involving 22,607 Blood Donors Covering the Period October 2021 to April 2022. Viruses. 2022;14:1877. doi: 10.3390/v14091877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Polack F.P., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menegale F., Manica M., Zardini A., Guzzetta G., Marziano V., d'Andrea V., Trentini F., Ajelli M., Poletti P., Merler S. Evaluation of Waning of SARS-CoV-2 Vaccine-Induced Immunity: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartof S.Y., Slezak J.M., Puzniak L., Hong V., Frankland T.B., Xie F., Ackerson B.K., Valluri S.R., Jodar L., McLaughlin J.M. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. Lancet Respir. Med. 2023;11:176–187. doi: 10.1016/S2213-2600(22)00354-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira E.D., Kitchin N., Xu X., Dychter S.S., Lockhart S., Gurtman A., Perez J.L., Zerbini C., Dever M.E., Jennings T.W., et al. Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. N. Engl. J. Med. 2022;386:1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu N., Joyal-Desmarais K., Ribeiro P.A.B., Vieira A.M., Stojanovic J., Sanuade C., Yip D., Bacon S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023;11:439–452. doi: 10.1016/S2213-2600(23)00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaitelly H., Ayoub H.H., Tang P., Coyle P., Yassine H.M., Al Thani A.A., Al-Khatib H.A., Hasan M.R., Al-Kanaani Z., Al-Kuwari E., et al. Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study. Lancet Infect. Dis. 2023;23:816–827. doi: 10.1016/S1473-3099(23)00058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosso A., Flacco M.E., Soldato G., Di Martino G., Acuti Martellucci C., Carota R., De Benedictis M., Di Marco G., Di Luzio R., Fiore M., et al. COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up. Vaccines (Basel) 2023;11 doi: 10.3390/vaccines11081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., Brooks N., Smaja M., Mircus G., Pan K., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macchia A., Ferrante D., Angeleri P., Biscayart C., Mariani J., Esteban S., Tablado M.R., de Quirós F.G.B. Evaluation of a COVID-19 Vaccine Campaign and SARS-CoV-2 Infection and Mortality Among Adults Aged 60 Years And Older in a Middle-Income Country. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas E.J., McLaughlin J.M., Khan F., Angulo F.J., Anis E., Lipsitch M., Singer S.R., Mircus G., Brooks N., Smaja M., et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect. Dis. 2022;22:357–366. doi: 10.1016/S1473-3099(21)00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos C.V.B.D., Noronha T.G.d., Werneck G.L., Struchiner C.J., Villela D.A.M. Estimated COVID-19 severe cases and deaths averted in the first year of the vaccination campaign in Brazil: A retrospective observational study. Lancet Reg. Health. Am. 2023;17 doi: 10.1016/j.lana.2022.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschiderer L., Seekircher L., Richter L., von Laer D., Lass-Flörl C., Forer L., Schönherr S., Krammer F., Embacher-Aichhorn S., Tilg H., et al. Ultra-rapid rollout vaccination with BNT162b2 to reduce SARS-CoV-2 infections in the general population. iScience. 2022;25 doi: 10.1016/j.isci.2022.105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braeye T., van Loenhout J.A.F., Brondeel R., Stouten V., Hubin P., Billuart M., Chung P.Y.J., Vandromme M., Wyndham-Thomas C., Blot K., Catteau L. COVID-19 vaccine effectiveness against symptomatic infection and hospitalisation in Belgium, July 2021 to May 2022. Euro Surveill. 2023;28 doi: 10.2807/1560-7917.ES.2023.28.26.2200768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattiuzzi C., Lippi G. Primary COVID-19 vaccine cycle and booster doses efficacy: analysis of Italian nationwide vaccination campaign. Eur. J. Public Health. 2022;32:328–330. doi: 10.1093/eurpub/ckab220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosa R.G., Falavigna M., Manfio J.L., de Araujo C.L.P., Cohen M., do Valle Barbosa G.R.G., de Souza A.P., Romeiro Silva F.K., Sganzerla D., Da Silva M.M.D., et al. BNT162b2 mRNA COVID-19 against symptomatic Omicron infection following a mass vaccination campaign in southern Brazil: A prospective test-negative design study. Vaccine. 2023;41:5461–5468. doi: 10.1016/j.vaccine.2023.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Abdullahi Y.A.M. COVID-19 Mass Vaccination Campaign: An International Comparison of Qatar With GCC Nations and Other Global Groups. Int. J. Public Health. 2023;68 doi: 10.3389/ijph.2023.1605614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira L.S., Darcie Marquitti F.M., Paixão da Silva R.L., Borges M.E., Ferreira da Costa Gomes M., Cruz O.G., Kraenkel R.A., Coutinho R.M., Prado P.I., Bastos L.S. Estimating the impact of implementation and timing of the COVID-19 vaccination programme in Brazil: a counterfactual analysis. Lancet Reg. Health. Am. 2023;17 doi: 10.1016/j.lana.2022.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., Huppert A. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavish N., Yaari R., Huppert A., Katriel G. Population-level implications of the Israeli booster campaign to curtail COVID-19 resurgence. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abn9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge Y., Zhang W.-B., Wu X., Ruktanonchai C.W., Liu H., Wang J., Song Y., Liu M., Yan W., Yang J., et al. Untangling the changing impact of non-pharmaceutical interventions and vaccination on European COVID-19 trajectories. Nat. Commun. 2022;13:3106. doi: 10.1038/s41467-022-30897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiemken T.L., Khan F., Puzniak L., Yang W., Simmering J., Polgreen P., Nguyen J.L., Jodar L., McLaughlin J.M. Seasonal trends in COVID-19 cases, hospitalizations, and mortality in the United States and Europe. Sci. Rep. 2023;13:3886. doi: 10.1038/s41598-023-31057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilboa M., Regev-Yochay G., Mandelboim M., Indenbaum V., Asraf K., Fluss R., Amit S., Mendelson E., Doolman R., Afek A., et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dedroogh S., Schmiedl S., Thürmann P.A., Graf K., Appelbaum S., Koß R., Theis C., Zia Z., Tebbenjohanns J., Thal S.C., Dedroogh M. Impact of timing and combination of different BNT162b2 and ChAdOx1-S COVID-19 basic and booster vaccinations on humoral immunogenicity and reactogenicity in adults. Sci. Rep. 2023;13:9036. doi: 10.1038/s41598-023-34961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemet I., Kliker L., Lustig Y., Zuckerman N., Erster O., Cohen C., Kreiss Y., Alroy-Preis S., Regev-Yochay G., Mendelson E., Mandelboim M. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siller A., Seekircher L., Astl M., Tschiderer L., Wachter G.A., Penz J., Pfeifer B., Huber A., Gaber M., Schennach H., Willeit P. Anti-SARS-CoV-2 IgG Seroprevalence in Tyrol, Austria, among 28,768 Blood Donors between May 2022 and March 2023. Vaccines (Basel) 2024;12 doi: 10.3390/vaccines12030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seekircher L., Astl M., Tschiderer L., Wachter G.A., Penz J., Pfeifer B., Huber A., Afonso P.M., Gaber M., Schennach H., et al. Anti-Spike IgG antibodies as correlates of protection against SARS-CoV-2 infection in the pre-Omicron and Omicron era. J. Med. Virol. 2024;96 doi: 10.1002/jmv.29839. [DOI] [PubMed] [Google Scholar]
- 32.GmbH . Österreichische Agentur für Gesundheit und Ernährungssicherheit. Gesundheit für Mensch, Tier & Pflanze: Coronavirus; 2022. https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus [Google Scholar]
- 33.GmbH . Österreichische Agentur für Gesundheit und Ernährungssicherheit. Gesundheit für Mensch, Tier & Pflanze: Coronavirus; 2023. https://www.ages.at/mensch/krankheit/krankheitserreger-von-a-bis-z/coronavirus [Google Scholar]
- 34.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., Al-Khatib H.A., Al Thani A.A., Coyle P., Al-Kanaani Z., et al. Effects of previous infection, vaccination, and hybrid immunity against symptomatic Alpha, Beta, and Delta SARS-CoV-2 infections: an observational study. EBioMedicine. 2023;95 doi: 10.1016/j.ebiom.2023.104734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall V.J., Insalata F., Foulkes S., Kirwan P., Sparkes D., Atti A., Cole M., de Lacy E., Price L., Corrigan D., et al. Effectiveness of BNT162b2 mRNA vaccine third doses and previous infection in protecting against SARS-CoV-2 infections during the Delta and Omicron variant waves; the UK SIREN cohort study September 2021 to February 2022. J. Infect. 2024;88:30–40. doi: 10.1016/j.jinf.2023.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Braunholtz D.A., Edwards S.J., Lilford R.J. Are randomized clinical trials good for us (in the short term)? Evidence for a "trial effect". J. Clin. Epidemiol. 2001;54:217–224. doi: 10.1016/S0895-4356(00)00305-X. [DOI] [PubMed] [Google Scholar]
- 37.Arbel R., Sergienko R., Friger M., Peretz A., Beckenstein T., Yaron S., Netzer D., Hammerman A. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat. Med. 2022;28:1486–1490. doi: 10.1038/s41591-022-01832-0. [DOI] [PubMed] [Google Scholar]
- 38.Canetti M., Barda N., Gilboa M., Indenbaum V., Asraf K., Gonen T., Weiss-Ottolenghi Y., Amit S., Doolman R., Mendelson E., et al. Six-Month Follow-up after a Fourth BNT162b2 Vaccine Dose. N. Engl. J. Med. 2022;387:2092–2094. doi: 10.1056/NEJMc2211283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalupka A., Richter L., Chakeri A., El-Khatib Z., Theiler-Schwetz V., Trummer C., Krause R., Willeit P., Benka B., Ioannidis J.P.A., Pilz S. Effectiveness of a fourth SARS-CoV-2 vaccine dose in previously infected individuals from Austria. Eur. J. Clin. Invest. 2024;54 doi: 10.1111/eci.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porru S., Monaco M.G.L., Spiteri G., Carta A., Caliskan G., Violán C., Torán-Monserrat P., Vimercati L., Tafuri S., Boffetta P., et al. Incidence and Determinants of Symptomatic and Asymptomatic SARS-CoV-2 Breakthrough Infections After Booster Dose in a Large European Multicentric Cohort of Health Workers-ORCHESTRA Project. J. Epidemiol. Glob. Health. 2023;13:577–588. doi: 10.1007/s44197-023-00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vivaldi G., Jolliffe D.A., Holt H., Tydeman F., Talaei M., Davies G.A., Lyons R.A., Griffiths C.J., Kee F., Sheikh A., et al. Risk factors for SARS-CoV-2 infection after primary vaccination with ChAdOx1 nCoV-19 or BNT162b2 and after booster vaccination with BNT162b2 or mRNA-1273: A population-based cohort study (COVIDENCE UK) Lancet Reg. Health. Eur. 2022;22 doi: 10.1016/j.lanepe.2022.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao M., Aveyard P., Lindson N., Hartmann-Boyce J., Watkinson P., Young D., Coupland C., Clift A.K., Harrison D., Gould D., et al. Association between smoking, e-cigarette use and severe COVID-19: a cohort study. Int. J. Epidemiol. 2022;51:1062–1072. doi: 10.1093/ije/dyac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Westen-Lagerweij N.A., Meijer E., Meeuwsen E.G., Chavannes N.H., Willemsen M.C., Croes E.A. Are smokers protected against SARS-CoV-2 infection (COVID-19)? The origins of the myth. NPJ Prim. Care Respir. Med. 2021;31:10. doi: 10.1038/s41533-021-00223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffith G.J., Morris T.T., Tudball M.J., Herbert A., Mancano G., Pike L., Sharp G.C., Sterne J., Palmer T.M., Davey Smith G., et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 2020;11:5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clift A.K., von Ende A., Tan P.S., Sallis H.M., Lindson N., Coupland C.A.C., Munafò M.R., Aveyard P., Hippisley-Cox J., Hopewell J.C. Smoking and COVID-19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax. 2022;77:65–73. doi: 10.1136/thoraxjnl-2021-217080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Medicines Agency EMA recommends first COVID-19 vaccine for authorisation in the EU. 2020. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu
- 48.data.gv.at - Open Data Österreich COVID-19: Zeitliche Darstellung von Daten zu Covid19-Fällen je Bezirk. 2020. https://www.data.gv.at/katalog/dataset/covid-19-zeitliche-darstellung-von-daten-zu-covid19-fallen-je-bezirk
- 49.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yahav D., Yelin D., Eckerle I., Eberhardt C.S., Wang J., Cao B., Kaiser L. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin. Microbiol. Infect. 2021;27:315–318. doi: 10.1016/j.cmi.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyzes existing, publicly available data. The websites where these datasets can be accessed are listed in the key resources table. In addition, the paper analyzes participant-level data, which cannot be deposited in a public repository because of data protection regulations.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.