Abstract
Mitochondrial bioenergetics in females and males is different. However, whether mitochondria from male and female brains display differences in enzymes of oxidative phosphorylation remains unknown. Therefore, we characterized mitochondrial complexes from the brains of male and female macaques (Macaca mulatta).
Cerebral tissue from male macaques exhibits elevated content and activity of mitochondrial complex I (NADH: ubiquinone oxidoreductase) and higher activity of complex II (succinate dehydrogenase) compared to females. No significant differences between sexes were found in the content of α-ketoglutarate dehydrogenase or in the activities of cytochrome c oxidase and F1Fo ATPase. Our results underscore the need for further investigations to elucidate sex-related mitochondrial differences in humans.
Keywords: Sex, Primates, Mitochondria, Mitochondrial complex I, α-Ketoglutarate dehydrogenase, Succinate dehydrogenase, TCA cycle, ATPase, Cytochrome c oxidase, NADH:ubiquinone oxidoreductase
Due to different hormonal statuses, adult healthy mammals exhibit sex differences in brain metabolism. Female hormone estradiol has a significant neuroprotective effect, increasing mitochondrial membrane fluidity and respiration [1]. In addition, estrogen receptors were found to be associated with brain mitochondria [2]. There are several, albeit controversial, reports on sex-dependence of the content and activity of brain mitochondrial enzymes in rodents [3–6] but very few reports on primates [7–9]. A recent meta-analysis [10] did not reveal any significant differences between sexes in humans, but tissues other than the brain were mostly considered. It is essential to perform an accurate evaluation of brain samples from non-human primates including both the content and function of the proteins involved in energy production. To address potential sex-related differences in the mitochondrial oxidative phosphorylation system, we analyzed the content and activity of respiratory chain enzymes in brain mitochondria from adult non-human primate Macaca mulatta using a novel approach recently developed in our laboratory [11,12].
The grey matter from snap-frozen prefrontal cortex was homogenized and processed for high-resolution Clear Native (hrCN) electrophoresis or activity measurements, as previously described [12,13] (see Supplementary data for details). Values of content and activity of several mitochondrial complexes for both sexes are shown in Fig. 1. After separation of the large membrane complexes by hrCN and fluorescent scanning of the gel, three main bands were determined: pyruvate dehydrogenase, α-ketoglutarate dehydrogenase and complex I (Fig. 1A). After calibration of the fluorescent signal [11], α-ketoglutarate dehydrogenase content was not significantly different between male (44.3 ± 12.0 pmol/mg of protein) and female (44.4 ± 5.8 pmol/mg of protein) brains (Fig. 1B). A key control point for oxidative phosphorylation is mitochondrial complex I or NADH:ubiquinone oxidoreductase [14,15], which governs the electron entry flux to the respiratory chain and also determines the availability of NAD+ for TCA cycle dehydrogenases, such as pyruvate and α-ketoglutarate dehydrogenase. In many pathologies, alterations of mitochondrial complex I function are a hallmark of central nervous system injury or degeneration. Macaque brains mitochondrial complex I content was found to be higher in males (6.9 ± 1.0 pmol/mg of protein) than in females (5.9 ± 0.4 pmol/mg of protein) (Fig. 1C). These values correspond to 81 ± 10 and 72 ± 11 pmol of complex I FMN per g of wet brain tissue in males and females, respectively (Supplementary data, Table 2S). Complex I content showed a trend towards increased levels in males and decreased levels in females with age (Fig. 1S). Specific complex I NADH oxidation activity with artificial acceptor hexaammineruthenium (HAR) was also significantly higher in male brain mitochondria (Fig. 1D), confirming the FMN content measurements. Next, we measured the activity of complex II (succinate dehydrogenase) and ATP hydrolysis by F1Fo ATP-ase. In male brains, the activity of succinate dehydrogenase was higher than in female brains, contrary to the results reported in humans [8]. No statistically significant differences were found for complex IV cytochrome c oxidase or ATP hydrolytic activity of complex V.
Fig. 1.
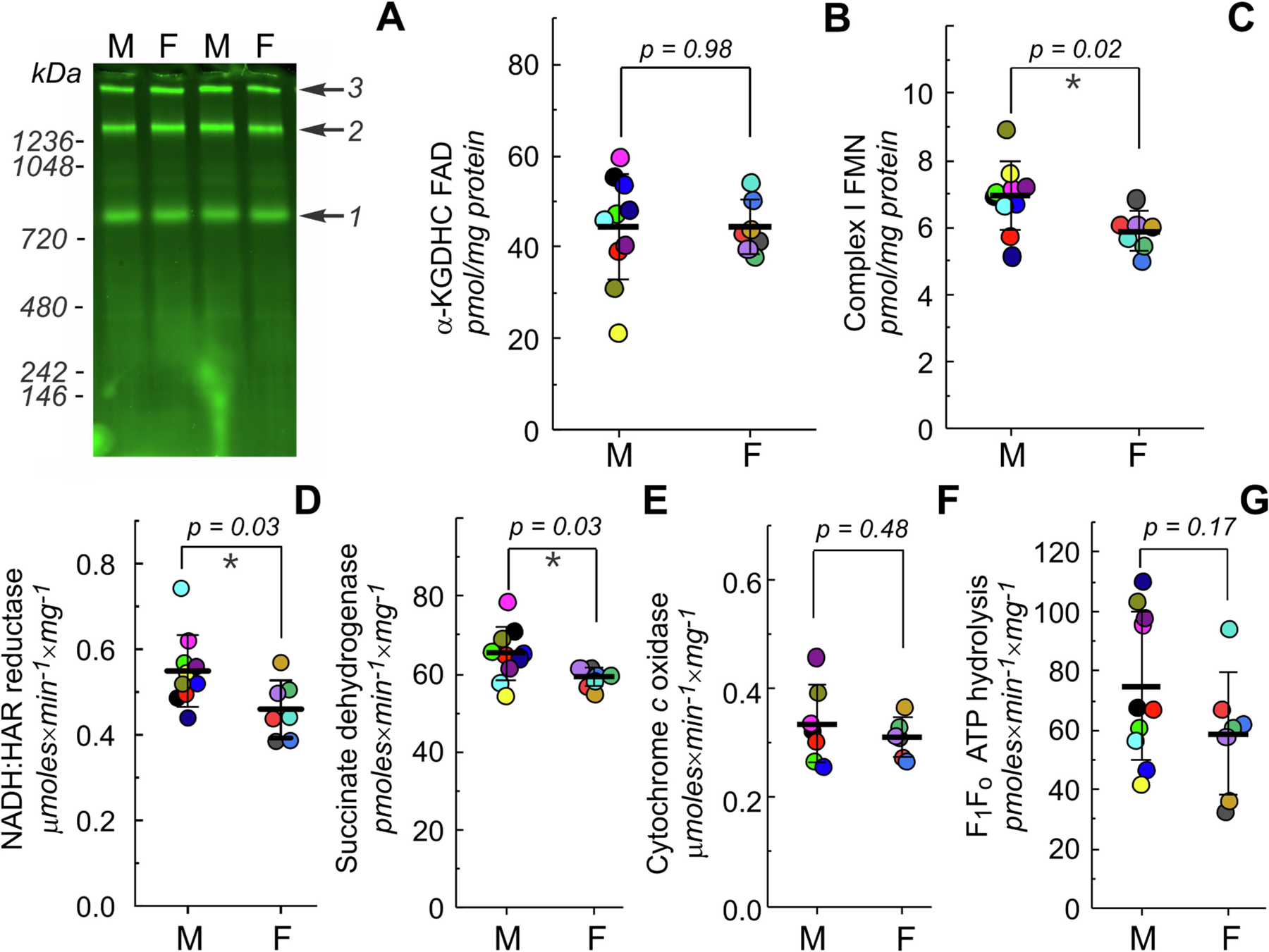
Functional measurements of mitochondrial oxidative phosphorylation in males (M) and females (F) monkey’s brain. A, representative image of an SDS-treated hrCN gel showing flavin fluorescence originated from complex I (1), α-ketoglutarate dehydrogenase (2) and pyruvate dehydrogenase (3) complexes. B, C, quantitative measurement of α-ketoglutarate dehydrogenase FAD (B) and complex I FMN (C) content. D–F, complex I NADH: hexaammineruthenium (HAR) reductase (D), complex II succinate dehydrogenase (E), complex IV ferrocytochrome c oxidase (F) activities and ATP hydrolysis by complex V (G) of brain mitochondria homogenates from males and females. Values of content and activity were normalized per tissue homogenate protein, mean ± SD, n = 7–10 for each group, Student’s t-test.
Most of the mitochondrial studies in mammals regarding sex differences have been performed in rodents, whereas data on sex-related mitochondrial differences in primates or humans are limited. In this study, we analyzed binary sex differences in specific components of the mitochondrial oxidative phosphorylation machinery in brains of adult non-human primates. Unlike previous reports in rodents [5,6] and humans [8], we did not observe the elevated function of mitochondria enzymes in females. On the contrary, we found that the content of mitochondrial complex I was higher in males. Complex I provides the electron entry to the respiratory chain and its activity defines overall flux through complexes I-IV as well as the rate of the TCA cycle via NAD+ availability. Since mitochondria complex I is one of the major contributors to the production of reactive oxygen species in mitochondria [16], higher content of this enzyme could underlie elevated levels of oxidative stress in males as proposed for the rat brain [17]. Previously, higher content of other brain mitochondria components, such as uncoupling protein 1 was also reported in male brains [7]. It is worth noting that studies like the present one have limitations due to the difference in postmortem period, initial sample processing, storage time, and possible tissue heterogeneity. For example, due to the high variability between samples, it was not possible to estimate the content of pyruvate dehydrogenase (Fig. 1A, band 3).
Undoubtedly, the evidence of sex-dependent differences in mitochondria enzymatic activities in primates including humans is growing. With an increasing number of new mitochondria-targeted medicines, the physiologic and mechanistic implications of these differences remain to be appreciated. Recognizing the unique sex-based dissimilarities in mitochondrial function would have a big impact on the design of personalized drugs or therapeutic protocols and ensure more targeted and effective treatments for both men and women.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health R01NS112381 and R21NS125466 (A. G.). This research was made possible in part using biomaterials from the NIA Nonhuman Primate Tissue Bank (https://www.nia.nih.gov/research/dab/nonhuman-primate-tissue-bank) at the Wisconsin National Primate Research Center, University of Wisconsin-Madison under contractual agreement with the National Institute on Aging (NIA). Work on the Typhoon scanner was partially supported by NIH grant S10OD030335.
Footnotes
CRediT authorship contribution statement
Ivan Guerrero: Writing – review & editing, Investigation, Data curation. Belem Yoval-Sánchez: Supervision, Methodology, Data curation. Csaba Konrad: Writing – review & editing, Methodology. Giovanni Manfredi: Writing – review & editing, Investigation. Ilka Wittig: Writing – review & editing, Methodology. Alexander Galkin: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbabio.2024.149494.
Data availability
Data will be made available on request.
References
- [1].Ventura-Clapier R, Moulin M, Piquereau J, Lemaire C, Mericskay M, Veksler V, Garnier A, Mitochondria: a central target for sex differences in pathologies, Clin. Sci. (Lond.) 131 (2017) 803–822. [DOI] [PubMed] [Google Scholar]
- [2].Burstein SR, Kim HJ, Fels JA, Qian L, Zhang S, Zhou P, Starkov AA, Iadecola C, Manfredi G, Estrogen receptor beta modulates permeability transition in brain mitochondria, Biochim. Biophys. Acta Bioenerg 2018 (1859) 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kiessling KH, Tilander K, The effect of prolonged alcohol treatment on the respiration of liver and brain mitochondria from male and female rats, Exp. Cell Res 30 (1963) 476–480. [DOI] [PubMed] [Google Scholar]
- [4].Guevara R, Gianotti M, Roca P, Oliver J, Age and sex-related changes in rat brain mitochondrial function, Cell. Physiol. Biochem 27 (2011) 201–206. [DOI] [PubMed] [Google Scholar]
- [5].Gaignard P, Savouroux S, Liere P, Pianos A, Therond P, Schumacher M, Slama A, Guennoun R, Effect of sex differences on brain mitochondrial function and its suppression by Ovariectomy and in aged mice, Endocrinology 156 (2015) 2893–2904. [DOI] [PubMed] [Google Scholar]
- [6].Escames G, Diaz-Casado ME, Doerrier C, Luna-Sanchez M, Lopez LC, Acuna-Castroviejo D, Early gender differences in the redox status of the brain mitochondria with age: effects of melatonin therapy, Horm Mol Biol, Clin. Investig 16 (2013) 91–100. [DOI] [PubMed] [Google Scholar]
- [7].Jamwal S, Blackburn JK, Elsworth JD, Age-associated sex difference in the expression of mitochondria-based redox sensitive proteins and effect of pioglitazone in nonhuman primate brain, Biol. Sex Differ 14 (2023) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harish G, Venkateshappa C, Mahadevan A, Pruthi N, Bharath MM, Shankar SK, Mitochondrial function in human brains is affected by pre- and post mortem factors, Neuropathol. Appl. Neurobiol 39 (2013) 298–315. [DOI] [PubMed] [Google Scholar]
- [9].Schwagerl A, Preservation of Succinic Dehydrogenase and Cytochrome Oxidase Activity in Aging Rhesus Monkeys (PhD thesis), School of Medicine, Boston University, Boston, 2003. [Google Scholar]
- [10].Junker A, Wang J, Gouspillou G, Ehinger JK, Elmer E, Sjovall F, Fisher-Wellman KH, Neufer PD, Molina AJA, Ferrucci L, Picard M, Human studies of mitochondrial biology demonstrate an overall lack of binary sex differences: a multivariate meta-analysis, FASEB J. 36 (2022) e22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ansari F, Yoval B, Niatsetskaya Z, Ten V, Wittig I, Galkin A, How many molecules of mitochondrial complex I are in a cell? Anal. Biochem 646 (2022) 114646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ansari F, Yoval-Sanchez B, Niatsetskaya Z, Sosunov S, Stepanova A, Garcia C, Owusu-Ansah E, Ten V, Wittig I, Galkin A, Quantification of NADH:ubiquinone oxidoreductase (complex I) content in biological samples, J. Biol. Chem 297 (2021) 101204–101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stepanova A, Sosunov S, Niatsetskaya Z, Konrad C, Starkov AA, Manfredi G, Wittig I, Ten V, Galkin A, Redox-dependent loss of flavin by mitochondrial complex I in brain ischemia/reperfusion injury, Antioxid. Redox Signal. 31 (2019) 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Genova ML, Castelluccio C, Fato R, Parenti Castelli G, Merlo Pich M, Formiggini G, Bovina C, Marchetti M, Lenaz G, Major changes in complex I activity in mitochondria from aged rats may not be detected by direct assay of NADH: coenzyme Q reductase, Biochem. J. 311 (1995) 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kuznetsov AV, Winkler K, Kirches E, Lins H, Feistner H, Kunz WS, Application of inhibitor titrations for the detection of oxidative phosphorylation defects in saponin-skinned muscle fibers of patients with mitochondrial diseases, Biochim. Biophys. Acta 1360 (1997) 142–150. [DOI] [PubMed] [Google Scholar]
- [16].Stepanova A, Galkin A, Measurement of mitochondrial H2O2 production under varying O2 tensions, Methods Cell Biol 155 (2020) 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guevara R, Santandreu FM, Valle A, Gianotti M, Oliver J, Roca P, Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress, Free Radic. Biol. Med 46 (2009) 169–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


