Abstract
The efficacy of vonoprazan‐based dual and triple therapy vs. lansoprazole‐based triple therapy in the treatment of H. pylori infection was largely consistent regardless of age, sex, race, ethnicity, BMI, alcohol intake, smoking status, and study drug compliance.
Keywords: clinical trial, Helicobacter pylori, potassium competitive acid blockers, proton pump inhibitors, vonoprazan
Introduction
Half of the world's population is infected with Helicobacter pylori (H. pylori), with wide regional variation in prevalence. 1 Approximately 80% of people with H. pylori infection are asymptomatic. However, all develop gastritis, 10% develop peptic ulcer disease, 2 and a small percentage develop gastric adenocarcinoma or gastric marginal zone B‐cell lymphoma of mucosa‐associated lymphoid tissue. 2
Since 1989, proton pump inhibitors (PPIs) have been considered part of H. pylori treatment regimens. By raising intragastric pH, they promote active replication of H. pylori, increasing the effectiveness of some antibiotics used to treat this infection. 2 Currently, the triple combination of a PPI, clarithromycin, and amoxicillin (or metronidazole) remains one of the most commonly used regimens for the treatment of H. pylori infection. 3 However, widespread use of macrolide antibiotics for other infections has led to an increase in the prevalence of H. pylori clarithromycin resistance, and a corresponding decline in eradication rates with clarithromycin‐based triple regimens. 4 It has been recommended that clarithromycin should only be used in regions where resistance is known to be <15% and that its use be restricted to patients with no previous history of macrolide exposure for any reason. 4
Vonoprazan, a potassium‐competitive acid blocker (P‐CAB), has been approved in Japan and several other countries for the treatment of H. pylori infection and other acid‐related diseases since 2014. In 2022, it was approved by the United States Food and Drug Administration (FDA) as a copackaged product with amoxicillin (dual regimen) or with clarithromycin and amoxicillin (triple regimen) for the treatment of H. pylori infection in adults. 5 P‐CABs are pharmacokinetically and pharmacodynamically distinct from PPIs; they produce more rapid and profound inhibition of gastric acid secretion and may be dosed independently of mealtimes. 6
The phase 3 pHalcon‐HP trial (NCT04167670) was conducted in treatment‐naive adults with H. pylori infection in the United States and five European countries. 7 Patients (N = 1046) were randomized to 14 days of open‐label vonoprazan dual therapy, or double‐blind vonoprazan‐ or lansoprazole‐based triple therapy. Vonoprazan‐based dual and triple therapy were noninferior to lansoprazole‐based triple therapy for eradication rates in patients with nonresistant strains; both were superior to lansoprazole‐based triple therapy in patients with clarithromycin‐resistant strains, and among all patients, regardless of resistance status. The size of the pHalcon‐HP program created the opportunity to understand whether the magnitude of the treatment effect for vonoprazan versus lansoprazole varies across different subgroups.
To explore the possibility of differences in treatment effect among specific subsets of patients enrolled in pHalcon‐HP, we performed subgroup analyses of eradication rates among all patients, regardless of resistance status. Subgroups assessed included those based on baseline characteristics (age, sex, race, ethnicity, body mass index [BMI], self‐reported smoking, and alcohol use), and study drug compliance. Eradication rates were summarized by treatment group for each level in each subgroup. Within each level, the point estimate and two‐sided 95% confidence interval (CI) of the difference in eradication rates between each vonoprazan group and the lansoprazole group were calculated via the Miettinen and Nurminen method. For each subgroup, logistic regression was performed, including factors for treatment, subgroup, and treatment by subgroup interaction for the comparisons of vonoprazan dual and triple therapy with lansoprazole triple therapy.
To identify factors that may have influenced eradication rates, we performed stepwise logistic regression to fit two models. The first included subjects who received vonoprazan dual therapy or lansoprazole triple therapy. The second included subjects who received vonoprazan triple therapy or lansoprazole triple therapy. For both models, factors considered for inclusion included treatment group, clarithromycin resistance status, and the subgroup variables listed above. Statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute, Cary, NC).
The full analysis set used for this subanalysis included 992 patients (vonoprazan dual therapy, n = 324; vonoprazan triple therapy, n = 338; lansoprazole triple therapy; n = 330). Baseline characteristics were well balanced among the groups.
Treatment group differences of vonoprazan dual and triple therapy compared with lansoprazole triple therapy across baseline demographic and clinical subgroups are shown in Figure 1a,b, respectively.
Figure 1.
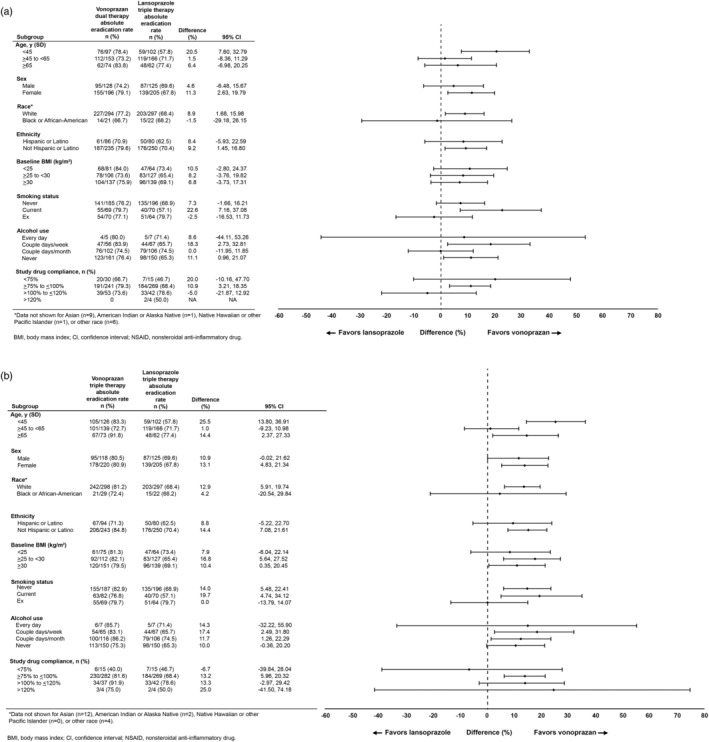
Differences in Helicobacter pylori eradication rates between (a) vonoprazan dual therapy and lansoprazole triple therapy or (b) vonoprazan triple therapy and lansoprazole triple therapy in all patients, according to baseline demographics and clinical characteristics.
Multivariate modeling to explore the impact of each subgroup on eradication rates revealed that in both models, eradication rates were higher in subjects with H. pylori strains not resistant to clarithromycin (P < 0.0001 for both) and in older subjects (P = 0.0157 and P = 0.0193 for vonoprazan dual and triple therapy, respectively). In the vonoprazan triple therapy model, compliance (P = 0.0026) and alcohol use (P = 0.0410) were also significant factors; eradication rates were higher in subjects with better study drug compliance, and in subjects who used alcohol ≥2 days/month versus than those who never used alcohol.
Discussion
pHalcon‐HP was the first trial to assess vonoprazan‐based dual and triple therapy in patients from the United States and Europe. 7 In this post hoc analysis, the magnitude of the treatment effect for vonoprazan versus lansoprazole in H. pylori eradication was generally comparable, regardless of most demographic and clinical characteristics, consistent with the results in the overall group of patients. Of note, age group significantly influenced relative eradication rates, with the greatest difference seen in patients aged <45 years in favor of vonoprazan‐based therapies. Although we can only speculate on the reason for this difference, the phenomenon of reduced adherence to medications among younger patients has been demonstrated in multiple studies. 8 , 9 PPIs require acid activation and thus should ideally be administered ~30 min prior to a meal, 10 whereas the relative timing of food intake does not influence vonoprazan pharmacodynamics. 4 Younger patients may have been less likely to adhere to dosing recommendations for lansoprazole relative to meals.
As all patients in this study were treatment‐naive, results cannot be generalized to patients with persistent H. pylori infection despite previous treatment. Some subgroups had relatively small numbers of patients, resulting in correspondingly wide confidence intervals. This precludes any firm conclusions regarding relative efficacy of vonoprazan‐based regimens versus lansoprazole triple therapy in these subgroups. The potential marginal benefit of alcohol use on eradication rates with vonoprazan‐based triple therapy has no obvious explanation and could even represent a chance finding among the multiple subgroup analyses.
In conclusion, the relative efficacy of vonoprazan‐based regimens versus lansoprazole triple therapy was consistent regardless of age, sex, race, ethnicity, BMI, alcohol intake, smoking status, and study drug compliance.
Ethical approval
The trial was approved by the institutional review boards of participating institutions, and written informed consent was obtained from participants before enrollment. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Acknowledgments
ClinicalTrials.gov: NCT04167670: Efficacy and safety of vonoprazan compared with lansoprazole in participants with Helicobacter pylori infection. Editorial support was provided by John Ferguson of Medical Leverage Communications, funded by Phathom Pharmaceuticals in accordance with Good Publications Practice (GPP 2022) guidelines.
Declaration of conflict of interest: WDC: Consultant, Phathom Pharmaceuticals; FM: Consultant, Phathom Pharmaceuticals; speaker for Biocodex; LL: Consultant, Phathom Pharmaceuticals; NS: Employee, stock/stock options—Phathom Pharmaceuticals; EL: Employee, stock/stock options—Phathom Pharmaceuticals; BH: Employee, stock/stock options—Phathom Pharmaceuticals; CWH: Consultant and speaker, Phathom Pharmaceuticals; consultant, ISOThrive; consultant EndoStim.
Author contribution: WDC, FM, LL, and BH contributed to study conceptualization. WDC, NS, EL, BH, and CWH contributed to formal analysis. All authors reviewed, edited, and approved the final manuscript.
Financial support: This study was supported by Phathom Pharmaceuticals.
Data availability statement
Individual participant data will not be shared.
References
- 1. Li Y, Choi H, Leung K, Jiang F, Graham DY, Leung WK. Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta‐analysis. Lancet Gastroenterol. Hepatol. 2023; 8: 553–564. [DOI] [PubMed] [Google Scholar]
- 2. Malfertheiner P, Camargo MC, El‐Omar E et al. Helicobacter pylori infection. Nat. Rev. Dis. Primers. 2023; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howden CW, Leifke E, Jacob R, Divino V, Fass R. Characterization of treatment practices for patients with newly diagnosed H. pylori Infection: a US population‐based study using claims and electronic medical record data. Poster presented at: Digestive Disease Week; 2021 May 22‐25; Washington, DC 2021.
- 4. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017; 112: 212–239. [DOI] [PubMed] [Google Scholar]
- 5. Phathom Pharmaceuticals, Inc . Vonoprazan [Prescribing Information]. Buffalo Grove, IL: Phathom Pharmaceuticals, Inc, 2023. [Google Scholar]
- 6. Laine L, Sharma P, Mulford DJ et al. Pharmacodynamics and pharmacokinetics of the potassium‐competitive acid blocker vonoprazan and the proton pump inhibitor lansoprazole in US subjects. Am. J. Gastroenterol. 2022; 117: 1158–1161. [DOI] [PubMed] [Google Scholar]
- 7. Chey WD, Megraud F, Laine L, Lopez LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology. 2022; 163: 608–619. [DOI] [PubMed] [Google Scholar]
- 8. Ge L, Heng BH, Yap CW. Understanding reasons and determinants of medication non‐adherence in community‐dwelling adults: a cross‐sectional study comparing young and older age groups. BMC Health Serv. Res. 2023; 23: 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeitouny S, Cheng L, Wong ST, Tadrous M, McGrail K, Law MR. Prevalence and predictors of primary nonadherence to medications prescribed in primary care. CMAJ. 2023; 195: e1000–e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hatlebakk JG, Katz PO, Camacho‐Lobato L, Castell DO. Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. Aliment. Pharmacol. Ther. 2000; 14: 1267–1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will not be shared.


