Abstract
People who inject drugs face many challenges which contribute to poor health outcomes, including drug overdose, HIV and Hepatitis C infections. These conditions require high quality prevention and treatment services. Syringe Services Programs are evidence-based harm reduction programs and have established track records with people who inject drugs, earning them deep trust within this community. In Baltimore, while many syringe support services were limited during the COVID-19 pandemic, the health department syringe service programs remained operational, allowing for the continuation of harm reduction services and naloxone distribution. This evaluation describes a collaborative effort to co-locate infectious disease testing and COVID-19 vaccinations with a syringe service program. Our evaluation demonstrated that co-location of important services with the utilization of trusted community partners can facilitate engagement, and is essential for service uptake. Maintaining adequate and consistent funding for these services is central to program success. Co-location of other services within syringe service programs, such as medications for opioid use disorder, wound care, and infectious disease treatment would further expand healthcare access for persons who inject drugs.
Introduction
People who inject drugs (PWID) face significant challenges that contribute to their poor health as well as increased morbidity and mortality. Challenges include drug overdoses, HIV, and Hepatitis C infections. PWID account for 7% of new HIV infections and 57% of new Hepatitis C infections annually in the United States.1,2 Additionally, recent surveillance data indicate that Hepatitis C infection rates continue to increase annually despite the availability of curative treatment.3 For these conditions, PWID require high quality prevention and treatment services, but traditional public health and healthcare systems are not always well-suited for delivering these services due to structural barriers and lack of trust.
PWID face many challenges and complications accessing traditional healthcare services. These include difficulties with obtaining health insurance, difficulty navigating complicated health systems, limited access to transportation, and internalized and enacted stigmatization.4 Alternative healthcare service delivery programs have helped PWID meet these challenges. These include Syringe Services Programs (SSPs), which have established track records with PWID and have earned their deep trust. The programs are often funded and managed by local health departments,5,6 and were initially designed to provide harm reduction in the form of offering education, clean needles, and a means of needle disposal. Newer SSP models have integrated the use and distribution of naloxone for opioid overdose reversal, wound care services and supplies, medication for opioid use disorder, and referrals to primary and specialty care services.5,7
During the COVID-19 pandemic, SSPs and other harm reduction programs suspended services initially but quickly made plans to re-open, recognizing the essential services they provide to PWID.8 Decreases in testing for infectious diseases and referrals to treatment during this time prompted concern among healthcare leaders that infectious disease outbreaks and increased morbidity from untreated disease would occur in this population.9 Additionally, PWID interviewed during the COVID-19 pandemic reported increased mental health issues, increased sharing of needles, polysubstance use, and decreased access to their usual harm reduction programs or SSPs for services.10
The burden of COVID-19 infection was also higher for people who use drugs compared to the general population, leading to increased hospitalization and mortality.11,12 However, PWID were not prioritized for COVID-19 vaccination, despite having co-occurring conditions that qualified them for vaccination prior to the general population (e.g., HIV infection and/or age).13 Venues where vaccines were offered, including large medical centers, and mass vaccine events that required online sign up, presented insurmountable logistical barriers for some PWID. Without targeted funding to co-locate vaccinations within healthcare sites that PWID trusted and commonly used, the barriers to healthcare services and stigma persisted among PWID.
To address unequal access to COVID-19 vaccines by PWID as well as the decreased access to wrap around infectious disease screening services for this population, we describe a collaboration between Johns Hopkins Medicine and the School of Nursing to co-locate infectious disease testing and linkage to treatment, and COVID-19 vaccinations with the Baltimore City Health Department’s SSP. This aim of this effort was to respond to the need to equitably distribute COVID-19 vaccinations and engage PWID for additional preventive services disrupted during the pandemic. In addition to describing the program, we present descriptive data on the population, number of vaccinations, infectious diseases tests and linkage outcomes, and syringe services to understand services accessed by PWID when co-located at a trusted source. Findings from this work can inform future delivery and expansions of preventive and low-barrier care co-located within SSPs, as the public health system reimagines how to meaningfully reach this population.
Program Description
Program description:
The Baltimore City Health Department (BCHD) SSP operated on a set schedule with a mobile van in Baltimore City neighborhoods with high overdose rates, providing needle exchange, harm reduction counseling, naloxone training and distribution, and overdose reversal. The SSP operated continuously during the COVID-19 pandemic to provide these essential services. The Center for Infectious Disease and Nursing Innovation (CIDNI), a Johns Hopkins University School of Nursing center that cultivates scientific and programmatic infectious disease expertise in nursing, and the Johns Hopkins Mobile Vaccine Unit (JH MVU) partnered with the BCHD SSP to conduct infectious disease testing and administer COVID-19 vaccinations at locations where SSP operated, leveraging the SSP program’s consistency in delivering services.
Between April 2021-June 2022, ten co-located clinics were conducted in conjunction with SSP services, and no appointments were required. Individuals receiving SSP services were offered free COVID-19 vaccinations and infectious disease testing. The JH MVU offered both mRNA (Moderna and Pfizer) and viral vector (Janssen) vaccines. Those interested in vaccination signed a consent for the vaccine and were registered within the JH MVU electronic health record to collect demographic information and document the vaccination. Their vaccine status was verified in the health information exchange linked to the electronic health record to determine if they were eligible for a first, second, or booster dose. Individuals who received the first dose of the mRNA series were scheduled to return to the SSP for their second vaccine during a scheduled co-located clinic and provided information on how to receive a COVID-19 vaccine at locations outside of SSP if they missed the co-located appointment. Gift card incentives were not initially offered for COVID-19 vaccinations, but later $10 gift cards were offered following full FDA approval for COVID-19 vaccinations (August 23, 2021).
CIDNI provided point of care testing for HIV, hepatitis C (HCV), and syphilis. PWID interested in testing provided written consent, completed a demographics questionnaire, and received testing results during the encounter. Individuals testing positive were offered linkage to a treatment provider and their appointment attendance confirmed. Self-collected oral, penile, vaginal, and rectal swabs were offered to test for gonorrhea and chlamydia, with a private tent available for on-site specimen collection. Vaginal testing also included trichomoniasis. Swabs were tested within 48 hours at an off-site lab. Participants were notified of their results by a CIDNI team member via telephone. Participants also received a card with the phone number of a CIDNI team member that they could call to receive their test results. For positive test results, participants were either linked to treatment (gonorrhea) or a prescription was sent to a local pharmacy for treatment (chlamydia and trichomoniasis). Prescriptions were billed to the participant’s insurance, and uninsured individuals were linked to the BCHD sexual health clinic for free treatment. Initially, participants received a $10 gift card for each completed infectious disease test. Following the first five clinics, CIDNI changed this incentive to a $20 gift card for any testing, regardless the number. Lessons learned from the first five clinics are published elsewhere.14
Partnership and Funding
The Baltimore-based JH MVU, a component of the program, was itself a partnership between the healthcare institution, local government, and health department. Comprised of healthcare personnel, including clinicians, pharmacists, logistics/administration staff, and students, this team was responsible for enacting mobile strategies to increase COVID-19 vaccination. To reach PWID for COVID-19 vaccinations, the JH MVU then partnered with the BCHD SSP. CIDNI’s partnership with the other two provided the infrastructure of field-based infectious testing, storage for the COVID-19 vaccinations while on site, and nursing student volunteers who assisted with vaccination, infectious disease testing, and directing PWID between the co-located clinics based on desired services.
This work was supported in part by braiding together grants, contacts, and center resources which also supported other efforts to independently provide infectious disease testing and COVID-19 vaccination. CIDNI utilized center funds to support the mobile van for infectious disease testing and vaccine storage, gas, WiFi, privacy tents, and clinical consumables such as gloves and wound care supplies. A grant from Urban Health Institute at Johns Hopkins University allowed for five additional clinics to be conducted in 2022 and supported these costs. Contracts to CIDNI from the Baltimore City Health Department supported the infectious disease testing services, including the cost of point of care and send out tests, and staff support. The JH MVU was funded through a combination of federal pandemic-relief funding support covering some personnel and essential supplies and BCHD provisioning vaccines and related supplies such as needles.
Data Collection and Analysis for Internal Evaluation
We present results of an internal evaluation of the program, including demographic characteristics of PWID and descriptive data on infectious disease tests completed by test type, frequency of positive test results, successful care linkage, and SSP services. Due to privacy reasons, SSP does not collect individual level data. SSP tracked aggregate data of syringes distributed and returned, naloxone training, and kits distributed at each co-located clinic. However, vaccine and infectious disease metrics were tracked separately and abstracted with procedures detailed below.
Vaccination data (including date and type of vaccine) for completing primary series and booster doses were tabulated for each individual receiving at least one COVID-19 vaccine at a co-located clinic. The data were extracted from the electronic health record and Maryland’s immunization registry to determine if individuals completed primary and booster doses outside the co-located clinics.
Infectious disease testing results were entered into CAREWare, the software maintained by the Health Resource and Services Administration (HRSA) Ryan White HIV/AIDS Program. Test results and demographic information were abstracted for each co-located clinic. Linkages between both datasets were made based on first and last name and date of birth.
Demographic information was collected on a strictly voluntary basis, so the record of demographic information is not complete. When possible, however, these data were abstracted from the two data sources if available. This evaluation was determined to be ‘not human subjects research’ by the Johns Hopkins University Medicine Institutional Review Board (IRB#00288290).
Evaluation Results
PWID Who Accessed Any Clinical Service
There were 347 unique PWID accessing SSP services who received at least one COVID-19 vaccine or infectious disease testing service at the ten co-located clinics from April 2021-June 2022. The majority of individuals were male (n=239, 69%), Black race (n=265, 76%), non-Hispanic or Latino (n=323, 93%), and a mean age of 51 years old (SD 13) (Exhibit 1). Of these individuals, 63% (n=218) accessed at least one dose of the COVID-19 vaccine and 58% (n=202) received one infectious disease test from a SSP co-located clinic.
Exhibit 1:
Characteristics of PWID receiving COVID-19 vaccination, infectious disease testing, or both at the Baltimore City Health Department Syringe Services Program April 2021-June 2022
| Total | Infectious disease testing only | COVID-19 vaccine only | Both services | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | n=347 | n=129 | n=145 | n=73 | ||||
|
| ||||||||
| n | % | n | % | n | % | n | % | |
|
| ||||||||
| Age (Mean, SD) | 5 | 13 | 49 | 12.5 | 51 | 13 | 50 | 13 |
|
| ||||||||
| Gender | ||||||||
|
| ||||||||
| Female | 108 | 31% | 46 | 36% | 47 | 32% | 15 | 20% |
|
| ||||||||
| Male | 239 | 69% | 83 | 64% | 98 | 68% | 58 | 80% |
|
| ||||||||
| Race | ||||||||
|
| ||||||||
| Black | 265 | 76% | 89 | 69% | 123 | 85% | 53 | 73% |
|
| ||||||||
| White | 51 | 15% | 19 | 15% | 15 | 10% | 17 | 23% |
|
| ||||||||
| Other | 16 | 5% | 11 | 8% | 2 | 2% | 3 | 4% |
|
| ||||||||
| Not specified | 15 | 4% | 10 | 8% | 5 | 3% | 0 | 0% |
|
| ||||||||
| Ethnicity | ||||||||
|
| ||||||||
| Hispanic or Latino | 9 | 3% | 4 | 3% | 3 | 2% | 2 | 3% |
|
| ||||||||
| Non-Hispanic or Latino | 323 | 93% | 115 | 89% | 137 | 95% | 71 | 97% |
|
| ||||||||
| Not specified | 15 | 4% | 10 | 8% | 5 | 3% | 0 | 0% |
|
| ||||||||
| Insurance | ||||||||
|
| ||||||||
| Insured | 138 | 40% | 0 | 0% | 82 | 57% | 56 | 77% |
|
| ||||||||
| Uninsured | 80 | 23% | 0 | 0% | 63 | 43% | 17 | 23% |
|
| ||||||||
| Not specified | 129 | 37% | 129 | 100% | 0 | 0% | 0 | 0% |
Notes: All values are number and percent except for Age, which is mean and standard deviation.
Sources: Author’s analysis of linked abstracted data from the electronic health record where COVID-19 vaccine data was entered and the CAREWare database (the software maintained by the Health Resource and Services Administration (HRSA) Ryan White HIV/AIDS Program). Please list the other data sources here
PWID Who Accessed Infectious Disease Testing Only
Of the 202 individuals who received at least one infectious disease test, the majority received a point-of-care HIV test (n=174, 86%), followed by Hepatitis C (HCV) (n= 129, 64%) (Exhibit 2). Syphilis testing (n=76, 38%), throat swab for gonorrhea and chlamydia (n=69, 34%), and genital swab for gonorrhea and chlamydia (n=50, 25%) were less frequent. All eight patients with positive HIV diagnoses (5%), one of whom was newly positive, were linked to treatment. An additional individual reported HIV exposure within the last 72 hours, tested negative with a rapid HIV test, was prescribed post-exposure prophylaxis, and provided linkage for follow-up clinical services. Of the 36% of those testing positive for HCV who required linkage to treatment, only six (28%) could be documented to have a successful linkage visit. All six individuals requiring follow-up for their presumptive point of care syphilis test were successfully linked for confirmatory testing and treatment if indicated. None of those tested for chlamydia or gonorrhea tested positive. One individual’s swab tested positive for trichomonas and was successfully treated.
Exhibit 2.
Characteristics and infectious disease testing results from 202 PWID Baltimore City Health Department Syringe Service Program April 2021-June 2022
| Characteristics | N | % |
|---|---|---|
| Age (Mean, SD) | 52 | 13 |
| Gender | ||
| Female | 61 | 30% |
| Male | 141 | 70% |
| Race | ||
| Black | 142 | 70% |
| White | 36 | 18% |
| Other | 13 | 6% |
| Not specified | 10 | 6% |
| Ethnicity | ||
| Hispanic or Latino | 6 | 3% |
| Non-Hispanic or Latino | 186 | 92% |
| Not specified | 10 | 5% |
| Hepatitis C results (n=129) | ||
| Negative | 77 | 60% |
| New positive, linked to care | 6 | 4% |
| New positive, unable to link to care | 15 | 12% |
| Previously diagnosed | 22 | 17% |
| Previously treated | 9 | 7% |
| HIV results (n=174) | ||
| Non-reactive | 166 | 95% |
| Previously diagnosed, linked to care | 7 | 4% |
| New positive, linked to care | 1 | 1% |
| Syphilis results (n=76) | ||
| Negative | 70 | 92% |
| Presumptive, confirmed positive | 6 | 8% |
| Chlamydia-Gonorrhea swab (n=119) | ||
| Negative | 119 | 100% |
| Positive | 0 | 0% |
| Trichomonas swab (n=24) | ||
| Negative | 23 | 95% |
| Positive | 1 | 5% |
Notes: All values are number and percent except for Age, which is mean and standard deviation.
Chlamydia-Gonorrhea reporting are inclusive of all sites (throat, vaginal, and penile). This data reflects the aggregate number of swabs collected and tested.
Sources: Author’s analysis of abstracted data from the CAREWare database (the software maintained by the Health Resource and Services Administration (HRSA) Ryan White HIV/AIDS Program)Please list all the other data sources here.
PWID Who Accessed COVID-19 Vaccination Services Only
There were 218 PWID (63%) who accessed at least one dose of the COVID-19 primary or booster series at the co-located clinics. The majority opted to receive a two-dose mRNA vaccine for their primary series (n=141, 65%). Of these, 128 (of 141 total, 91%) completed the two-dose series (Exhibit 3). Booster doses had lower uptake. All PWID were eligible for a first booster vaccine at the time of data abstraction, but only 30% (n=66) received at least one additional dose.
Exhibit 3.
Characteristics and COVID-19 vaccine metrics from 218 PWID, Syringe Services Program Clients, Baltimore City Health Department, April 2021-June 2022.
| Characteristics | N | % |
|---|---|---|
| Age (Mean, SD) | 52 | 13 |
| Gender | ||
| Female | 62 | 28% |
| Male | 156 | 72% |
| Race | ||
| Black | 176 | 81% |
| White | 21 | 15% |
| Other | 5 | 2% |
| Not Specified | 4 | 2% |
| Ethnicity | ||
| Hispanic or Latino | 5 | 2.5% |
| Non-Hispanic or Latino | 208 | 95% |
| Not Specified | 5 | 2.5% |
| Dose 1 COVID-19 vaccine (n=218) | ||
| Viral vector | 77 | 35% |
| mRNA | 141 | 65% |
| Dose 2 COVID-19 vaccine (n=133) | ||
| Viral vector | 4 | 3% |
| mRNA | 128 | 96.5% |
| Unspecified | 1 | 0.5% |
| Booster 1 COVID-19 vaccine (n=66) | ||
| Viral vector | 1 | 1.5% |
| mRNA | 65 | 98.5% |
| Booster 2 COVID-19 vaccine (n=9) | ||
| mRNA | 9 | 100% |
Notes: All values are number and percent except for Age, which is mean and standard deviation.
Source: Author’s analysis of abstracted data from the electronic health record where COVID-19 vaccine data was entered Please list sources here.
PWID Who Accessed Harm Reduction Services
Counts of syringes distributed and returned, and naloxone training conducted and kits distributed are presented in Exhibit 4. Across the eight co-located clinics with SSP data available, an average of 3057 syringes were distributed, 1437 returned, and 24 naloxone kits distributed with training.
Exhibit 4.
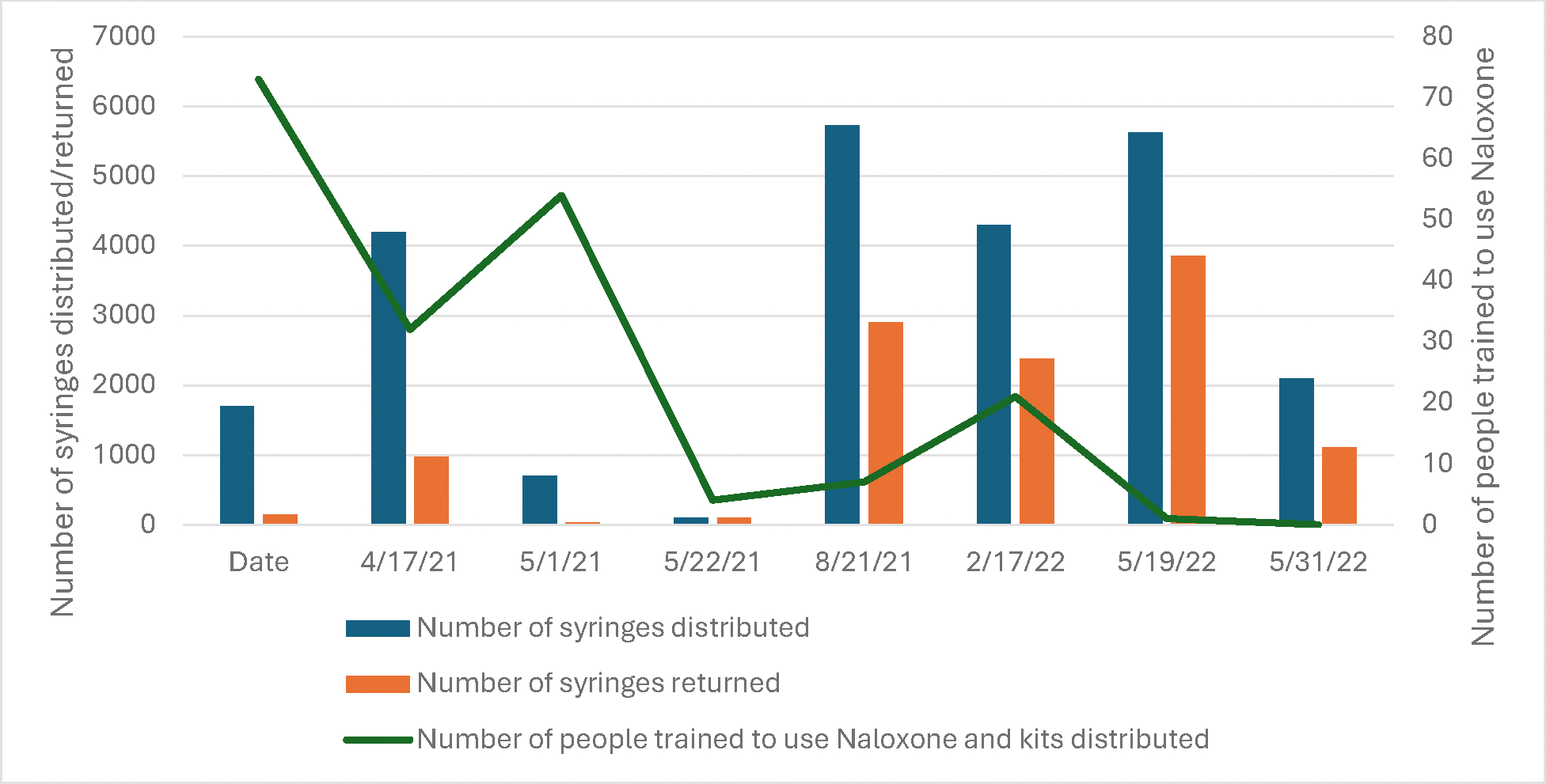
Aggregate count of SSP services from n=8 co-located clinics in Baltimore with available data April 2021-June 2202
Notes: All values are counts of services provided
Source: Author’s analysis of data collected and reported by the Baltimore City Health Department’s Syringe Services Program (SSP)
Discussion
Utilizing co-location of COVID-19 vaccination, infectious disease testing and care linkage services with a SSP works. Particularly striking was the rate of second dose uptake of the primary COVID-19 vaccination series (205/218, 94%) which exceeded that of the general population.15 Given the well documented low uptake by PWID of services and prevention interventions16,17, this high completion rate, and the high completion rate for a two-dose series (91%), strongly indicates the vital role inclusion of trusted partners plays. It is also noteworthy that booster dosing declined to 30% with the return to a traditional service model, lower than the uptake of first booster doses of the general US population during the same time period (50%).18
Low uptake of healthcare services makes PWID a priority population. The reasons for this low uptake are complex and interwoven but include poverty, poor healthcare experiences, stigma, racism, and lack of transportation.16 SSPs have proven to be an important vehicle for service provision, confirmed by their high utilization and effectiveness in preventing disease transmission, decreasing overdose deaths, and facilitating treatment access.19 By co-locating other clinical services within SSPs, our evaluation suggest that this relationship can be leveraged to provide other essential public health services, including HIV and STI testing, and COVID-19 vaccination. Our data contradicts concerns regarding treatment adherence and vaccine completion rates for this population18, as over 91% completed the two-dose series of COVID-19 vaccinations, potentially driven by the co-location with a SSP.20–22
The multifaceted approach in our intervention for PWID is justified based upon the documented intersection between injection drug use and transmission of many bloodborne, and respiratory infections. Globally, PWID account for approximately 10% of all new HIV diagnoses and an estimated 23–39% of new HCV diagnoses.23 Comprehensive SSPs have demonstrated their effectiveness in preventing the transmission of blood-borne diseases such as HIV Hepatitis C, infectious endocarditis, as well as resources for collaborative prevention of STIs.5 Offering additional services – including infectious disease testing and linkage to treatment, and COVID vaccination - is an essential innovation responsive to the ongoing high incidence of HIV, HCV and STIs in this population, while providing the opportunity for mitigating COVID risk. This model of co-location of services could prevent further disease transmission and ensure newly diagnosed individuals are treated promptly, thus saving money while improving the quality and quantity of life for affected individuals.
Adequate and consistent funding for these expanded services within SSPs and other harm reduction sites is central to success. Programs that start and then stop due to funding disruptions are perceived as unreliable, and thus will not exhibit the necessary consistency that builds trust w for successful service provision. This project utilized a variety of funding sources, including Ending the HIV Epidemic funding through HRSA, allowing for the creation of seamlessly (from the perspective of the end user) interwoven prevention and treatment services. However, this was not without constraints. The collaborators had to ensure that pools of money which were tapped supported services for which the funding was allocated. This created additional administrative and reporting difficulties and constrained the leadership’s ability to be nimble when responding to the needs of PWID in administering these co-located clinics. BCHD’s long history of forward-facing services, including the SSP, proved an important infrastructure for rapidly assembling and administering these co-located clinics.24 Academic partnerships allowed for leveraging funding, content experts, and volunteers to participate in this effort, and were key to administer this project. However, without sustained funding directed toward supporting co-location of services to be delivered to this population, relying on relationships alone is unsustainable. Even with a motivated group, only ten clinics were conducted, less than one per month during the entire period of this partnership. Future research including costing analyses would be helpful in determining the level of funding needed to sustain these forward-facing prevention and treatment services at SSPs for PWID.
Funding from local, state, and federal partners focused on providing effective prevention, care, and treatment services for people who use drugs must also have funding that allows for the expansion of care services provided by SSPs and harm reduction programs. The Comprehensive Addiction Resources Emergency (CARE) Act proposed creation of programmatic infrastructure and support for individuals diagnosed with a substance use disorder.25 The extent of program support needed by this population is extensive, spanning from social service intervention to case management, medical care, drug treatment, care linkage and more. Funding for comprehensive and holistic care services can be channeled through care sites like ours that already have proven their acceptability and trustworthiness for people who use drugs. Finally, we need these program interventions to initiate remediation of the disparities experienced by this population. Health disparities unsustainably cost the US billions annually.26 Co-located program interventions cost far less than the $400–900 billion dollars health disparities cost annually.
The success of providing comprehensive services with a SSP, such as described in this manuscript, highlight the benefits of programs that are inclusive and nonjudgmental, especially when serving under-resourced communities such as PWID. To enhance services for PWID, novel approaches that leverage service co-location and use of trusted partners can be expanded to include other healthcare interventions. These range from pre-exposure prophylaxis(PrEP), HIV and HCV treatment, providing wound care and dispensing supplies, diabetes and hypertension screening and treatment, mental health counseling and medications for opioid use disorder to name a few. PWID view SSPs as a potential “one-stop shop” that could provide other healthcare services.27 These services could be quickly leveraged during future public health emergencies to deliver testing and other critical medical counter measures, facilitating better healthcare access and public health control measures.28 During the COVID-19 pandemic, integrating COVID-19 vaccines into existing SSP services not only reduced barriers to vaccine access but provided an opportunity for dissemination of factually accurate and important information during a time of great misinformation.29 Co-location of preventive and healthcare services in SSPs and harm reduction programs offers a unique and effective way to address the needs of PWID, as part of the remediation of decades of neglect.
Acknowledgements:
OH, VMC, and JEF would like to acknowledge the Baltimore City Health Department, Johns Hopkins Mobile Vaccine Unit, Ms. Lisa Parker, the Johns Hopkins nursing student volunteers, and co-authors KL, DH, JL, and AB. Without them, these clinics would not have been possible. Finally, all authors would like to acknowledge the individuals who access SSP services in Baltimore City, all of whom have enriched our lives and deserve the same dignity and respect as everyone else from public health and health care systems. It is a pleasure to serve you all.
Contributor Information
Omeid Heidari, University of Washington.
Diane Meyer, Johns Hopkins University.
Kelly Lowensen, Johns Hopkins University.
Amita Patil, Johns Hopkins University.
Katie J. O’Conor, Johns Hopkins University Jess LaRicci, CIDNI
Derrick Hunt, Baltimore City Health Department.
Adam Boek, Johns Hopkins University.
Victoria M Cargill, Milken Institute.
Jason E Farley, Johns Hopkins University.
Notes
- 1.HIV Diagnoses. [Internet]. US Centers for Disease Control and Prevention. 2023. Jun 21. [cited 2023 Dec 27]. Available from: https://www.cdc.gov/hiv/statistics/overview/in-us/diagnoses.html [Google Scholar]
- 2.2021 Viral Hepatitis Surveillance report. [Internet]. US Centers for Disease Control and Prevention. 2023. Aug 7. [cited 2023 Dec 27]. Available from: https://www.cdc.gov/hepatitis/statistics/2021surveillance/index.htm [Google Scholar]
- 3.2022 National Virus Hepatitis Progress Report. [Internet]. US Centers for Disease Control and Prevention. 2022. Sept 1. [cited 2023 Dec 27]. Available from: https://www.cdc.gov/hepatitis/policy/npr/2022/reduce-estimated-hepatitis-c-infections.htm [Google Scholar]
- 4.Heidari O, Tormohlen K, Dangerfield DT 2nd, Tobin KE, Farley JE, Aronowitz SV. Barriers and facilitators to primary care engagement for people who inject drugs: A systematic review. J Nurs Scholarsh. 2023;55(3):605–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakubowski A, Fowler S, & Fox AD. Three decades of research in substance use disorder treatment for syringe services program participants: A scoping review of the literature. Addict Sci Clin Pract. 2023;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JM. Making the case for syringe services programs. Public Health Rep. 2020;135(1_suppl):10S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood JE, Banta-Green CJ, Duchin JS, Breuner J, Dell W, Finegood B, et al. Engaging an unstably housed population with low-barrier buprenorphine treatment at a syringe services program: Lessons learned from Seattle, Washington. Subst Abus. 2020;41(3):356–64. [DOI] [PubMed] [Google Scholar]
- 8.Glick JL, Grieb SM, Harris SJ, Weir BW, Smith KC, Puryear T, et al. Exploring the impact of the COVID-19 pandemic on syringe services programs in rural Kentucky. Harm Reduct J. 2022;19(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasylyeva TI, Smyrnov P, Strathdee S, Friedman SR. Challenges posed by COVID-19 to people who inject drugs and lessons from other outbreaks. J Int AIDS Soc. 2020;23(7):e25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aponte-Melendez Y, Mateu-Gelabert P, Fong C, Eckhardt B, Kapadia S, Marks K. The impact of COVID-19 on people who inject drugs in New York City: Increased risk and decreased access to services. Harm Reduct J. 2021;18(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baillargeon J, Polychronopoulou E, Kuo YF, Raji MA. the impact of substance use disorder on COVID-19 outcomes. Psychiatric Serv. 2021;72(5):578–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strathdee SA, Abramovitz D, Harvey-Vera A, Vera CF, Rangel G, Artamonova I, et al. Prevalence and correlates of SARS-CoV-2 seropositivity among people who inject drugs in the San Diego-Tijuana border region. PLoS One. 2021;16(11):e0260286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Interim list of categories of essential workers mapped to standardized industry codes and titles. [Internet]. US Centers for Disease Control and Prevention. 2021. Mar 29. [cited 2023 Dec 27]. Available from: https://www.cdc.gov/vaccines/covid-19/categories-essential-workers.html#:~:text=Phase%201a%20includes%20healthcare%20personnel,in%20Phase%201a%20or%201b. [Google Scholar]
- 14.Heidari O, Meyer D, O’Conor KJ, Cargill V, Patch M, Farley JE. COVID-19 vaccination and communicable disease testing services’ integration within a syringe services program: A program brief. J Assoc Nurses AIDS Care. 2022;33(3):348–352. [DOI] [PubMed] [Google Scholar]
- 15.Kriss JL, Reynolds LE, Wang A, Stokley S, Cole MM, Harris LQ, et al. COVID-19 vaccine second-dose completion and interval between first and second doses among vaccinated persons — United States, December 14, 2020−February 14, 2021. MMWR Morb Mortal Wkly Rep 2021;70(11):389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motavalli D, Taylor JL, Childs E, Valente PK, Salhaney P, Olson J, et al. “Health Is on the Back Burner:” Multilevel Barriers and Facilitators to Primary Care Among People Who Inject Drugs. J Gen Intern Med. 2021;36(1):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier MG, Drobeniuc J, Cuevas-Mota J, Garfein RS, Kamili S, Teshale EH. Hepatitis A and B among young persons who inject drugs--vaccination, past, and present infection. Vaccine. 2015;33(24):2808–12. [DOI] [PubMed] [Google Scholar]
- 18.Fast HE, Murthy BP, Zell E, Meng L, Murthy N, Saelee R, et al. Booster COVID-19 vaccinations among persons aged ≥5 years and second booster COVID-19 vaccinations among persons aged ≥50 years — United States, August 13, 2021–August 5, 2022. MMWR Morb Mortal Wkly Rep 2022;71(35):1121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barocas JA, Eftekhari Yazdi G, Savinkina A, Nolen S, Savitzky C, Samet JH, et al. Long-term infective endocarditis mortality associated with injection opioid use in the United States: A modeling study. Clin Infect Dis. 2021;73(11):e3661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strathdee SA, Abramovitz D, Vera CF, Artamonova I, Patterson TL, Smith DM, et al. Predictors of COVID-19 vaccine uptake among people who inject drugs. Vaccine. 2023;41(12):1916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cioffi CC, Kosty D, Nachbar S, Capron CG, Mauricio AM, Tavalire HF. COVID-19 vaccine deliberation among people who inject drugs. Drug Alcohol Depend Rep. 2022;3:100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cepeda JA, Feder KA, Astemborski J, Schluth CK, Kirk GD, Mehta SH, et al. COVID-19 vaccine hesitancy and vaccination status in a community-based cohort of people who inject drugs in Baltimore, Maryland, March-June 2021. Public Health Rep. 2022;137(5):1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global HIV, hepatitis and STIs programmes. [Internet]. World Health Organization. 2023. [cited 2023 Dec 27]. Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/populations/people-who-inject-drugs [Google Scholar]
- 24.Heidari O, Shah H, Bhagwat A, Ahmad NJ, Whaley S, Sherman SG, et al. Changes in opioid treatment programs and harm reduction provider services during the COVID-19 pandemic: Findings from 10 states. Psychol Serv. 2023; Oct 10. DOI: 10.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosecrans A, Harris R, Saxton RE, Cotterell M, Zoltick M, Willman C, et al. Mobile low-threshold buprenorphine integrated with infectious disease services. J Subst Abuse Treat. 2022;133:108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren, Cummings, and more than 95 colleagues in Senate and House reintroduce comprehensive CARE Act to combat the opioid and substance use epidemic. [Internet]. Elizabeth Warren Press Release. 2019. May 8. [cited 27 Dec 2023]. Available from: https://www.warren.senate.gov/newsroom/press-releases/warren-cummings-and-more-than-95-colleagues-in-senate-and-house-reintroduce-comprehensive-care-act-to-combat-the-opioid-and-substance-use-epidemic [Google Scholar]
- 27.LaVeist TA, Pérez-Stable EJ, Richard P, et al. The Economic Burden of Racial, Ethnic, and Educational Health Inequities in the US. JAMA. 2023;329(19):1682–92. [DOI] [PubMed] [Google Scholar]
- 28.Bartholomew TS, Andraka-Cristou B, Totaram RK, Harris S, Doblecki-Lewis S, Ostrer L, et al. “We want everything in a one-stop shop”: acceptability and feasibility of PrEP and buprenorphine implementation with mobile syringe services for Black people who inject drugs. Harm Reduct J. 2022;19(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery MP, Zhong Y, Roberts E, Asher A, Bixler D, Doshani M, et al. Vaccination barriers and opportunities at syringe services programs in the United States, June-August 2021-A cross-sectional survey. Drug Alcohol Depend. 2022;237:109540. [DOI] [PMC free article] [PubMed] [Google Scholar]


