Abstract
Background and Aim
Endoscopic submucosal dissection (ESD) has become the treatment of choice for many superficial gastric neoplasms. Clinical outcomes are increasingly comparable between Japanese and Western series; however, data are lacking on the validity of risk stratification tools in Western cohorts. We aimed to evaluate clinical outcomes, explore risk stratification, and compare our data with published Western series.
Methods
We conducted a retrospective, observational cohort study in a single tertiary referral center over a 13‐year period. Primary outcomes were rates of en bloc, complete (R0) and curative resection. Secondary outcomes included adverse events, recurrence, metachronous lesions, eCura grades, and ESGE criteria. A comparative analysis was performed with existing published series from Western centers.
Results
Totally 112 patients were included in the study cohort. 50.9% were male, 87.5% Caucasian, and median age was 75.5 years (IQR 14.3 years). Lesions were predominantly antral (36.6%) or body (35.7%); median size 20 mm (IQR 15 mm). Rates of en bloc, R0 resection, and curative resection were 96.4%, 89.3%, and 78.6% (identical between eCura and ESGE), respectively. Adverse events occurred in 5.8%, recurrence in 0%, and metachronous lesions in 9.9%. Our data compared favorably with a review existing Western series, which illustrates increasing adoption of ESD and stable outcomes over time.
Conclusion
ESD represents a safe and effective method of treatment for gastric neoplasia in the Western setting. This study highlights the potential for excellent outcomes in a single center with a heterogeneous patient cohort and supports the use of eCura in guiding post procedural management.
Keywords: endoscopic submucosal dissection, ESD, gastric cancer, stomach
We present the outcomes for endoscopic submucosal dissection (ESD) for early gastric cancer (EGC) in a single tertiary referral centre, with positive chronological comparison made to international Western series.
Introduction
Since its inception in Japan in the 1990s, endoscopic submucosal dissection (ESD) has become the treatment of choice for gastric neoplasia with low risk of lymph node metastasis (LNM). 1 , 2 , 3 Early gastric cancer (EGC) is defined as carcinoma invading no deeper than the gastric submucosa, regardless of lymph node status. 4 ESD for EGC results in superior technical and clinical outcomes when compared with endoscopic mucosal resection (EMR) and gastrectomy, in addition to fewer adverse events and enhanced health‐related quality of life. 3 , 5 , 6
After initial concerns over safety and efficacy, multiple series have demonstrated that outcomes from Western centres have become generally comparable with those from East Asia. 3 , 7 , 8 , 9 , 10 However, differences in demographics, natural history and healthcare utilization raise the possibility that Japanese data may not be entirely applicable to a Western population.
Prior to resection, image enhanced endoscopy (IEE) has been shown to be accurate in predicting lesion characteristics, including depth of invasion. Yet, approximately 20% of ESD result in non‐curative resections. 3 , 11 This can occur despite complete or R0 removal of a lesion due to the histopathological presence of features associated with higher risk of LNM, for example, poor differentiation, lymphovascular invasion, or ulceration. To guide further management in these cases, scoring systems such as eCura have been developed to define and predict ‘curability’, that is, risk of LNM and recurrence. 12 This system was validated in a Japanese population, and so data on its utility in Western centers remain limited. Similar risk stratification tools have been developed by the European Society of Gastrointestinal Endoscopy (ESGE), and efforts are underway to explore dedicated tools for Western populations. 3 , 13
To support an evidence‐based expansion of ESD for EGC, real‐world data on safety and efficacy from Western centers are essential. This study aimed to describe outcomes of gastric ESD in a multicultural, tertiary referral center in Australia and to explore the utility of risk stratification tools in this cohort.
Methods
We conducted a retrospective, single‐center, observational cohort study of patients who underwent ESD for EGC from January 2010 to September 2023. This study took place in a single tertiary referral center in Perth, Australia. Demographics and relevant clinical data were collected and analyzed from patient records. These included Helicobacter Pylori (H pylori) status (as defined presence of H pylori on histopathological analysis during diagnostic workup or on ESD specimen), previous gastric surgery history, prior ESD and EMR history, and history of autoimmune gastritis. Endoscopic, pathological, and procedural characteristics were also recorded. Histopathology was defined according to the World Health Organization (WHO) classification. 14
Primary and secondary outcomes
The primary outcomes for this study were endoscopic en bloc, R0, and curative resection rates in all ESDs performed. En bloc resections were those where the lesion was endoscopically judged to be fully removed in a single piece. R0 or complete resection was where lateral and deep margins were clear of neoplasm. Curative lesions were those described by eCura A or B criteria, with all other lesions categorized as eCura C1 or 2.
Secondary outcomes included procedural complications, post ESD surgery, local recurrence, and metachronous lesions. Local recurrence was defined as the presence of endoscopic or histological evidence of gastric neoplasm at the site of index ESD during follow‐up endoscopy. Metachronous lesion was defined as any new neoplasm in an area other than the site of index ESD during follow‐up endoscopy. Recorded complications included perforation during ESD, acute bleeding (within the first 24 h), and delayed bleeding (greater than 1 week following ESD). Other events such as follow‐up times, death from gastric cancer, and other causes were also described.
Inclusion and exclusion criteria
Cases were included if ESD was performed for lesions that were diagnosed endoscopically and histologically as EGC. EGC was defined according to the Japanese Gastroenterological Endoscopy Society (JGES) definition of carcinoma invading no deeper than the submucosa, regardless of lymph node status. 4 Low‐grade (LGD) or high‐grade dysplasia (HGD) corresponded to Tis. Expanded criteria for resection were defined by the JGES guidelines 2016 1 (Fig. 1). ESD for gastric lesions that did not meet these histopathological definitions were not included in this study. Lesions were described according to location, size, and Paris classification.
Figure 1.
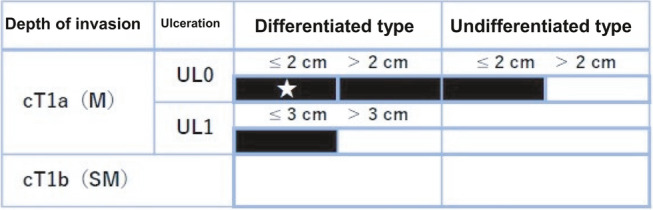
Absolute Indications and Expanded Indications for Treating EGC with ESD. Figure depicting indications and expanded indications for ESD of early gastric cancer as per Japanese Gastroenterological Endoscopy Society (JGES) guidelines. cT1a, 
 , cT1b,
, cT1b,  .
.
ESD procedure
ESD was performed by two operators: MC (2010–2023) and NM (2016–2023). Referrals for ESD underwent additional endoscopic evaluation to assess for ESD suitability as per the absolute and expanded criteria. ESD was performed using a Fujinon 360Z adult gastroscope (ELUXEO processor). DualKnife‐J (Olympus Medical) and IT knife (Olympus Medical) were used for resection. All lesions were marked 2–3 mm outside the demarcation line of the lesion prior to resection and lifted using indigo carmine and gelofusine. Dilute Adrenaline was used in the lifting solution at the periphery of the lesion prior to initial mucosal incision.
Various traction methods were used at the endoscopist's discretion including clip+snare, cli + suture line, etc. Patients were admitted to hospital post ESD and were started on either proton pump inhibitor (PPI) infusion or twice daily PPI. Patients were started on clear fluids on the day of the procedure and slowly upgraded to a normal diet over 3–4 days. If the patients were started on bd PPI, they were discharged home the following day, and if the patients were on PPI infusion, they were admitted to hospital for 72 h.
Histopathological assessment and staging
Following ESD, all specimens were assessed by one of two pathologists with a special interest in GI pathology. Endoscopic curability of EGC was determined according to the eCura staging system (A, B, C1, C2) as described in the JGES guidelines 2020 (Fig. 2). 1 , 12 Lesions were considered curative if they were eCura A or B.
Figure 2.
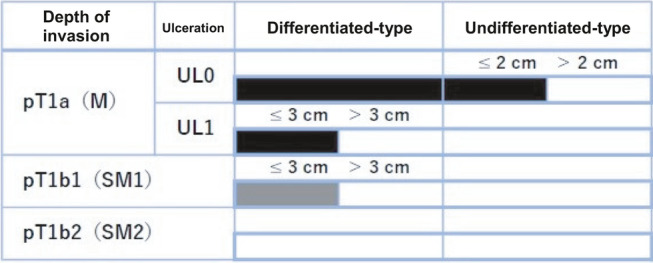
eCura criteria for curability. Figure depicting eCura grade according to Japanese Gastroenterological Endoscopy Society (JGES) Guidelines 2020. Lesions are confined to en bloc resection and HM0, VM0, Ly0, and V0. pT1a (M), intramucosal cancer (histopathological diagnosis); pT1b (SM), submucosally invasive cancer (histopathological diagnosis). UL, finding of ulceration (or ulcer scar); UL0, absence of ulceration or ulcer scar; UL1, presence of ulceration or ulcer scar (1). Note is made of misspelling of eCura as ‘eCure.’ eCureA*,  ; eCureB*,
; eCureB*,  ; eCureC‐2,
; eCureC‐2,  .
.
Lesions were considered eCura A if they were resected en bloc and met the following conditions according to JGES guidelines: (i) predominantly differentiated type, pT1a, UL0, HM0 VM0, Ly0, V0, regardless of size; (ii) long diameter ≤2 cm, predominantly undifferentiated type, pT1a, UL0, HM0, VM0, Ly0, V0; or (iii) long diameter ≤3 cm, predominantly differentiated type, pT1a, UL1, HM0, VM0, Ly0, V0. 1 Lesions were considered eCura B if they were resected en bloc and were ≤3 cm in long diameter, predominantly of the differentiated type, and satisfy the following criteria: pT1b1 (SM1) (within <500 μm from the muscularis mucosae), HM0, VM0, Ly0, and V0. Lesions that did not meet the criteria for eCuraA or B were eCuraC. If lesions met criteria for eCuraA or B but were not resected en bloc or had positive horizontal margins, they were defined as eCuraC‐1. All other lesions were eCuraC‐2. Further management was based on JGES guidelines (Fig. 3). 1
Figure 3.
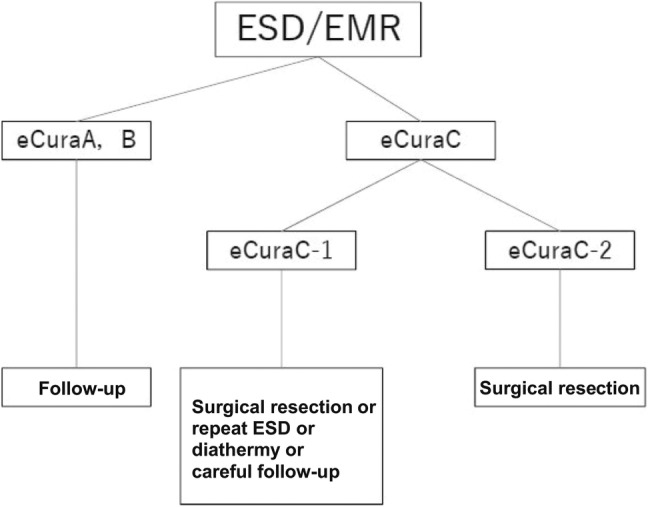
Japanese Gastroenterological Endoscopy Society (JGES) algorithm following ESD. JGES guidelines on management of lesions following ESD.
Patients with eCura grades A and B were followed up with an initial 3‐month gastroscopy followed by annual gastroscopy and computed tomography (CT) surveillance for recurrence and metastasis. Patients with an eCuraC‐1 and 2 scores were routinely referred for surgery in line with JGES guidelines (Fig. 3). However, a similar surveillance strategy was also offered in patients who were unfit for surgery or declined further treatment.
We also looked at other risk stratification tools to determine if there were differences in outcomes. The ESGE guidelines on ESD for superficial gastrointestinal lesions categorize patients into very low/low (curative), low and high (non‐curative) categories. W‐eCura is a modified version of the eCura system that has been described recently to apply to a non‐Western cohort. The most notable difference with eCura is that lesions demonstrating submucosal invasion are considered to be low risk until they display depth of invasion of >1000um. This scoring system has yet to be validated. We calculated ESGE and W‐eCura grades to determine if there were any significant differences between the scoring systems.
Review and comparison of existing data
A literature review of published data was conducted using Pubmed and Embase using the search terms ‘gastric’ and ‘ESD’. Relevant systematic reviews (SR) were included and combined with subsequent published data from inception until December 2023. A systematic review and meta‐analysis by Zullo et al. was explored and granular data extracted which reported outcomes to October 2019. 15 A review of subsequently published series was added from this date until December ‘23. Studies were eligible for inclusion if they were from Western centers examining outcomes of ESD for EGC and were full articles available in English, Italian, or Spanish; on humans only; with at least five patients. Articles comparing ESD with different types of resection or comparing different subtypes of ESD technique were excluded. Articles containing combined outcomes for ESD of epithelial and non‐epithelial lesions were assessed and outcomes for EGC was extracted where possible. Studies were then analyzed and compared over time periods (2008–2014, 2015–2019 and 2020‐December 2023). These were then compared with our series to assess differences in outcomes.
Statistical analysis
For all outcomes assessed, absolute (n) and percentages (%) were calculated for categorical variables and mean ± SD, median and interquartile ranges were calculated for continuous variables. Chi‐square or Fisher's exact tests were used to assess differences in categorical variables, and Student's t test or Mann–Whitney tests were used for continuous variables. Regression analysis was used to determine relationships between variables. A significant P value was defined as <0.05.
Results
Patient and lesion characteristics
A total of 112 ESDs were attempted for EGC over a 13‐year period. 50.9% of patients were male, with a median age of 75.5 years [IQR 14.3] (Table 1). Most patients were Caucasian (87.5%) or East Asian (8.9%); 19.8% had an existing or previous diagnosis of Helicobacter pylori, and 26.4% had a history of autoimmune gastritis. The majority of lesions were in the antrum (36.6%) or body (35.7%) and were flat in morphology (Paris OIIa – 67.3%). The median size was 20 mm, ranging from 9 to 130 mm. Most lesions were adenocarcinoma (75.9%) and were well or moderately differentiated (86.7%), and most were confined to the mucosa (pT1a – 61.3%, or intraepithelial neoplasia – 24.3%).
Table 1.
Demographics and lesions characteristics
| Demographics + Lesion characteristics (n = 112) | |
|---|---|
| Age at procedure, median [IQR] | 75.5 [14.3] |
| Gender, n (%) | |
| Male | 57 (50.9%) |
| Female | 55 (49.1%) |
| Ethnicity | |
| Aboriginal or Torres strait | 1 (0.9%) |
| African | 1 (0.9%) |
| Caucasian | 98 (87.5%) |
| East Asian | 10 (8.9%) |
| Indian | 1 (0.9%) |
| Middle Eastern | 1 (0.9%) |
| History of Helicobacter pylori, n (%) | 19 (19.8%) |
| Autoimmune gastritis, n (%) | 29 (26.4%) |
| Anticoagulation, n (%) | |
| Yes | 18 (17%) |
| DOAC | 13 (12%) |
| Warfarin | 5 (5%) |
| No | 88 (83%) |
| Antiplatelets, n (%) | |
| Yes | 23 (22%) |
| Aspirin | 18 (17%) |
| Other | 2 (2%) |
| Dual antiplatelet | 3 (3%) |
| No | 82 (77%) |
| Previous gastric surgery, n (%) | 4 (4%) |
| Lesion characteristics | |
| Lesion location, n (%) | |
| Antrum | 41 (36.6%) |
| Body | 40 (35.7%) |
| Incisura | 16 (14.3%) |
| Cardia | 13 (11.6%) |
| Fundus | 2 (1.8%) |
| Polyp morphology (Paris), n (%) | |
| Is | 15 (15.3%) |
| IIa | 66 (67.3%) |
| IIb | 12 (12.2%) |
| IIc | 5 (5.1%) |
| Lesion size, mm, median [IQR] | 20 mm [15 mm) |
| Histopathology, n (%) | |
| Low grade dysplasia | 9 (8%) |
| High grade dysplasia | 18 (16.1%) |
| Adenocarcinoma | 85 (75.9%) |
| Tumor differentiation, n = 83 | |
| Well/Moderate | 72 (86.7%) |
| Poor | 11 (13.3%) |
| Tumor stage, n = 111 | |
| T1a | 69 (62.2%) |
| T1b | 14 (12.6%) |
| T2 | 1 (0.9%) |
| Intraepithelial Neoplasia | 27 (24.1%) |
| Depth of Invasion, um, [IQR] | 900um [1100um] |
| Duration, mins, median [IQR] | 120 min [80 min] |
| Type of anesthetic | |
| General anesthetic | 112 (100%) |
Table describing characteristics of patients, lesions and procedures. Staging based on WHO TNM staging system.
Procedural data
All resections were performed by one of two interventional endoscopists. All patients received a general anesthetic. Median duration was 120 min, ranging from 24 to 510 min. Three procedures were abandoned due to severe submucosal fibrosis preventing safe resection.
Outcomes
Over the study period, rates of en bloc, R0, and curative resection (as per eCura) were 96.4%, 89.3%, and 77.7%, respectively (Table 2).
Table 2.
Outcomes of lesions and patients post ESD
| Outcomes | |
|---|---|
| En bloc resection, n (%) | 108 (96.4%) |
| Resection, n (%) | |
| R0 | 100 (89.3%) |
| R1 | 12 (10.7%) |
| Curative resection (JGES), n (%) | 88 (78.6%) |
| eCura grade (JGES), n = 112 | |
| eCura A | 86 (76.8%) |
| eCura B | 2 (1.8%) |
| eCura C1 | 2 (1.8%) |
| eCura C2 | 22 (19.6%) |
| ESGE risk grade, n = 112 | |
| Curative (low/very low risk) | 88 (78.6%) |
| Local risk resection | 2 (1.8%) |
| Non‐curative/high risk | 22 (19.6%) |
| W‐eCura, n = 112 | |
| A | 86 (76.8%) |
| B | 2 (1.8%) |
| C1 | 3 (2.7%) |
| C2 | 21 (18.8%) |
| Surgery | |
| Referrals for surgery | 18 (16.1%) |
| Surgical resection | 4 (3.6%) |
| LNM on resection | 0% |
| Residual disease on resection | 1/4 (25%) |
| New lesions, n (%) | 11 (9.9%), P = 0.85 |
| eCura A | 8 (7.2%) |
| eCura B | 0 |
| eCura C1 | 0 |
| eCura C2 | 3 (2.7%) |
| Recurrence, n = 96 | 0% |
| Complications | 7 (5.8%) |
| Intraprocedural perforation | 2 (1.8%) |
| Delayed bleeding | 4 (3.6%) |
| Thrombophlebitis | 1 (0.9%) |
| Duration of follow‐up, weeks [SD] | 158 [119 weeks], P = 0.006 |
| eCura A | 201 [125] |
| eCura B | 213 [191] |
| eCura C1 | 87 [2] |
| eCura C2 | 131 [71] |
Table describing outcomes including resection status, curative status and classification according to eCura, W‐eCura, and ESGE systems.
Complications occurred in seven cases (5.8%), including intraprocedural perforation (n = 2), delayed bleeding (n = 4), and thrombophlebitis (n = 1). Intraprocedural perforations were endoscopically clipped shut, administered intravenous antibiotics, and the ESDs were completed.
Of the 24 patients with non‐curative resection, 18 (16.1%) were referred for surgery, with the others declining referral due to frailty or patient preference. Of the 18 referred for surgery, four (3.6%) patients underwent gastric surgery post ESD (including one resection which was abandoned due to submucosal scarring). No patients (0%) had positive lymph nodes. One patient with an eCuraC2 lesion was found to have residual adenocarcinoma in the surgically resected stomach; however, margins were clear and there was no lymphovascular invasion. There was no residual disease or involved LN in the resection specimens of the remaining three patients that underwent surgery. Of the 14 patients who were referred but did not undergo surgery, the median age was 72.5 years [9.7 years], with a mortality rate of 35.3% (n = 6) over a median duration of 7.5 months, reflecting the highly comorbid nature of this group. During continuous follow‐up of this cohort, none (0%) of the surviving patients have developed recurrence or metastatic disease.
Ten (8.9%) patients developed metachronous lesions. Seven patients (six adenocarcinoma and one HGD) had repeat curative ESD; one patient had a repeat ESD which was abandoned, and the patient proceeded to surgery; one patient was diagnosed with a new LGD; one patient refused further treatment and returned to their country of origin.
All surviving patients (100%) were reviewed within 3 months of the end of the study period, with the median duration of follow‐up (surveillance gastroscopy, CT, or clinic appointment) being 158 weeks, and the maximum duration being 579 weeks (11.1 years). There were 16 deaths during the course of follow‐up, none of which were related to gastric cancer (heart failure 30.8%, kidney disease 7.7%, and cholangiocarcinoma 7.7%).
Risk stratification scores
The median eCura score was 0, with 86 (76.8%) in the eCura A, 2 (1.8%) in eCura B, 2 (1.8%) in eCura C1, and 22 (19.6%) in the eCura C2 categories (Table 2). Descriptions of non‐curative lesions are seen in Tables 3 and 4. There were no differences in categorization between eCura or ESGE. One additional patient was changed from eCura C2 to W‐eCura C1 due to a depth of invasion of 600 nm, resulting in a down‐grade. This would not have made any influence on post‐resection strategy.
Table 3.
Comparison of outcomes by curative status
| Variables | Curative (n = 88) | Non‐curative (n = 24) | P value |
|---|---|---|---|
| Age, median, [IQR] | 76 years [12 years] | 72 years [14 years] | 0.88 |
| Size, median, [IQR] | 20 m [8.9 mm] | 33 mm [27.2 mm] | <0.001* |
| Previous/existing H pylori | 58 (22.7%) | 2 (9.5%) | 0.23 |
| Autoimmune Gastritis | 20 (23.5%) | 9 (36%) | 0.21 |
| Previous gastric surgery | 3 (3.6%) | 2 (8.3%) | 0.31 |
| Location | 0.41 | ||
| Antrum | 35 (39.3%) | 6 (26.1%) | |
| Body | 29 (32.6%) | 11 (47.8%) | |
| Incisura | 14 (15.7%) | 2 (8.7%) | |
| Cardia | 9 (10.1%) | 4 (17.4%) | |
| Fundus | 2 (2.2%) | – | |
| Paris classification | 0.64 | ||
| Is | 13 (16.7) | 2 (10%) | |
| IIa | 50 (64.1%) | 16 (80%) | |
| IIb | 11 (14.1%) | 1 (5%) | |
| IIc | 4 (5.1%) | 1 (5%) | |
| Stage | <0.001* | ||
| pT1a | 53 (59.6%) | 10 (45.5%) | |
| pT1b | 3 (3.4%) | 11 (50%) | |
| pT2 | – | 1 (4.5%) | |
| IEN | 33 (37.1%) | – | |
| Differentiation | <0.001* | ||
| Well/moderate | 52 (96.3%) | 12 (60%) | |
| Poor | 2 (3.7%) | 8 (40%) | |
| Duration | 240 min [124 min] | 120 min [56.2 min] | <0.001* |
| Complications | 12 (13.5%) | 2 (9.1%) | 0.73 |
| Recurrence | 0% | 0% | — |
| Metachronous Lesions | 8 (9.4%) | 3 (13.6%) | 0.85 |
| Mortality | 8 (10.8%) | 7 (31.8%) | 0.02* |
| Disease related | 0% | 0% | — |
| Time to death | 140 [123] | 151 [113] | 0.73 |
| Duration of follow‐up | 202 [125 weeks] | 131 [71 weeks] | 0.006* |
Table comparing outcomes between groups defined as curative versus non‐curative.
IEN, intraepithelial neoplasia.
Table 4.
Characteristics of individual non‐curative lesions
| Individual lesions | En bloc | R0 | Size | Differentiation | Ulcer | LVI | Submucosal Invasion |
|---|---|---|---|---|---|---|---|
| eCura C1 | |||||||
| #1 | Yes | No | 15 mm | Poor | No | No | No |
| #2 | No | No | 130 mm | Well | No | No | No |
| eCura C2 | |||||||
| #1 | Yes | Yes | 50 mm | Poor | No | No | No |
| #2 | No | No | 25 mm | Poor | Yes | No | 1400 μm |
| #3 | Yes | Yes | 35 mm | Poor | No | No | No |
| #4 | Yes | Yes | 35 mm | Well | No | Yes | 1300 μm |
| #5 | No | No | 50 mm | Poor | No | No | T2 |
| #6 | Yes | Yes | 12 mm | Well | No | Yes | 100 μm |
| #7 | Yes | Yes | 10 mm | Well | Yes | Yes | No |
| #8 | Yes | Yes | 70 mm | Well | No | No | 100 μm |
| #9 | Yes | Yes | 30 mm | Well | No | Yes | 500 μm |
| #10 | Yes | Yes | 17 mm | Well | No | Yes | No |
| #11 | Yes | No | 33 mm | Moderate | No | No | 600 μm |
| #12 | Yes | Yes | 70 mm | Poor | No | No | No |
| #13 | Yes | Yes | 50 mm | Poor | No | Yes | No |
| #14 | Yes | Yes | 80 mm | Poor | Yes | No | No |
| #15 | Yes | Yes | 15 mm | Yes | No | Yes | 1200 μm |
| #16 | Yes | No | 20 mm | Well | No | No | 1700 μm |
| #17 | Yes | Yes | 25 mm | Poor | No | Yes | 300 μm |
| #18 | Yes | Yes | 25 mm | Poor | No | No | No |
| #19 | Yes | Yes | 30 mm | Poor | No | No | No |
| #20 | Yes | No | 30 mm | Well | No | Yes | 1900 μm |
| #21 | Yes | Yes | 60 mm | Well | Yes | No | No |
| #22 | No | No | 46 mm | Moderate | No | Yes | 500 μm |
Table describing characteristics of individual ‘non‐curative’ lesions.
Comparison between curative and non‐curative lesions
Comparison of the characteristics of curative and non‐curative lesions can be seen in Table 3. Univariate and multivariate analysis of the factors associated with resection, complications, metachronous lesions, and mortality are seen in Table 5. Multivariate analysis demonstrated that longer duration was associated with non‐curative resection.
Table 5.
Univariate and multivariate analysis of associations between outcomes and lesion characteristics
| Factors | OR (95% CI) | P value |
|---|---|---|
| En bloc resection | ||
| Size | 1.06 (1.02–1.11) | 0.009* |
| R1 resection | ||
| Size | 1.05 (1.02–1.09) | 0.003* |
| Duration | 1.02 (1–1.04) | 0.018* |
| Previous gastric surgery | 6.89 (1.01–46.78) | 0.048* |
| Multivariate analysis | ||
| Size | 1.06 (0.93–1.2) | 0.39 |
| Duration | 1.01 (0.98–1.04) | 0.5 |
| Previous gastric surgery | 22.6 (0.37–1376.5) | 0.14 |
| Non‐curative resection (eCura) | ||
| Size | 1.09 (1.04–1.13) | <0.001* |
| Duration | 1.04 (1.01–1.07) | 0.004* |
| Differentiation | 4.92 (2.14–11.29) | <0.001* |
| Multivariate analysis | ||
| Duration | 1.04 (1.003–1.07) | 0.03* |
| Size | 1.06 (0.95–1.17) | 0.31 |
| Differentiation | 15 808 (0 – Inf) | 0.99 |
| Complications | ||
| Autoimmune gastritis | 4.69 (1.47–15.1) | 0.009* |
| Metachronous lesions | ||
| H. pylori | 5.07 (1.29–19.87) | 0.02* |
= p < 0.05.
Comparison with Western data
Review of available published data on Western outcomes from gastric ESD revealed a total of 35 relevant articles from 2008 to 2023 10 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 (Table 6). There was a total of 3589 lesions, with an analysis of outcomes over the entire period returning a mean en bloc resection rate of 94.8%, R0 resection rate of 84.2%, and curative resection of 69.8%. When divided by period, there was an increase in the number of lesions studied between 2008 and 14 (209), 2015 and 19 (1001), and 2020 and 23 (2379). When evaluating en bloc rates between periods, there were no differences in mean rates (90.2%, 93.4% and 91.7% respectively, P = 0.718). Similarly, there were no differences in R0 resection rates (82.4%, 79.3% and 78.9% respectively, P = 0.88) or curative resection rates (75%, 73.8% and 66.7% respectively, P = 0.95). By comparison, our data demonstrated en bloc rates of 100%, 94.9% and 97% (P = 0.75) across the same time period (Fig. 4). R0 rates were 85.7%, 89.7% and 89.4% (P = 0.95) and curative resection rates were 85.7%, 87.2% and 72.7% (P = 0.19).
Table 6.
Literature review of outcomes of gastric ESD
| Author | Year | Lesions (n) | En Bloc (%) | R0 (%) | Curative (%) |
|---|---|---|---|---|---|
| Cardoso | 2008 | 15 | 80 | 60 | 75 |
| Catalano | 2009 | 12 | 91.7 | 91.7 | — |
| Coda | 2010 | 7 | 85.7 | 85.7 | — |
| Hulagu | 2011 | 24 | 91.7 | 91.7 | — |
| Schumaker | 2012 | 30 | 90 | 60 | — |
| Baldaque‐Silva | 2013 | 17 | 100 | 100 | — |
| Repici | 2013 | 42 | 100 | 92.9 | — |
| Chavez | 2013 | 62 | 82.3 | 77.4 | — |
| Jaques | 2015 | 6 | 100 | 83.3 | — |
| Donoso | 2015 | 16 | 100 | 87.5 | 87.5 |
| Emura | 2015 | 54 | 98.1 | 92.6 | — |
| Najmeh | 2016 | 30 | 100 | 86.7 | — |
| Karpinska | 2016 | 58 | 96.6 | 81 | 70.7 |
| Sooltangos | 2017 | 25 | 60 | 32 | — |
| Libanio | 2017 | 194 | 95.4 | 93.8 | — |
| Probst | 2017 | 191 | 92.1 | 75.9 | 63.9 |
| Chirinos Vega | 2018 | 13 | 84.6 | 84.6 | — |
| Mendonca | 2018 | 51 | 92.2 | 72.5 | 71.1 |
| Petruzzielo | 2018 | 70 | 97.1 | 62.9 | — |
| Mocker | 2019 | 26 | 100 | 80.8 | 76 |
| Costa | 2019 | 114 | 96.5 | 86.8 | — |
| Libanio | 2019 | 153 | 94.8 | 90.2 | — |
| Chen | 2020 | 42 | 81 | 76.2 | — |
| Maselli | 2020 | 502 | 84.5 | 82.3 | — |
| Canete Ruiz | 2020 | 35 | 85.7 | 80 | 77.1 |
| Manta | 2020 | 299 | 97.7 | 89 | 72.5 |
| Kim | 2020 | 46 | 89.1 | 23.9 | 18.9 |
| Dragonov | 2021 | 101 | 98 | 82.2 | — |
| Fernandez‐ Esparrach | 2021 | 230 | 91.3 | 75.2 | — |
| Doumbe‐Mandengue | 2021 | 19 | 89.5 | 78.9 | 63.2 |
| Ngamruengphong | 2021 | 311 | 92.3 | 83 | 58.7 |
| Fleischmann | 2021 | 236 | 92.4 | 80.5 | — |
| Mejia | 2021 | 102 | 98 | 93.1 | 87 |
| Da Silva Costa | 2022 | 41 | 97.6 | 97.6 | 80.5 |
| Bhandari | 2023 | 415 | 94.7 | 83.4 | 75.8 |
* = p < 0.05. Outcomes of published data of outcomes of gastric ESD by time, number of lesions, en bloc, complete resection and curative resection.
Figure 4.
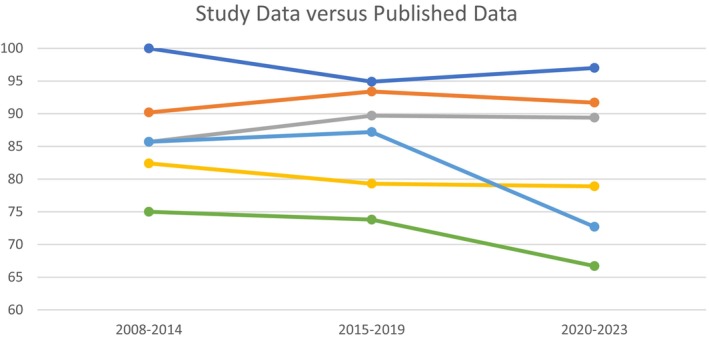
Comparative data between current study and existing published data. Comparison between rates of en bloc, complete and curative resection between the current study and published series, when compared over time. RPH = Royal Perth Hospital, R0 = complete resection with negative margins. RPH en bloc,  ; RPH R0,
; RPH R0,  ; RPH Curative,
; RPH Curative,  ; Pooled en bloc,
; Pooled en bloc,  ; Pooled R0,
; Pooled R0,  ; Pooled Curative,
; Pooled Curative,  .
.
Discussion
This study demonstrates that ESD for gastric neoplasia is associated with high rates of technical and clinical success in a multicultural tertiary referral center in Australia.
Historically, East Asian outcomes have been superior to those seen in Western centers, as demonstrated in a systematic review and meta‐analysis by Daoud et al. 7 However, as Western experience continues to develop, so too have outcomes improved. Recent studies from Europe and North America have demonstrated comparable rates of en bloc resection, R0 resection and adverse events. 9 , 36 Our study reports rates of en bloc resection (96.4%), complete or R0 resection (89.3%), and curative resection (78.6%) comparable with Asian and international series, with a slight decline in curative resection rates in the latter tertile, thought to be due to increasing complexity of cases accepted for resection. 48
Previous studies has reported the rate of non‐curative ESD for EGC at approximately 20%. This is associated with an increased risk of LNM or parietal recurrence. 3 , 11 Regression analysis of our cohort demonstrated that on multivariate analysis, duration of procedure was the only factor associated with non‐curative resection. This may reflect a more difficult procedure or potentially larger lesions, although this was not borne out on analysis. None of location, Paris classification, age, ethnicity, H pylori, or autoimmune gastritis status incurred greater risk of non‐curative resection, which is of interest and warrants further exploration.
The eCura system was validated in 2017 and is incorporated into JGES guidelines to direct post‐resection management. 12 Since its inception, our center has used eCura to define curativeness and guide management. This study demonstrates that among patients with curative resection as defined by eCura A or B, there were no cases of recurrence and 11 metachronous lesions, with a median duration of follow‐up of 3.9 years. In high‐risk lesions (eCura C1/2) who underwent surgery, there was one case of residual tumor, no LNM, no recurrence and three metachronous lesions. In our cohort of high‐ risk lesions who did not undergo surgery, we followed these patients up endoscopically and with CT imaging. In this cohort, seven (31.8%) died from non‐disease related comorbidities. We would advise against surgery for non‐curative lesions in the very elderly and those with significant comorbidities.
As yet, there is no validated system comparable to eCura for use in the Western setting. However, a recent study by Morais et al. has proposed a modified version following evaluation of over 300 non‐curative ESD across European and Australian centers. 13 On analysis of risk factors for LNM, submucosal invasion ≥1 mm was found to correlate better than >0.5 mm. Reflecting this, the W‐eCura system was created. The W‐eCura score was associated with an AUC‐ROC for LNM (0.916, 95% CI 0.870–0.961), significantly better than the original in this cohort. 13
We aimed to assess the applicability of eCura, W‐eCura, and also ESGE to determine if there were any differences between the scoring systems. We determined that one patient was downgraded from eCura C2 to W‐eCura C1 on the basis of submucosal invasion of 600 nm. They were referred to surgery but declined due to frailty and age. While this would not result in a practical difference in strategy, evidence does suggest that the risk of metastasis in C1 lesions is low. JGES suggest that repeat endotherapy or follow‐up are reasonable strategies. There were no other relevant differences between the systems. This is reassuring and suggests that familiarity with a single tool may be equally beneficial in guiding post‐resection management but is a space which requires further larger studies to evaluate potential subtle differences that may influence outcomes for patients in Western centers.
The incidence of metachronous lesions requires regular follow‐up of patients post ESD. This phenomenon is likely as a result of a field defect, such as the ongoing risk of gastric cancer from autoimmune gastritis. Our rate of metachronous lesions was 9.9% among all cases, which is in line with international series such as that by Abe et al. who demonstrated a 5‐year incidence of 9.5%. 49 It also highlights the importance of surveillance gastroscopy post ESD to detect such lesions, even where resections have been deemed curative.
Initial rates of adverse events during Western gastric ESD were significantly higher than corresponding Japanese series. 50 This has been considered as one the reasons influencing the more gradual adoption of ESD outside of East Asia. Our study is in line with more recent data supporting the safety of ESD. Our rates of adverse events are 5.8% overall, with the majority of these complications relating to delayed bleeding (3.4%). These figures are comparable with other Western data and once again support the safety of ESD for EGC outside of Japan. 51
Our study benefits from being a single‐center study with a large number of EGC, thereby limiting heterogeneity and adding to the real‐world data on ESD for such lesions outside of Japan. As such, the cohort is multicultural with a mix of ethnicities, with Caucasians making up the majority. We also benefit from reporting on over 13 years of data, with regular follow‐up in all patients. We aimed to compare our data with other Western series through a comparative literature review. This demonstrated favorable comparability. The volume of cases and lesions has steadily increased over time, reflecting Western adoption and experience. Interestingly despite this, neither our series nor the pooled international data show a significant change in outcomes over time. While acknowledging this review does not amount to a formal meta‐analysis, it could be seen to illustrate a combination of increased caution during early procedures, bias, or perhaps that target outcomes may be more user‐dependent rather than reliant on advancements in technology.
Whilst this was a retrospective, single‐center observational study, we sought to limit bias by establishing a comprehensive, standardized template for documentation of procedural and patient data, and a standard histopathological reporting template in line with WHO guidance. We compared several different risk stratification tools including eCura, W‐eCura, and ESGE guidelines and found that outcomes correlated well with risk of recurrence or LNM. We sought to compare our data with other series to demonstrate comparability. Our numbers of surgical resections were low, which limits conclusions relating to non‐curative resections; however, close surveillance with CT and gastroscopy failed to detect any recurrence or LNM to date.
In conclusion, this study demonstrates that high rates of safe, clinically, and technically successful ESD for EGC can be achieved in the Western setting. Numbers of lesions resected have steadily increased over time, with outcomes comparable across time periods. Risk stratification tool such as eCura can be used to practically and successfully direct management in patients post ESD. Further long‐term prospective research utilizing evaluating the validity of risk stratification tools in a Western cohort will be useful.
Acknowledgment
Open access publishing facilitated by Curtin University, as part of the Wiley ‐ Curtin University agreement via the Council of Australian University Librarians.
Declaration of conflict of interest: None.
Krish Ragunath and Marcus Chin Jointly senior authors.
References
- 1. Ono H, Yao K, Fujishiro M et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig. Endosc. 2021; 33: 4–20. [DOI] [PubMed] [Google Scholar]
- 2. Banks M, Graham D, Jansen M et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019; 68: 1545–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pimentel‐Nunes P, Libânio D, Bastiaansen BAJ et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline ‐ Update 2022. Endoscopy. 2022; 54: 591–622. [DOI] [PubMed] [Google Scholar]
- 4. Murakami T. Pathological diagnosis‐definition and gross classification of early gastric cancer. Gann. Monogr. Cancer Res. 1971; 11: 53–55. [Google Scholar]
- 5. Chung IK, Lee JH, Lee SH et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest. Endosc. 2009; 69: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 6. Chiu PWY, Teoh AYB, To KF et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg. Endosc. 2012; 26: 3584–3591. [DOI] [PubMed] [Google Scholar]
- 7. Daoud DC, Suter N, Durand M, Bouin M, Faulques B, von Renteln D. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: a systematic review and meta‐analysis. World J. Gastroenterol. 2018; 24: 2518–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pimentel‐Nunes P, Pioche M, Albéniz E et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2019; 51: 980–992. [DOI] [PubMed] [Google Scholar]
- 9. Catalano F, Mengardo V, Trecca A et al. The impact of experience on short‐ and long‐term outcomes on gastric ESD: a western series. Updates Surg. 2019; 71: 359–365. [DOI] [PubMed] [Google Scholar]
- 10. Kim Y, Kuan JY, Ratcliffe E et al. Long‐term follow‐up of endoscopic submucosal dissection of gastric dysplasia and early neoplasia in a United Kingdom Caucasian population ‐ a tertiary centre experience. Scand. J. Gastroenterol. 2020; 55: 18–26. [DOI] [PubMed] [Google Scholar]
- 11. Santos‐Antunes J, Pioche M, Ramos‐Zabala F et al. Risk of residual neoplasia after a noncurative colorectal endoscopic submucosal dissection for malignant lesions: a multinational study. Endoscopy. 2023; 55: 235–244. [DOI] [PubMed] [Google Scholar]
- 12. Hatta W, Gotoda T, Oyama T et al. A Scoring system to stratify curability after endoscopic submucosal dissection for early gastric cancer: “eCura system”. Am. J. Gastroenterol. 2017; 112: 874–881. [DOI] [PubMed] [Google Scholar]
- 13. Morais R, Libanio D, Ribeiro MD et al. Predicting residual neoplasia after a non‐curative gastric ESD: validation and modification of the eCura system in the Western setting: the W‐eCura score. Gut. 2024; 73: 105–117. [DOI] [PubMed] [Google Scholar]
- 14. Nagtegaal ID, Odze RD, Klimstra D et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020; 76: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zullo A, Manta R, De Francesco V et al. Endoscopic submucosal dissection of gastric neoplastic lesions in Western countries: systematic review and meta‐analysis. Eur. J. Gastroenterol. Hepatol. 2021; 33: e1–e6. [DOI] [PubMed] [Google Scholar]
- 16. Cardoso DMM, de Campoli PMO, Yokoi C et al. Initial experience in Brazil with endoscopic submucosal dissection for early gastric cancer using insulation‐tipped knife: a safety and feasibility study. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2008; 11: 226–232. [DOI] [PubMed] [Google Scholar]
- 17. Catalano F, Trecca A, Rodella L et al. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg. Endosc. 2009; 23: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 18. Coda S, Trentino P, Antonellis F et al. A Western single‐center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2010; 13: 258–263. [DOI] [PubMed] [Google Scholar]
- 19. Hulagu S, Senturk O, Aygun C et al. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J Gastroenterol. 2011; 17: 1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet‐assisted knife: a Western, single‐center experience. Gastrointest. Endosc. 2012; 75: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 21. Baldaque‐Silva F, Marques M, Andrade AP et al. Endoscopic submucosal dissection of gastrointestinal lesions on an outpatient basis. United Eur. Gastroenterol J. 2019; 7: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Repici A, Zullo A, Hassan C et al. Endoscopic submucosal dissection of early gastric neoplastic lesions: a western series. Eur. J. Gastroenterol. Hepatol. 2013; 25: 1261–1264. [DOI] [PubMed] [Google Scholar]
- 23. Chaves DM, Moura EGH, Milhomem D et al. Initial experience of endoscopic submucosal dissection in Brazil to treat early gastric and esophagheal cancer: a multi‐institutional analysis. Arq. Gastroenterol. 2013; 50: 148–152. [DOI] [PubMed] [Google Scholar]
- 24. Donoso DA, Sharp A, Parra‐Blanco A et al. Disección submucosa endoscópica en cáncer gástrico incipiente: experiencia inicial en el Hospital Clínico de la Pontificia Universidad Católica de Chile. Rev. Méd. Chile. 2015; 143: 1277–1285. [DOI] [PubMed] [Google Scholar]
- 25. Emura F, Mejía J, Donneys A et al. Therapeutic outcomes of endoscopic submucosal dissection of differentiated early gastric cancer in a Western endoscopy setting (with video). Gastrointest. Endosc. 2015; 82: 804–811. [DOI] [PubMed] [Google Scholar]
- 26. Najmeh S, Cools‐Lartigue J, Mueller C, Ferri LE. Comparing Laparoscopic to Endoscopic Resections for Early Gastric Cancer in a High Volume North American Center. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2016; 20: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 27. Sooltangos A, Davenport M, McGrath S et al. Gastric endoscopic submucosal dissection as a treatment for early neoplasia and for accurate staging of early cancers in a United Kingdom Caucasian population. World J. Gastrointest Endosc. 2017; 9: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Probst A, Schneider A, Schaller T, Anthuber M, Ebigbo A, Messmann H. Endoscopic submucosal dissection for early gastric cancer: are expanded resection criteria safe for Western patients? Endoscopy. 2017; 49: 855–865. [DOI] [PubMed] [Google Scholar]
- 29. Chirinos Vega JA, García Delgado C, Delgado VA. Disección submucosa endoscópica de cáncer gástrico temprano transpilórico: Reporte de caso y revisión de la literatura. Rev. Gastroenterol. Perú. 2018; 38: 72–77. [PubMed] [Google Scholar]
- 30. Mendonça EQ, Pessorrusso FCS, Ramos MFKP et al. Validation of classic and expanded criteria for endoscopic submucosal dissection of early gastric cancer: 7 years of experience in a Western tertiary cancer center. Clinics. 2018; 73: e553s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petruzziello L, Campanale M, Spada C et al. Endoscopic submucosal dissection of gastric superficial neoplastic lesions: a single Western center experience. United Eur. Gastroenterol. J. 2018; 6: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costa RS, Ferreira A, Leal T, Costa D, Rolanda C, Gonçalves R. Endoscopic Submucosal Dissection for the Treatment of Superficial Epithelial Gastric Neoplasia in a Portuguese Centre. GE Port. J. Gastroenterol. 2019; 26: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen A, Chen M, Trepanier M et al. Endoscopic Submucosal Dissection for Upper Gastrointestinal Neoplasia—a North American Perspective. J. Gastrointest. Surg. 2020; 24: 2456–2465. [DOI] [PubMed] [Google Scholar]
- 34. Maselli R, Iacopini F, Azzolini F et al. Endoscopic submucosal dissection: Italian national survey on current practices, training and outcomes. Dig. Liver Dis. Off J. Ital Soc. Gastroenterol. Ital Assoc. Study Liver. 2020; 52: 64–71. [DOI] [PubMed] [Google Scholar]
- 35. Manta R, Galloro G, Pugliese F et al. Endoscopic submucosal dissection of gastric neoplastic lesions: an Italian, Multicenter Study. J. Clin. Med. 2020; 9: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Draganov PV, Aihara H, Karasik MS et al. Endoscopic submucosal dissection in North America: a large prospective multicenter study. Gastroenterology. 2021; 160: 2317–2327.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doumbe‐Mandengue P, Beuvon F, Belle A et al. Outcomes of endoscopic submucosal dissection for early esophageal and gastric cardia adenocarcinomas. Clin. Res. Hepatol. Gastroenterol. 2021; 45: 101700. [DOI] [PubMed] [Google Scholar]
- 38. Ngamruengphong S, Ferri L, Aihara H et al. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin. Gastroenterol. Hepatol. Off Clin. Pract. J. Am. Gastroenterol. Assoc. 2021; 19: 1611–1619.e1. [DOI] [PubMed] [Google Scholar]
- 39. Fleischmann C, Probst A, Ebigbo A et al. Endoscopic submucosal dissection in Europe: results of 1000 neoplastic lesions from the German endoscopic submucosal dissection registry. Gastroenterology. 2021; 161: 1168–1178. [DOI] [PubMed] [Google Scholar]
- 40. Costa LC d S, Santos JOM, Miyajima NT, Montes CG, Andreollo NA, Lopes LR. Efficacy analysis of endoscopic submucosal dissection for the early gastric cancer and precancerous lesions. Arq. Gastroenterol. 2022; 59: 421–427. [DOI] [PubMed] [Google Scholar]
- 41. Bhandari P, Abdelrahim M, Alkandari AA et al. Predictors of long‐term outcomes of endoscopic submucosal dissection of early gastric neoplasia in the West: a multicenter study. Endoscopy. 2023; 55: 898–906. [DOI] [PubMed] [Google Scholar]
- 42. Mejía R, Sáez J, Norero E et al. Long‐term Results of Endoscopic Submucosal Dissection (ESD) for the Treatment of Early Gastric Cancer (EGC) in a High‐volume Latin American Center. Surg. Laparosc. Endosc. Percutan. Tech. 2020; 31: 165–169. [DOI] [PubMed] [Google Scholar]
- 43. Fernández‐Esparrach G, Marín‐Gabriel JC, de Tejada AH et al. Implementation of endoscopic submucosal dissection in a country with a low incidence of gastric cancer: results from a prospective national registry. United Eur. Gastroenterol. J. 2021; 9: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cañete Ruiz Á, Arribas Anta J, Alvarez‐Nava Torrego T et al. Endoscopic submucosal dissection for gastric epithelial lesions: long‐term results in a Spanish cohort. Rev. Esp. Enferm. Dig. 2020; 112: 189–194. [DOI] [PubMed] [Google Scholar]
- 45. Libânio D, Pimentel‐Nunes P, Afonso LP, Henrique R, Dinis‐Ribeiro M. Long‐Term outcomes of gastric endoscopic submucosal dissection: focus on metachronous and non‐curative resection management. GE Port. J. Gastroenterol. 2017; 24: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karpińska‐Kaczmarczyk K, Białek A, Lewandowska M, Dobak E, Ławniczak M, Urasińska E. Histomorphologic features of early gastric carcinoma treated by endoscopic submucosal dissection: relation to efficiency of endoscopic resection. Scand. J. Gastroenterol. 2016; 51: 1495–1501. [DOI] [PubMed] [Google Scholar]
- 47. Mocker L, Hildenbrand R, Oyama T, Sido B, Yahagi N, Dumoulin FL. Implementation of endoscopic submucosal dissection for early upper gastrointestinal tract cancer after primary experience in colorectal endoscopic submucosal dissection. Endosc. Int. Open. 2019; 7: E446–E451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oda I, Gotoda T, Hamanaka H et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig. Endosc. 2005; 17: 54–58. [Google Scholar]
- 49. Abe S, Oda I, Suzuki H et al. Long‐term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015; 47: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 50. Friedel D, Stavropoulos SN. Introduction of endoscopic submucosal dissection in the West. World J. Gastrointest. Endosc. 2018; 10: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palacios‐Salas F, Benites‐Goñi H, Marin‐Calderón L et al. Efficacy and safety of endoscopic submucosal dissection for superficial gastric neoplasms: a Latin American Cohort Study. Clin. Endosc. 2022; 55: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]


