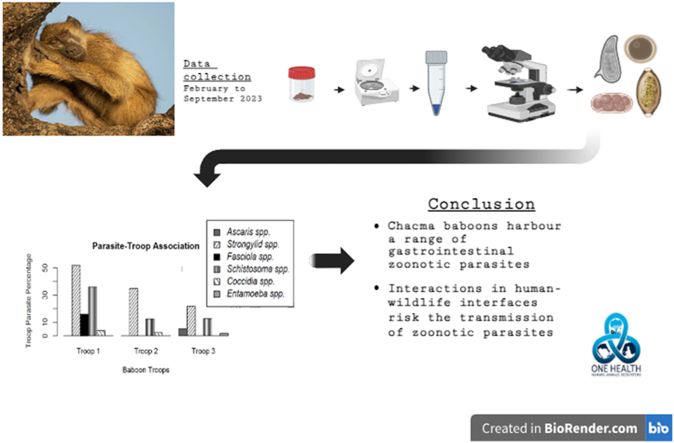
Abstract
Free-ranging Chacma baboon species are known to harbour a wide range of zoonotic parasites, and their frequent close interactions with humans pose a risk of transmission of zoonotic parasites between the two species. This research study focused on understanding parasite dynamics in free-ranging baboon populations that inhabit human-wildlife interface areas, a case of Gwanda State University's Epoch Mine campus in Filabusi at Insiza district. A descriptive and analytical cross-sectional design was used to investigate the prevalence, diversity and association of gastrointestinal parasites among three baboon troops found at the Epoch Mine campus. One hundred and twenty (120) fresh faecal samples were collected from the three troops between February and September 2023. The centrifugal floatation technique was used to process and analyse faecal samples, and parasite ova and cysts were identified using morphological features like shape and size. The prevalence of the parasite taxa and a chi-squared multiple comparison test was computed. Results showed significant differences among most parasite taxa except Coccidia spp and Entamoeba spp among the three troops. The Shannon–Wiener (H′) index was used to calculate diversity and graphs were utilized to present the association. The Kruskal-Wallis tests showed no significant difference in parasite diversity among the three troops. Although troop-parasite association showed different parasite species for each troop, helminths Strongylid spp. and Schistosoma spp. were highly common across all three troops. Troop 1 and 3 were associated with four parasite taxa, whereas Troop 2 had only three. The study reveals the presence of gastro-intestinal parasites of public health concern, as they are capable of causing diseases in humans and domestic animals. The study, therefore, underscores the importance of understanding parasite-host dynamics in mitigating zoonotic disease transmission and suggests the need to generate baseline data for mitigating zoonotic diseases and maintaining a healthy ecosystem.
Keywords: Baboons, Emerging diseases, Human-wildlife interface, Parasite-host interactions, Zoonotic diseases, Zoonotic parasites
Graphical abstract
Gastrointestinal parasite prevalence and diversity in free-ranging Chacma baboon troops in a semi-arid savanna ecosystem of Zimbabwe.
Highlights
-
•
Chacma baboons harbour a wide range of gastrointestinal zoonotic parasites.
-
•
Interactions in human-wildlife interfaces risk the transmission of zoonotic parasites.
-
•
Strongylid spp. and Schistosoma spp. were highly prevalent across all three troops.
-
•
Troop-parasite associations showed different parasite species for each troop.
1. Introduction
Baboons are among the Non-Human Primates (NHP) that interact more with human populations which makes humans susceptible to disease transmissions including those of parasitic origins (Weyher et al., 2006; Ebbert et al., 2013) many of which have a substantial negative impact on human public health. Morphological, physiological, genetic and behavioural similarities between humans and baboons facilitate the bi-directional transmission of zoonotic parasites between the two species (Fedrick, 2019). These old-world monkeys (Cercopithecidae) harbour parasites and are often asymptomatic, which promotes the transmission of particular infectious parasites including those of zoonotic importance such as the roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura) and hookworm (Necator spp., Ancylostoma duodenale) (Legesse and Erko, 2004; Howells et al., 2011; Nakayima et al., 2014; Larbi et al., 2020). Infections caused by gastrointestinal parasites are a threat to public health (Cupertino et al., 2020; Ahmed et al., 2023). These parasites can cause infections in both humans and NHP, impairing the physical health of their hosts, lowering reproductive success and foraging rates consequently lowering the fitness and overall health of the host (Mason et al., 2022). Most gastrointestinal parasitic infections in wild animals are often asymptomatic however, severe pathology can occur (Limantara et al., 2023; Sangpeng et al., 2023). It is, therefore, crucial to identify parasites present within NHP populations, understand parasite dynamics, and ascertain their role in parasite transmission (Obanda et al., 2019; Larbi et al., 2020).
Anthropogenic habitats serve as highly prone areas for disease transmission as changes force humans and NHP to have persistent contact (Nunn et al., 2016; Mason et al., 2022). Understanding the nature of zoonotic disease transmission has become essential, especially in areas where humans and animals interact frequently (Bowden and Drake, 2013; Nakayima et al., 2014). Increased rates of human and livestock encroachment into wildlife habitats, along with the interaction and exposure that results in the shared habitat, can have serious epidemiological repercussions, particularly new emerging infectious diseases, cross-transmission, and pathogen evolution (Obanda et al., 2019; Mason et al., 2022). As a result of human encroachment, baboons seek shelter in altered habitat mosaics of human settlements, agricultural lands, and forest fragments (Chapman et al., 2005; Nakayima et al., 2014). Human encroachment modifies the dynamics of parasite-host interactions (Weyher et al., 2006; Ebbert et al., 2013; Mason et al., 2022) which can lead to a myriad of health issues in wild animals. Frequent interaction in a human-wildlife interface is a gateway to introducing new emerging diseases in a previously naive population (Weyher et al., 2006). The spatial overlap of terrestrial environments promotes the transmission of parasites (Wells et al., 2018) and parasite spill-over from humans to wildlife and vice versa can occur through dependence on shared resources like water sources; the risk is further heightened by poor human sanitation, especially in developing countries (Fedrick, 2019; Mason et al., 2022).
Parasite prevalence, diversity and host-parasite associations vary depending on the host, social dynamics, and environmental conditions. The diversity and prevalence of parasites within an ecosystem are strongly influenced by environmental factors (Chapman et al., 2010), including temperature, humidity, food and host availability, as well as biotic and abiotic elements. Parasites that are well-suited to specific conditions or niches are more likely to thrive in environments that meet their requirements, while those with broader environmental tolerances may be more widely distributed. Environmental moisture is correlated to the prevalence and abundance of parasitic infections (Appleton and Henzi, 1993; Chapman et al., 2010). Certain parasites thrive more in damp or wet conditions where they can move easily from host to host, while others may require dry environments to complete their life cycle. For example, soil-transmitted parasites, such as hookworms and Ascaris, require moisture to survive and can be more prevalent in areas with higher soil moisture. High humidity can increase the survival and reproductive rate of vectors that spread parasitic infections. This, understanding this relationship is crucial for disease prevention and control strategies, especially in areas with high humidity or prone to flooding.
The impact of anthropogenic activities in natural habitats has led to changes in the ecology of host-parasite associations, increasing the risk of disease transmission (Appleton and Henzi, 1993; Chapman et al., 2005; Hussain et al., 2013; Banda et al., 2021; Mason et al., 2022). Research indicates that forest fragmentation contributes to the increased prevalence of gastrointestinal parasites in NHP, particularly directly transmitted parasites (Mason et al., 2022). Thus, it is paramount to carry out studies on parasite infection patterns to assess the different parasite taxa separately. The outcome of host-parasite associations is also influenced by the host's nutritional status. For instance, baboons consuming high-nutritional-value crops from agriculture fields exhibit improved overall nutrition and immunity, reducing their susceptibility to parasites (Hahn et al., 2003). The contact between humans and NHP in human-wildlife interfaces further exposes primates to a higher diversity of parasites (Valenta et al., 2017). Therefore, studies on parasite prevalence, diversity, and host associations provide crucial insights into the complex relationships among parasites, hosts, and the environment.
Drewe et al. (2012) did a study to determine the health risks harboured by baboons in Peninsula, South Africa. Twenty-seven baboons from 5 troops were screened for 10 infections in April 2010. Baboons were live caged, euthanized, samples collected, blood and faeces and then later released when stable. Blood samples serum was used to detect the presence of antibodies, while for the faecal samples culturing was done to detect the presence of specific parasites. The study showed evidence of potential for cross-species trafficking between humans and baboons.
Mason et al. (2022) carried out a study to determine the relationship between parasite infection and human disturbances among yellow baboons living outside the national park in Tanzania. 135 samples from 9 troops were collected occupying varying levels of human disturbances. They evaluated parasite prevalence and abundance of Isotrichid ciliates and Strongylida. Seven protozoan taxa and four helminth taxa were identified. Taxa showed varied associations with human disturbance, baboon troop size and host age. In four taxa they observed a positive relationship between prevalence and troop size. They reported a trend towards higher parasite prevalence of two taxa in less disturbed areas. Contrary, high levels of human disturbance predicted an increased abundance of Isotrichid ciliates; however, no association was found between disturbance and Strongylida abundance. Their results provide mixed evidence that human disturbance is associated with NHP parasite infections. Further on their study highlights the need to consider monitoring parasite infections when developing NHP conservation strategies.
The free-ranging Chacma baboons (Papio ursinus griseipes) at the Gwanda State University, Epoch Mine campus, Filabusi, Matabeleland South, Zimbabwe have effectively been forced into ever closer contact with humans. More land is continuously being cleared for infrastructural development at the institution. Changes to the natural habitat of baboons due to human interferences may result in the alteration of parasite transmission rates, host range, and virulence (Patz et al., 2000; Fedrick, 2019; Mason et al., 2022). Therefore, there is a need to manage both the health of the baboon population and human population living close to them thereby effectively maintaining the health of the ecosystem. Baseline data on parasite infections must be generated which will be used to investigate and prevent potential cross-infection and identify potential points for intervention (Larbi et al., 2020). Human-baboon interactions and conflicts amplify incidences of parasitic infections in both species increasing risks of spillover, zoonoses and anthroponoses due to environmental modifications (Ryan et al., 2012; Mason et al., 2022). This is a cause for concern for both baboon management conservation and human public health matters. Although Chacma baboons are categorised as Least Concern by the IUCN Red data list, their conservation is imperative in guarding against extinction (Wallis, 2023). The One Health approach also recognizes that the health of animals, humans and ecosystems is interconnected and seeks to encourage holistic approaches when investigating zoonotic and anthroponotic diseases, thus improving global health (Mackenzie and Jeggo, 2019; Aggarwal and Ramachandran, 2020). For the current study we focused on three troops that live within the Gwanda State University Filabusi campus vicinity. The three troops have distinct sleeping sites; one near the Trukumb mine on a mountain, the second on the northwestern side of the campus (staff quarters and the library) and the third on the southern side (close to the recreational facility) in a restricted disused collapsed mine area. When leaving their sleeping den, these troops travel in different directions. There is extensive documentation on Papio parasite infections, however, there is scarce information on investigating baboon troops utilizing different trails but inhabiting a similar agroecological region IV zone of Zimbabwe and living within the vicinity of Gwanda State University campus. Noteworthy travelling in, out and around the Gwanda State University campus. This campus is endowed with human, domestic and wild animals sharing inhabitant spaces. Considering that baboons are generalists in their feeding behaviour (Mason et al., 2022), it is paramount to appreciate the parasites they harbour, since they can serve as the bridging gap in disease transmission among animals (humans, domestic and wild animals). A few studies also have been done on species co-occurrence (association). Species co-occurrence theory is a novel theory in the ecological networks that wants to explain some of the causes of species distribution revealed by biotic effects instead (Cazelles et al., 2016). The distribution of species is proposed not only to be influenced by the ability of the organism's physiological tolerance to environmental conditions but also by interactions with other species (Hutchinson, 1957). The previous hypothesize, i.e., physiological tolerance to environmental conditions has been well researched and well documented, thereby leaving a gap for species co-occurrence, i.e., the interactions with other organisms (Cazelles et al., 2016). For one to be able to interpret association data, express hypothesis for unlike community assemblages mechanisms, and incorporate the analysis of species distributions currently focused on the relationship between occurrences and abiotic factors, there is a need to rally forward the theory of species co-occurrence (Cazelles et al., 2016). Research contributing to the theory of co-occurrence is scarce; this study therefore wants to add to this novel theory.
The study compared gastro-intestinal parasites of Chacma baboons occupying a similar habitat but travelling in different trails. We hypothesized that different trail directions (different troops) would be related to different parasite taxa prevalence and diversity due to assorted human anthropogenic activities. Further on the study predicted the respective troops to be associated with different parasite taxa. Against this background, this study aims to investigate the prevalence, diversity and host-parasite associations of gastrointestinal parasites among free-ranging Chacma baboon troops at Gwanda State University, Epoch Mine campus.
2. Materials and methods
2.1. Study area
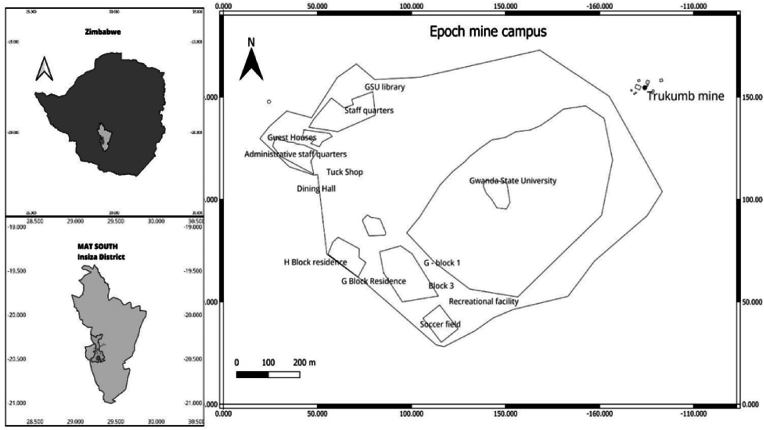
The study was conducted at Gwanda State University, Epoch Mine campus (20°26′11.37''S; 29°16′08.64''E) in Filabusi, Insiza district (Fig. 1) Epoch Mine is positioned at an altitude of 1420 m–1500 m above sea level (Kileshye Onema et al., 2006). Insiza district is one of the seven districts located in the province of Matabeleland South in Zimbabwe. It is located about 106 km to the Southeast of Bulawayo in Zimbabwe and is bordered by Gwanda district to the South, Zvishavane district to the east, and Umzingwane district to the West (Kileshye Onema et al., 2006; Pirajno and González-Álvarez, 2013). This region receives a mean annual rainfall of 350 mm and is subject to periodic seasonal droughts and severe dry spells during the rainy season (Musara et al., 2021). Thus, the district is mostly in natural agro-region IV. Temperatures range from 12 °C to 29 °C, with the lowest and highest in June and October, respectively (Kileshye Onema et al., 2006).
Fig. 1.
Location of Gwanda State University, Epoch Mine campus, Insiza District, Matabeleland South, Zimbabwe.
Baboons have established their habitat sharing inhabitants with other primates like Vervet monkeys (Chlorocebus pygerythrus) at this derelict mine which was later revived to pave the way for a tertiary institute. They spend considerable time roaming around the campus, especially at the staff and students' quarters, foraging for food from fruit trees, in refuse bins, and defecating around the campus (Fig. 2) thus predisposing inhabitants to infections especially those of parasitic origins.
Fig. 2.
Human-wildlife-livestock interface, baboons and goats foraging under a mango fruit tree at the staff quarters at Epoch Mine campus, Insiza District, Matabeleland South (Photo credits: Nokubonga Ncube, April 2023).
2.2. Data collection
The study utilized a descriptive and analytical cross-sectional design (Larbi et al., 2020) to investigate the prevalence, diversity and association of gastrointestinal parasites with baboons among three different baboon troops at Gwanda State University, Epoch Mine campus, Filabusi.
A total of one hundred and twenty (120) fresh faecal samples were collected between February and September 2023, at least twice per week from three (3) baboon troops, with an average of two samples per day. Many samples were collected to increase the chances of parasite detection. Ten days before data collection, the troops were followed on foot from their sleeping sites to distinguish the troops and make the baboons accustomed to the observer (Ndagurwa, 2007). The three troops have distinct sleeping sites; One at the Trukumb mine, the second on the northwestern side of the campus (staff quarters and the library), and the third on the southern side (close to the recreational facility). With the aid of binoculars (Sterrlah 12x50 Optics, China), troops were trailed in the morning (from 5.30 to 9.00 a.m.) collecting the fresh faecal samples immediately after defecation or when the baboons had left the immediate foraging area (Katsvanga et al., 2009; Adrus et al., 2019; Larbi et al., 2020).
Even though samples were randomly collected within a troop, if the faecal matter were less than 10 m apart, only one was collected assuming each faecal sample represents a unique individual thereby minimising the probability of repeated sampling, however, it cannot be denied that some individuals might have been sampled more than once on repeat days (Codron et al., 2006; Ryan et al., 2012; Mafuyai et al., 2013). To minimize repeated sampling of the same individual within a group, distinguishing features such as sex, size and behaviour were observed to identify separate individuals (Ryan et al., 2012; Matthews et al., 2020). Individual behaviour such as dominance traits was taken into account. Dominant males were often identified as being aggressive and often displaced lower-ranking baboons from food and water sources (Alberts, 2019). Size was also taken into account to differentiate between young and adult baboons (Phillips et al., 2004). Sex-specific characteristics: female baboons exhibit pink to red swollen vagina during their oestrus period (Magden et al., 2015). Based on the above-mentioned characteristics alternating sampling would be done i.e. if the first sample was collected from a young baboon the next would be intentionally collected from an adult and vice versa. However, age and sex were not part of this study's objectives hence not recorded. Troop sizes were estimated by counting all visible individuals before sampling (Kiffner et al., 2022). The three troops comprised 27, 40, and 64 individuals.
Using a clean and sterilized spatula, an estimate of 2 g (4g) of faecal droppings were collected from the middle to avoid environmental contamination (Larbi et al., 2020) and immediately preserved in 10% formalin solution in airtight vials (labelled with the date, time of collection, sample number and troop number) and stored in the laboratory until taken for laboratory analysis at the Bulawayo Provincial Veterinary Laboratory, Division of Veterinary Technical Services, Bulawayo, Zimbabwe.
2.3. Parasitological analysis of faecal samples
The centrifugal floatation technique was used to process and analyse samples (Hansen and Perry, 1990) as follows. Each sample vial was separately analysed, i.e., not pooled. One gram of faeces from each sample was weighed using an analytical balance (with an accuracy of 0.1 g) and mixed with ten (10) ml of normal saline solution. The resultant faecal suspension was filtered through a 150 μm sieve into a 15 ml centrifuge tube. The faecal suspension was centrifuged for 5 min at 1500 revolutions per minute (RPM). The resulting supernatant was decanted leaving the sediment behind. The sediment was re-suspended completely, to a volume of 15 ml with concentrated Ammonium Nitrate solution (with specific gravity 1300 which allows eggs to float and faecal matter to sink (Hansen and Perry, 1990). Both chambers of the McMaster slide were filled with the sub-sample for observation under a microscope (Compound light microscope, Optika brand, Italy) at x100 magnification.
2.4. Parasite identification
Parasites were microscopically identified with the help of Bulawayo Provincial Veterinary Laboratory, Zimbabwe experts. Identification was based on morphological characteristics i.e. shape, size, hooks on eggs or the presence of polar filaments on cysts following identification guidelines, keys and photographs in literature as reference (Hasegawa et al., 2009; Taylor et al., 2015). Eggs of Strongylid spp (60–80 μm; 30–40 μm wide) are elongated, oval, or cylindrical with a smooth surface and a prominent polar plug or operculum at one end (Taylor et al., 2015) surface. Due to the low reliability of the identification of parasite eggs by microscopic analysis (Gillespie, 2006), the parasites were identified to suborder and genus level.
2.5. Data analysis
2.5.1. Parasite prevalence
Parasite prevalence per troop was calculated as a percentage of the total number of individuals of a host species infected with a particular parasitic species divided by the number of hosts examined (Margolis et al., 1982; Bush et al., 1997; Kouassi et al., 2015). Chi-squared test was conducted at 0.05 level of significance to test if there was a statistically significant difference in parasite prevalence among the three troops.
Where, P is parasite prevalence in troop i for parasite j where i = 1; 2; 3 and j = 1; 2; … 6.
2.5.2. Parasite diversity
The diversity of parasites was computed for each baboon troop. The Shannon–Wiener (H′) index was used to measure diversity (Poulin, 2015). Kruskal-Wallis test was performed to test if there was any statistical significant difference in parasite diversity among the three troops.
2.5.3. Parasite-troop association
Assessment of the association of parasite taxa to troops was done using graphical analysis (Banda et al., 2021) using the R Studio version 4.3.2 (R Core Team, 2022).
2.6. Ethics statement
The research was approved and cleared by the Gwanda State University research board.
3. Results
3.1. Parasite frequency
A total number of 120 samples were collected. Among the samples collected a total of 21 samples were positive with one or more parasite taxa in troop 1 samples troop 2 had 18 positive samples while troop 3 had 19 (Table 1). Six parasite taxa were identified, five to genus level and one to suborder (Strongylida). Strongylid nematodes often occur in complex communities and it is difficult to identify individual strongylid species using coproscopy analysis (Pafčo et al., 2018). Therefore, in this study the strongylid nematodes were grouped (under the suborder Strongylida) (Frias et al., 2019). There were four helminths taxa and two protozoan taxa.
Table 1.
Frequency of positive parasites identified in each troop at Epoch Mine campus, Insiza district.
| Parasite taxa |
|||||||
|---|---|---|---|---|---|---|---|
| Troop | Total no. of +ve samples with one or more parasite taxa | Ascaris spp | Strongylid spp | Fasciola spp | Schistosoma spp | Coccidia spp | Entamoeba spp |
| One | 21 | – | 13 | 4 | 9 | 1 | – |
| Two | 18 | – | 14 | – | 5 | 1 | – |
| Three | 19 | 3 | 12 | – | 7 | – | 1 |
NB this is raw data before multiplying by 50 as a pre-requisite for using the McMaster method.
3.2. Parasite prevalence
Strongylid spp. had the highest prevalence among the three troops (Table 2) with troop 1 having a prevalence rate of 52%. Although Schistosoma spp. was also reported across the three troops, its prevalence was lower than Strongylid spp. Ascaris spp. and Entamoeba spp was only reported in troop 3 with a prevalence of 5.5% and 1.8%, respectively.
Table 2.
Prevalence of gastrointestinal parasites in free-ranging baboons (Papio spp.).
| Parasite species | Troops |
Chi-squared value (χ2) | P-value | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Helminths | |||||
| Ascaris spp. | – | – | 5.5 | 7.547 | 0.02297152 ∗ |
| Strongylid spp. | 52 | 35 | 21.8 | 19.833 | 0.00004935 ∗ |
| Fasciola spp. | 16 | – | – | 33.803 | 0.000005 ∗ |
| Schistosoma spp. | 36 | 12.5 | 12.7 | 22.481 | 0.00001313 ∗ |
| Protozoa | |||||
| Coccidia spp. | 4 | 2.5 | – | 2.162 | 0.3392561 |
| Entamoeba spp. | – | – | 1.8 | 0.855 | 0.6521374 |
NB: asterisks (∗) represent No significant difference.
A chi-squared multiple comparison test (χ2) indicated that the prevalence of Ascaris spp from troop 3 was significantly different (χ2 = 7.547, P = 0.023) from the other troops. Similarly, Fasciola spp. prevalence of troop 1 was significantly different (χ2 = 33.803, P = 0.000005) from the other troops. There was a significant difference among the three troops for Strongylid spp. and Schistosoma spp (χ2 = 19.833, P = 0.00005), (χ2 = 22.481, P = 0.00001) respectively.
There were no significant differences in parasite prevalences' for Coccidia spp. (χ2 = 2.162, P = 0.339), and Entamoeba spp. (χ2 = 0.855, P = 0.652) among the three troops.
3.3. Parasite diversity
Despite the Kruskal-Wallis test indicating no significant difference in parasite diversity among the three troops (P = 0.3841), troop 1 had the highest parasite diversity based on the Shannon-Weiner Index (H′) (see Table 3), followed by troop 3 and then troop 2.
Table 3.
Shannon–Wiener (H′) index among the three troops.
| Group | Shannon–Wiener (H′) Diversity index |
|---|---|
| Troop 1 | 0.801522 |
| Troop 2 | 0.746032 |
| Troop 3 | 0.753921 |
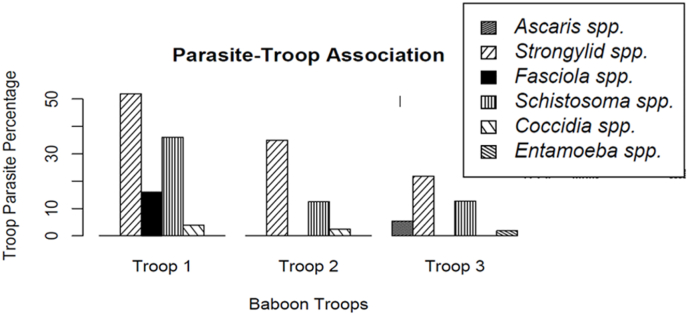
3.4. Parasite-troop association
Troop 1 was associated with four parasite taxa (Fig. 3), namely Strongylid spp., Schistosoma spp., Fasciola spp. and Coccidia spp. Troop 2 was associated with three parasite taxa, namely Strongylid spp, Schistosoma spp. and Coccidia spp. Troop 3 was associated with four parasite taxa, which are Ascaris spp., Strongylid spp., Schistosoma spp. and Entamoeba spp.
Fig. 3.
Parasites and baboon troops association at Epoch Mine campus, Insiza District.
4. Discussion
Six parasite taxa were observed in this study. Strongylid spp. and Schistosoma spp. were present in all three troops with the former recording the highest prevalence range (20%–55%). There was significant differences in prevalence among the three troops for four parasite taxa, except Coccidia spp and Entamoeba spp. No significant difference was noted in parasite diversity among the troops. Troops were associated with different parasite taxa. Fasciola spp. was unique to troop 1, while troop 3 had Ascaris spp. and Entamoeba spp. unique to it.
Strongylid nematodes are part of the most reported parasites in wild primate studies (Pafčo et al., 2018; Frias et al., 2019). Despite the many observations, studies are limited by that strongylid nematodes often occur in complex communities, and are difficult to distinguish individual species using faecal analysis (Ebbert et al., 2015; Uppal et al., 2017; Pafčo et al., 2018). The use of molecular analysis has led to the identification of new parasite strains showing new levels of diversity (Frias et al., 2019). Understanding parasite prevalence and diversity within each troop provides valuable insights into the distribution of parasites and overall ecological dynamics and interactions within the baboon troop (Chapman et al., 2005; Mason et al., 2022). Strongylid often infects livestock, particularly cattle (N’da et al., 2022). Furthermore, the close phylogenetic relation between humans and NHP causes a potential partial overlap of diseases, and strongylid spillover could be a major worry for the conservation of baboons and a health threat to humans (Pafčo et al., 2018). In cases where parasite prevalence ranges between, 20%–50%, it is considered a moderate risk to communities and the World Health Organization (WHO) recommends action such as mass drug administration twice yearly; above 50% is high risk (Deka et al., 2021).
Despite the moderately high prevalence of Schistosoma spp. (12.5%–36%) the presence of the parasite in baboons calls for health monitoring of baboons and strategies to prevent transmission of schistosomiasis disease to humans (Howells et al., 2011) even though identification was to a generic level in this study. For instance, Schistosoma mansoni and Schistosoma mattheei infect humans (human schistosomiasis) (Weyher et al., 2010; Richards et al., 2019) causing systemic morbidities including anaemia, malnutrition, and impaired childhood development (King and Dangerfield-Cha, 2008; Colley et al., 2014). Given that Schistosoma spp. is waterborne and present in the Chacma baboon populations studied, there is a potential for zoonotic transmission between humans and baboons. Therefore, it is crucial to implement safe water practices and sanitation measures in affected areas. It is also important to educate communities about the risks and to regularly monitor the shared water sources to prevent the spread of the parasites from baboons to humans. Schistosomiasis disease in humans caused by Schistosoma mansoni is one of the prioritized neglected tropical diseases (Richards et al., 2019) however, the presence of similar parasites between humans and baboons does not automatically prove zoonosis but a potential risk (Mafuyai et al., 2013).
The study observed similar parasites to other previous studies on gastrointestinal parasites in baboons such as Ascaris spp., Schistosoma spp., Strongylid and Entamoeba spp. (Hahn et al., 2003; Weyher et al., 2006; Ravasi et al., 2012; Larbi et al., 2020; N’da et al., 2022). Of particular interest in this study was the presence of Fasciola spp., a parasite rarely observed in baboon studies (N’da et al., 2022; Mason et al., 2022; Larbi et al., 2020; Ravasi et al., 2012) and the absence of Trichuris spp. in the observed parasites of this study. In several Papio studies, Trichuris spp. has been observed as one of the most common parasites (Müller-Graf et al., 1996; Ravasi et al., 2012; Moxley, 2013). However, a study by Larbi et al. (2021) on intestinal parasites of free-ranging baboons Trichuris spp. was not observed. Similarly, another study by Mafuyai et al. (2013) results observed recorded the presence of Fasciola spp. and the absence of Trichuris spp. Both of these studies involve free-ranging baboons living in proximity to humans, similar to the study site being discussed. There hasn't been much documentation on the presence of Fasciola spp. in baboon studies. However, Fasciola spp. presence has been well-documented particularly where livestock and wild animals overlap (Lalor et al., 2021; Mas-Coma et al., 2022). The presence of Fasciola spp. observed in this study may be due to the area being inhabited by domestic (cattle, goats), humans and wild animals who mingle with baboons. Their unrestricted defecation creates the probability of cross-contamination (Atuman et al., 2019; Lalor et al., 2021). Trichuris spp. has free-living stages in its life cycle which do not thrive in hot and dry environments, such as the conditions of the study area, the semi-arid savanna ecosystem (Kalousová et al., 2014; Manz et al., 2017; Mason et al., 2022)
Ascaris spp. was present in troop 3 only but with a low prevalence (5.5%). Ascaris spp. is a commonly identified helminth in Non-Human Primates (NHP) studies where humans live in proximity (Ocaido et al., 2003; Howells et al., 2011; Adrus et al., 2019). The parasite causes ascariasis disease in humans and the infection can lead to death (Howells et al., 2011). The One-health approach classifies Ascaris lumbricoides (roundworms) as soil-transmitted helminth of great public health importance (Ellwanger and Cavallero, 2023). According to Adrus et al. (2019) Entamoeba spp. and Coccidia spp. are often asymptomatic, however, in larger quantities may cause dysentery and/or diarrhoea (Adrus et al., 2019; Almeria et al., 2019). According to Bezjian et al. (2008) parasite prevalence and diversity are dependent on weather and habitat. A limitation of the current study is that the effect of seasonality on parasite prevalence and diversity was not studied however it is a potential future research area.
Humans and livestock living near baboons and monkeys are at risk of being infected with interspecific parasitic disease (Ocaido et al., 2003; Richards et al., 2019). Baboons and humans can be infected by the same multi-host parasites (Muriuki et al., 1998; Adrus et al., 2019). Interactions in human-baboon interfaces provide ample opportunities for the cross-transmission of parasitic infections such as zoonotic schistosomiasis particularly where there is a sharing of water sources and crop raiding (Bezjian et al., 2008; Richards et al., 2019). In the study area, human-livestock-baboon interactions occurred through baboons raiding crop fields, and waste bins. The variations in host-parasite prevalence may be attributed to the differences in home range sizes, forage area, level of contact with humans and/neighbouring troops, troop genetics, habitat exposure and parasite characteristics (Müller-Graf et al., 1996; Mason et al., 2022).
Parasite diversity may vary due to differences in the host's susceptibility to parasites and different geographic locations (Tabasshum et al., 2022). The trails of the troops in this study lead in different directions, hence impacting the parasite diversity variability. Bordes et al. (2009) reported a negative correlation between directly transmitted helminths and home range sizes because larger home ranges have less environmental contamination (faecal matter) which in turn reduces infection and re-infection rates mostly for directly transmitted parasites (Nunn et al., 2011). On the contrary Kalousová et al. (2014) mentioned that larger home ranges might increase baboon encounters with other host individuals, thereby acquiring various parasite species and introducing them to their sleeping sites resulting in higher parasite diversity.
The observed high parasite diversity in this study might imply an increase in the type of parasites that can potentially infect humans and livestock living in close proximity (Larbi et al., 2020) thereby increasing risks of zoonotic transmission either directly or indirectly through shared environmental resources (Akinyi et al., 2019; Mason et al., 2022). In humans, parasitic diseases might range from mild symptoms to severe and potentially life-threatening infections, particularly in children (Larbi et al., 2020; Mason et al., 2022). In livestock, parasitic infections affect productivity by reducing growth, milk production and fertility (Strydom et al., 2023).
Encounter rate and host-parasite compatibility also regulate host-parasite dynamics (Budria and Candolin, 2014). There are several open sewage points in the study area that are situated along the trails of troop 1 and troop 3 (Ncube, 2023 personal observations). These points might be the source attributed to the presence of parasites such as Ascaris spp., Fasciola spp., and Coccidia spp. in the troops. Sewage wastewater is known to be associated with soil-transmitted helminthiase infections (Pham-Duc et al., 2013). There is a mine sinkhole in the vicinity of the troops' sleeping sites which has since been a stagnant water body that might harbour parasites. Proximity to a water body with infected vectors and infective larvae increases the risk factor for parasitic infections (Ponce-Terashima et al., 2014; Mason et al., 2022).
Fasciola spp. and Ascaris spp. are zoonotic parasites causing Ascariasis and Fasciolosis diseases respectively, particularly in cattle, and small ruminants (Torgerson, 2013; Cwiklinski et al., 2015). These zoonotic parasitic diseases not only have negative repercussions on human health but also result in significant economic losses in livestock production, affecting people's socio-economic well-being (Torgerson, 2013; Kanyari et al., 2017). Ascaris spp. is a soil-transmitted helminth, both humans and baboons are prone to these infections. For instance, some pregnant women and children often consume soil (Kawai et al., 2009; Mbuya et al., 2015), and baboons also practice geophagy (Pebsworth et al., 2012) therefore, ingestion or direct contact with an infective stage of the parasite results in infection. Geophagy practice greatly increases the risk of infection with Ascaris lumbricoides (Kawai et al., 2009). In a study on Chacma baboons in South Africa, the authors attributed the presence of Ascaris spp. in baboons to human-baboon interactions (Ravasi et al., 2012). The variations in micro-habitat factors like soil moisture and abundance of intermediate hosts have a bearing on host-parasite associations (Müller-Graf et al., 1996) and survival of parasite infectious stages (Ravasi et al., 2012).
Some of the reported parasites in this study were found to have a low to moderate prevalence. One potential resultant for this could be that baboons consume plants with anthelminthic properties as a form of ethnomedicine, which may be altering their internal parasite load (Huffman, 1997, 2003; Kirabo et al., 2018). Furthermore, the troops in this study often raid bins from staff and student areas of residence, thus, nutrient-fortified foods from these bins improve the physical conditions of the baboons and reduce the intensity of parasitic infections (Hahn et al., 2003; Weyher et al., 2006).
5. Conclusion
The Chacma baboon populations’ gastro-intestinal research exhibited moderate to high prevalence of some parasites that may pose a risk to public health. There was high to moderate prevalence of Strongylid spp and Schistosoma spp in all the troops and the two parasite taxa were common in all the troops. There were significant differences noted in the prevalence of the parasites among the troops except for Coccidia spp and Entamoeba spp. Noteworthy was the observation of Fasciola spp in troop 1 and Ascaris spp and Entamoeba spp in troop 3. Parasite diversity was high in all three troops, however, troop 1 had the highest. There was no significant difference in parasite diversity. Troop 1 and 3 were associated with 4 parasite species, while troop 2 had three parasite species. Therefore, it is essential to comprehend parasite dynamics in wild baboon populations near areas of human-wildlife interaction so that efforts to prevent and control infections can be prioritized.
Recommendations
Based on the research findings, regular monitoring of baboon defecation to detect any changes in parasite dynamics is critical. Awareness campaigns on zoonotic and anthroponotic diseases should be done regularly. Given the potential impact of gastrointestinal parasites on both human and baboon health, it is recommended that measures be taken to improve sanitation and hygiene in the study area i.e. teaching residents about the effects of open defecation and coprophagy for instance. There is a need to put in place baboon-proof trash cans and advise people to remove attractants like food sources and trash at their houses. The baboon population needs to be monitored on campus so as to reduce the incidence of parasite transmission to humans and domestic animals and vice versa. A policy has to be developed and implemented supporting the coexistence and conflict mitigation between humans and baboons. Future studies should consider blood and ectoparasite research and research on genetic and molecular techniques to identify and distinguish parasite species and strains particularly those shared among humans, livestock and baboons. Overall, the research findings of this study underscore the need for One health approaches that integrate human, animal, and environmental health to promote coexistence and address emerging diseases in dynamic relationships between humans and NHP. Sampling from unknown individuals like the one used in this study limit the conclusions that can be derived from findings regarding specific baboon-parasite interaction, for example how interaction/association of certain parasites is influenced by sex or age of the study animal (Gillespie, 2006).
CRediT authorship contribution statement
Annabel Banda: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Formal analysis, Conceptualization, Data curation. Doreen Z. Moyo: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization. Nokubonga Ncube: Writing – original draft, Project administration, Methodology, Formal analysis, Data curation, Conceptualization, Visualization, Writing – review & editing. Edmore Utete: Writing – original draft, Software, Methodology, Conceptualization, Data curation, Visualization, Writing – review & editing. James Machingura: Writing – original draft, Writing – review & editing. Tapiwa Gumbo: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Edson Gandiwa: Writing – review & editing.
Data availability
Available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to Gwanda State University for aiding the study to take off. We acknowledge Mr Mziwakhe Nyathi and Mr Maqhawe Masuku for their invaluable assistance during data collection. We appreciate the Bulawayo Provincial Veterinary Laboratory, Division of Veterinary Technical Services, Zimbabwe, for assisting with parasitology analysis particularly Dr Clifford Tshuma and Mr Zwelabo Sibanda.
References
- Adrus M., Zainudin R., Ahamad M., Jayasilan M., Abdullah M.T. Gastrointestinal parasites of zoonotic importance observed in the wild, urban, and captive populations of non‐human primates in Malaysia. J. Med. Primatol. 2019;48:22–31. doi: 10.1111/jmp.12389. [DOI] [PubMed] [Google Scholar]
- Aggarwal D., Ramachandran A. One health approach to address zoonotic diseases. Indian J. Community Med. 2020;45:S6–S8. doi: 10.4103/ijcm.IJCM_398_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.A., Kotepui M., Masangkay F.R., Milanez G.D., Karanis P. Gastrointestinal parasites in Africa: a review. Adv. Parasitol. 2023;119:1–64. doi: 10.1016/bs.apar.2022.10.001. [DOI] [PubMed] [Google Scholar]
- Akinyi M.Y., Jansen D., Habig B., Gesquiere L.R., Alberts S.C., Archie E.A. Costs and drivers of helminth parasite infection in wild female baboons. J. Anim. Ecol. 2019;88:1029–1043. doi: 10.1111/1365-2656.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts S.C. Social influences on survival and reproduction: insights from a long‐term study of wild baboons. J. Anim. Ecol. 2019;88:47–66. doi: 10.1111/1365-2656.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria S., Cinar H.N., Dubey J.P. Coccidiosis in Livestock, Poultry, Companion Animals, and Humans. CRC Press; 2019. Coccidiosis in humans; pp. 267–312. [Google Scholar]
- Appleton C.C., Henzi S.P. Environmental correlates of gastrointestinal parasitism in montane and lowland baboons in Natal, South Africa. Int. J. Primatol. 1993;14:623–635. [Google Scholar]
- Atuman Y.J., Kudi C.A., Abdu P., Abubakar A. Prevalence of parasites of wildlife in Yankari game reserve and Sumu wildlife park in Bauchi State, Nigeria. Sokoto J. Vet. Sci. 2019;17:70–79. [Google Scholar]
- Banda A., Gandiwa E., Muboko N., Muposhi V.K. 2021. An Assessment of Rodent-Flea Diversity and Association in a Semi-arid Tropical Ecosystem of South-Western Zimbabwe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezjian M., Gillespie T.R., Chapman C.A., Greiner E.C. Coprologic evidence of gastrointestinal helminths of forest baboons, Papio anubis, in Kibale National Park, Uganda. J. Wildl. Dis. 2008;44:878–887. doi: 10.7589/0090-3558-44.4.878. [DOI] [PubMed] [Google Scholar]
- Bordes F., Morand S., Kelt D.A., Van Vuren D.H. Home range and parasite diversity in mammals. Am. Nat. 2009;173:467–474. doi: 10.1086/597227. [DOI] [PubMed] [Google Scholar]
- Bowden S.E., Drake J.M. Ecology of multi-host pathogens of animals. National Education Knowledge. 2013;4 [Google Scholar]
- Budria A., Candolin U. How does human-induced environmental change influence host-parasite interactions? Parasitology. 2014;141:462–474. doi: 10.1017/S0031182013001881. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997:575–583. [PubMed] [Google Scholar]
- Cazelles K., Araújo M.B., Mouquet N., Gravel D. A theory for species co-occurrence in interaction networks. Theor. Ecol. 2016;9:39–48. [Google Scholar]
- Chapman C.A., Gillespie T.R., Goldberg T.L. Primates and the ecology of their infectious diseases: how will anthropogenic change affect host‐parasite interactions? Evol. Anthropol.: Issues, News, and Reviews: Issues, News, and Reviews. 2005;14:134–144. [Google Scholar]
- Chapman C.A., Speirs M.L., Hodder S.A., Rothman J.M. Colobus monkey parasite infections in wet and dry habitats: implications for climate change. Afr. J. Ecol. 2010;48(2):555–558. [Google Scholar]
- Codron D., Lee-Thorp J.A., Sponheimer M., De Ruiter D., Codron J. Inter- and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N, and %N. Am. J. Phys. Anthropol. 2006;129:204–214. doi: 10.1002/ajpa.20253. [DOI] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupertino M.C., Resende M.B., Mayer N.A.J., Carvalho L.M., Siqueira-Batista R. Emerging and re-emerging human infectious diseases: a systematic review of the role of wild animals with a focus on public health impact. Asian Pac. J. Tropical Med. 2020;13:99–106. [Google Scholar]
- Cwiklinski K., de la Torre-Escudero E., Trelis M., Bernal D., Dufresne P., Brennan G., O'Neill S., Tort J., Paterson S., Marcilla A. The extracellular vesicles of the helminth pathogen, Fasciola hepatica: biogenesis pathways and cargo molecules involved in parasite pathogenesis∗[S] Mol. Cell. Proteomics. 2015;14:3258–3273. doi: 10.1074/mcp.M115.053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka S., Barua D., Bahurupi Y., Kalita D. Assessment of the prevalence of soil-transmitted helminth infections and associated risk factors among school-aged children in a flood-affected area of Northeast India. Am. J. Trop. Med. Hyg. 2021;105:480. doi: 10.4269/ajtmh.20-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe J.A., O'Riain M.J., Beamish E., Currie H., Parsons S. Survey of infections transmissible between baboons and humans, Cape Town, South Africa. Emerg. Infect. Dis. 2012;18:298. doi: 10.3201/eid1802.111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert M.A., McGrew W.C., Marchant L.F. Community composition, correlations among taxa, prevalence, and richness in gastrointestinal parasites of baboons in Senegal, West Africa. Primates. 2013;54:183–189. doi: 10.1007/s10329-012-0339-x. [DOI] [PubMed] [Google Scholar]
- Ebbert M.A., McGrew W.C., Marchant L.F. Differences between chimpanzee and baboon gastrointestinal parasite communities. Parasitology. 2015;142:958–967. doi: 10.1017/S0031182015000104. [DOI] [PubMed] [Google Scholar]
- Ellwanger J.H., Cavallero S. Soil-transmitted helminth infections from a One Health perspective. Front. Med. 2023 doi: 10.3389/fmed.2023.1167812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedrick M. Zoonotic hemoparasites of baboons (Papio anubis) at the human-wildlife interface in Kenya. Int. J. Zool. Animal Biol. 2019;2:1–9. [Google Scholar]
- Frias L., Stark D.J., Salgado Lynn M., Nathan S., Goossens B., Okamoto M., MacIntosh A.J.J. Molecular characterization of nodule worm in a community of Bornean primates. Ecol. Evol. 2019;9:3937–3945. doi: 10.1002/ece3.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie T.R. Non-invasive assessment of gastrointestinal parasite infections in free-ranging primates. Int. J. Primatol. 2006;27:1129–1143. [Google Scholar]
- Hahn N.E., Proulx D., Muruthi P.M., Alberts S., Altmann J. Gastrointestinal parasites in free-ranging Kenyan baboons (Papio cynocephalus and P. anubis) Int. J. Primatol. 2003;24:271–279. [Google Scholar]
- Hansen J., Perry B.D. ILRI (aka ILCA and ILRAD); 1990. The Epidemiology, Diagnosis and Control of Gastro-Intestinal Parasites of Ruminants in Africa: A Handbook. [Google Scholar]
- Hasegawa H., Huffman M.A., Chapman C.A. Useful diagnostic references and images of protozoans, helminths, and nematodes commonly found in wild primates. Primate Parasite Ecology. 2009:507–513. [Google Scholar]
- Howells M.E., Pruetz J., Gillespie T.R. Patterns of gastro‐intestinal parasites and commensals as an index of population and ecosystem health: the case of sympatric Western chimpanzees (Pan troglodytes verus) and Guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. Am. J. Primatol. 2011;73:173–179. doi: 10.1002/ajp.20884. [DOI] [PubMed] [Google Scholar]
- Huffman M.A. Current evidence for self‐medication in primates: a multidisciplinary perspective. Am. J. Phys. Anthropol. 1997;104:171–200. The Official Publication of the American Association of Physical Anthropologists. [Google Scholar]
- Huffman M.A. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 2003;62:371–381. doi: 10.1079/pns2003257. [DOI] [PubMed] [Google Scholar]
- Hussain S., Ram M.S., Kumar A., Shivaji S., Umapathy G. 2013. Human Presence Increases Parasitic Load in Endangered Lion-Tailed Macaques. Macaca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E.E. Concluding remarks. Cold Spring Harbor Symp. Quant. Biol. 1957;22:415–427. [Google Scholar]
- Kalousová B., Piel A.K., Pomajbíková K., Modrý D., Stewart F.A., Petrželková K.J. Gastrointestinal parasites of savanna chimpanzees (Pan troglodytes schweinfurthii) in Ugalla, Tanzania. Int. J. Primatol. 2014;35:463–475. [Google Scholar]
- Kanyari P.W.N., Kagira J.M., Mhoma R.J. 2017. Prevalence of Endoparasites in Cattle with Zoonotic Potential within Urban and Peri-Urban Areas of Lake Victoria Basin. Kenya. [Google Scholar]
- Katsvanga C.A.T., Jimu L., Zinner D., Mupangwa J.F. Diet of pine plantation and non-plantation ranging baboon (Papio ursinus) groups with reference to bark consumption in the eastern highlands of Zimbabwe. J. Hortic. For. 2009;1:168–175. [Google Scholar]
- Kawai K., Saathoff E., Antelman G., Msamanga G., Fawzi W.W. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV–infected pregnant women in Tanzania. Am. J. Trop. Med. Hyg. 2009;80:36. [PMC free article] [PubMed] [Google Scholar]
- Kiffner C., Paciência F.M.D., Henrich G., Kaitila R., Chuma I.S., Mbaryo P., Knauf S., Kioko J., Zinner D. Road-based line distance surveys overestimate densities of olive baboons. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kileshye Onema J.-M., Mazvimavi D., Love D., Mul M.L. Effects of selected dams on river flows of Insiza River, Zimbabwe. Phys. Chem. Earth. 2006;31:870–875. [Google Scholar]
- King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chron. Illness. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Kirabo I., Mabiki F.P., Mdegela R.H., Obbo C.J.D. In vitro antibacterial potential of extracts of Sterculia africana, Acacia sieberiana, and Cassia abbreviata ssp. abbreviata used by yellow baboons (Papio cynocephalus) for possible self‐medication in Mikumi National Park, Tanzania. Int J Zool. 2018;2018 [Google Scholar]
- Kouassi R.Y.W., McGraw S.W., Yao P.K., Abou-Bacar A., Brunet J., Pesson B., Bonfoh B., N’goran E.K., Candolfi E. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d'Ivoire. Parasite. 2015;22 doi: 10.1051/parasite/2015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi J.A., Akyeampong S., Abubakari A., Offei Addo S., Okoto D., Hanson H. Zoonotic gastrointestinal parasites of baboons (Papio anubis) in the shai hill reserve in Ghana. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/1083251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larbi J.A., Akyeampong S., Addo S.O., Dakwa K.B., Boampong K., Opoku‐Nketiah B. Distribution of intestinal parasites of baboons (Papio anubis) and warthogs (Phacochoerus aethiopicus) at the Mole National Park, Ghana. Vet. Med. Sci. 2021;7:251–255. doi: 10.1002/vms3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor R., Cwiklinski K., Calvani N.E.D., Dorey A., Hamon S., Corrales J.L., Dalton J.P., De Marco Verissimo C. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis Fasciolosis. Virulence. 2021;12:2839–2867. doi: 10.1080/21505594.2021.1996520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse M., Erko B. Zoonotic intestinal parasites in Papio anubis (baboon) and Cercopithecus aethiops (vervet) from four localities in Ethiopia. Acta Trop. 2004;90:231–236. doi: 10.1016/j.actatropica.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Limantara M.A.Y., Purbantoro S.D., Govendan P.N., Christiani Z., Ridwan I.A., Maharani N. Bali; Indonesia: 2023. Gastrointestinal Parasites of Captive Reptiles. [Google Scholar]
- Mackenzie J.S., Jeggo M. The one health approach—why is it so important? Trav. Med. Infect. Dis. 2019 doi: 10.3390/tropicalmed4020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafuyai H.B., Barshep Y., Audu B.S., Kumbak D., Ojobe T.O. Baboons as potential reservoirs of zoonotic gastrointestinal parasite infections at Yankari National Park, Nigeria. Afr. Health Sci. 2013;13:252–254. doi: 10.4314/ahs.v13i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magden E.R., Mansfield K.G., Simmons J.H., Abee C.R. Laboratory Animal Medicine. Elsevier; 2015. Nonhuman primates; pp. 771–930. [Google Scholar]
- Manz K.M., Clowes P., Kroidl I., Kowuor D.O., Geldmacher C., Ntinginya N.E., Maboko L., Hoelscher M., Saathoff E. Trichuris trichiura infection and its relation to environmental factors in Mbeya region, Tanzania: a cross-sectional, population-based study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis L., Esch G.W., Holmes J.C., Kuris A.M., Schad Ga. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists) J. Parasitol. 1982;68:131–133. [Google Scholar]
- Mason B., Piel A.K., Modrý D., Petrželková K.J., Stewart F.A., Pafčo B. Association of human disturbance and gastrointestinal parasite infection of yellow baboons in western Tanzania. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Coma S., Valero M.A., Bargues M.D. Human and animal fascioliasis: origins and worldwide evolving scenario. Clin. Microbiol. Rev. 2022;35(e00088–19) doi: 10.1128/cmr.00088-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.K., Ridley A., Kaplin B.A., Grueter C.C. A comparison of fecal sampling and direct feeding observations for quantifying the diet of a frugivorous primate. Curr Zool. 2020;66:333–343. doi: 10.1093/cz/zoz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbuya M.N.N., Tavengwa N.V., Stoltzfus R.J., Curtis V., Pelto G.H., Ntozini R., Kambarami R.A., Fundira D., Malaba T.R., Maunze D. Design of an intervention to minimize ingestion of fecal microbes by young children in rural Zimbabwe. Clin. Infect. Dis. 2015;61:S703–S709. doi: 10.1093/cid/civ845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley C. Genotypes within and Among Baboon (Papio ursinus) Troops on the Cape Peninsula. 2013. Infection of two distinct Trichuris sp. South Africa. [Google Scholar]
- Müller-Graf C.D.M., Collins D.A., Woolhouse M.E.J. Intestinal parasite burden in five troops of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology. 1996;112:489–497. doi: 10.1017/s0031182000076952. [DOI] [PubMed] [Google Scholar]
- Muriuki S.M.K., Murugu R.K., Munene E., Karere G.M., Chai D.C. Some gastro-intestinal parasites of zoonotic (public health) importance commonly observed in old world non-human primates in Kenya. Acta Trop. 1998;71:73–82. doi: 10.1016/s0001-706x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Musara J.P., Tibugari H., Moyo B., Mutizira C. Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: hedging against climate change-induced shocks in semi-arid Zimbabwe. Open Life Sci. 2021;16:1330–1340. doi: 10.1515/biol-2021-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayima J., Hayashida K., Nakao R., Ishii A., Ogawa H., Nakamura I., Moonga L., Hang’ombe B.M., Mweene A.S., Thomas Y. Detection and characterization of zoonotic pathogens of free-ranging non-human primates from Zambia. Parasites Vectors. 2014;7:1–7. doi: 10.1186/s13071-014-0490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’da K.M., Dahourou L.D., Ndiaye P.I., Lindshield S., Gbati O.B., Traore A. Gastrointestinal parasites of baboons (Papio papio) in Niokolo-Koba National Park, Senegal. Open Vet. J. 2022;12(4):481–488. doi: 10.5455/OVJ.2022.v12.i4.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndagurwa H.G.T. 2007. Bark Stripping by Chacma Baboons (Papio ursinus) in Relation to Daily Patterns of Activity, Feeding Behaviour and Home Range in a Pine Plantation in Eastern Zimbabwe. [Google Scholar]
- Nunn C.L., Gillespie T.R., Wich S., Marshall A. An Introduction to Primate Conservation. 2016. Infectious disease and primate conservation; pp. 157–173. [Google Scholar]
- Nunn C.L., Thrall P.H., Leendertz F.H., Boesch C. The spread of fecally transmitted parasites in socially-structured populations. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obanda V., Maingi N., Muchemi G., Ng’ang’a C.J., Angelone S., Archie E.A. Infection dynamics of gastrointestinal helminths in sympatric non-human primates, livestock and wild ruminants in Kenya. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaido M., Dranzoa C., Cheli P. Notes and records gastrointestinal parasites of baboons (Papio anubis) interacting with humans in west bugwe forest reserve, Uganda. Afr. J. Ecol. 2003;41 [Google Scholar]
- Pafčo B., Čížková D., Kreisinger J., Hasegawa H., Vallo P., Shutt K., Todd A., Petrželková K.J., Modrý D. Metabarcoding analysis of strongylid nematode diversity in two sympatric primate species. Sci. Rep. 2018;8:5933. doi: 10.1038/s41598-018-24126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A., Graczyk T.K., Geller N., Vittor A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Pebsworth P.A., Bardi M., Huffman M.A. Geophagy in chacma baboons: patterns of soil consumption by age class, sex, and reproductive state. Am. J. Primatol. 2012;74:48–57. doi: 10.1002/ajp.21008. [DOI] [PubMed] [Google Scholar]
- Pham-Duc P., Nguyen-Viet H., Hattendorf J., Zinsstag J., Phung-Dac C., Zurbrügg C., Odermatt P. Ascaris lumbricoides and Trichuris trichiura infections associated with wastewater and human excreta use in agriculture in Vietnam. Parasitol. Int. 2013;62:172–180. doi: 10.1016/j.parint.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Phillips K.A., Haas M.E., Grafton B.W., Yrivarren M. Survey of the gastrointestinal parasites of the primate community at Tambopata National Reserve, Peru. J. Zool. 2004;264:149–151. [Google Scholar]
- Pirajno F., González-Álvarez I. A re-appraisal of the Epoch nickel sulphide deposit, Filabusi Greenstone Belt, Zimbabwe: a hydrothermal nickel mineral system? Ore. Geol. Rev. 2013;52:58–65. [Google Scholar]
- Ponce-Terashima R., Koskey A.M., Reis M.G., McLellan S.L., Blanton R.E. Sources and distribution of surface water fecal contamination and prevalence of schistosomiasis in a Brazilian village. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics 9–26. 2015. Quantifying parasite diversity. [Google Scholar]
- Ravasi D.F., O'Riain M.J., Adams V.J., Appleton C.C. A coprological survey of protozoan and nematode parasites of free-ranging chacma baboons (Papio ursinus) in the southwestern Cape, South Africa. South African Journal of Wildlife Research-24-month delayed open access. 2012;42:35–44. [Google Scholar]
- Richards L., Erko B., Ponpetch K., Ryan S.J., Liang S. Assessing the nonhuman primate reservoir of Schistosoma mansoni in Africa: a systematic review. Infect Dis Poverty. 2019;8:10–20. doi: 10.1186/s40249-019-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S.J., Brashares J.S., Walsh C., Milbers K., Kilroy C., Chapman C.A. A survey of gastrointestinal parasites of olive baboons (Papio anubis) in human settlement areas of Mole National Park, Ghana. J. Parasitol. 2012;98:885–888. doi: 10.1645/GE-2976.1. [DOI] [PubMed] [Google Scholar]
- Sangpeng J., Eamudomkarn C., Hongsrichan N., Artchayasawat A., Chaisongkram C., Ponsrila K., Kimkamkaew S., Laoprom N., Boonmars T., Sithithaworn P. Prevalence of gastrointestinal parasites in captive mammals at Khon Kaen Zoo, Thailand. Vet. World. 2023;16:2416. doi: 10.14202/vetworld.2023.2416-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom T., Lavan R.P., Torres S., Heaney K. The economic impact of parasitism from nematodes, trematodes and ticks on beef cattle production. Animals. 2023;13:1599. doi: 10.3390/ani13101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabasshum T., Liza F.T., Rabbe M.F., Mukutmoni M., Alam M.M., Begum A. Occurrence of gastrointestinal (GI) parasites in captive olive baboon and common langur in Bangladesh. Animal Diseases. 2022;2:4. [Google Scholar]
- Taylor M.A., Coop R.L., Wall R. John Wiley & Sons; 2015. Veterinary Parasitology. [Google Scholar]
- Torgerson P.R. One world health: socioeconomic burden and parasitic disease control priorities. Vet. Parasitol. 2013;195:223–232. doi: 10.1016/j.vetpar.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Uppal H.S., Bal M.S., Singla L.D., Kaur P., Sandhu B.S. Morphometric and scanning electron microscopy based identification of Ancylostoma caninum parasites in dog. J. Parasit. Dis. 2017;41:517–522. doi: 10.1007/s12639-016-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta K., Twinomugisha D., Godfrey K., Liu C., Schoof V.A.M., Goldberg T.L., Chapman C.A. Comparison of gastrointestinal parasite communities in vervet monkeys. Integr. Zool. 2017;12:512–520. doi: 10.1111/1749-4877.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J. Vol. 17. Afr Primates; 2023. (Conservation Status of African Primates: Updates to the IUCN Red List for 2020-2023). [Google Scholar]
- Wells K., Gibson D.I., Clark N.J., Ribas A., Morand S., McCallum H.I. Global spread of helminth parasites at the human–domestic animal–wildlife interface. Global Change Biol. 2018;24:3254–3265. doi: 10.1111/gcb.14064. [DOI] [PubMed] [Google Scholar]
- Weyher A.H., Ross C., Semple S. Gastrointestinal parasites in crop raiding and wild foraging Papio anubis in Nigeria. Int. J. Primatol. 2006;27:1519–1534. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on request.