Abstract
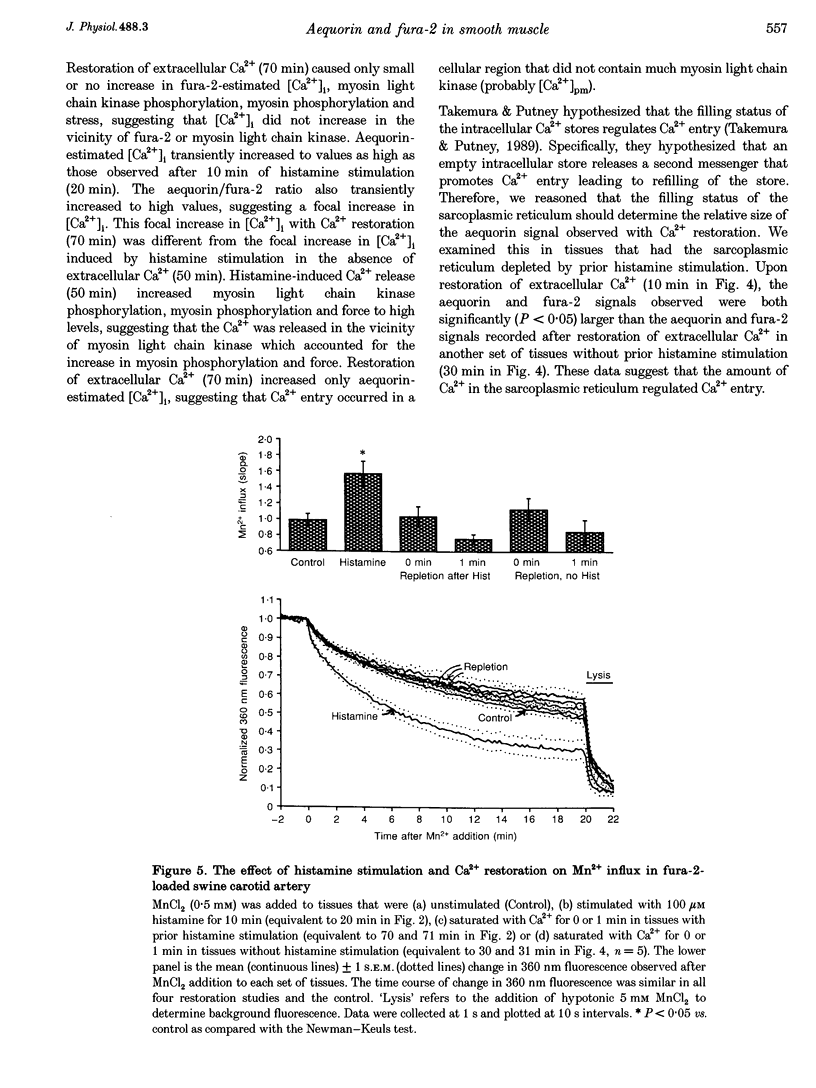
1. We hypothesized that the homogeneity of intracellular [Ca2+] ([Ca2+]i) varies and is regulated in arterial smooth muscle. 2. We evaluated this hypothesis by exploiting the different characteristics of several [Ca2+]i indicators: (1) aequorin, which theoretically can measure focal increases in [Ca2+]i, (2) fura-2, which is predominantly a measure of mean cytoplasmic [Ca2+], and (3) myosin light chain phosphorylation and force, which reflect increases in [Ca2+] near the contractile apparatus. 3. From the differences in the observed aequorin and fura-2 signals, we developed an index of the relative degree of [Ca2+]i homogeneity as the ratio of the aequorin signal and fura-2 signal. 4. Stimulation with intermediate concentrations of histamine (1 and 10 microM) or high [K+]o (25 and 40 mM) increased [Ca2+]i and contractile stress. Relative [Ca2+]i homogeneity, estimated from the aequorin/fura-2 ratio, remained similar to levels observed in unstimulated tissues. 5. Higher concentrations of histamine (100 microM) also increased [Ca2+]i and stress, but the aequorin/fura 2 ratio declined , indicating increased [Ca2+]i homogeneity. Similarly, the aequorin/fura-2 ratio decreased when extracellular Ca2+ was removed. 6. Stimulation with histamine in low extracellular [Ca2+] transiently increased [Ca2+]i and the aequorin/fura-2 ratio. Similarly, in tissues treated with low extracellular [Ca2+], restoration of extracellular Ca2+ transiently increased both [Ca2+]i and the aequorin/fura-2 ratio. Although both of these experiments demonstrated a transient decrease in [Ca2+]i homogeneity, only histamine stimulation led to increased myosin light chain phosphorylation and force. These results indicate that the focal increases in [Ca2+]i observed with histamine stimulation and Ca2+ restoration occurred in different cellular regions. 7. Addition of caffeine (20 mM) increased [Ca2+]i and [cAMP], but this was not accompanied by sustained increased myosin light chain phosphorylation or contraction. Phosphorylation of myosin light chain kinase did not appear to underlie the lack of increase in myosin light chain phosphorylation. Rather, caffeine induced a sustained increase in the aequorin/fura-2 ratio, suggesting that caffeine inhibits smooth muscle contraction by localizing increases in [Ca2+]i to a region distant from the contractile apparatus. 8. These data suggest that there can be transient and sustained focal increases in [Ca2+]i. Aequorin detected increased [Ca2+]i in small regions of the cytoplasm during release from and refilling of the intracellular Ca2+ store and with caffeine stimulation. Dual use of aequorin and fura-2 permits determination of relative [Ca2+]i homogeneity in smooth muscle.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Peskoff A. Diffusion around a cardiac calcium channel and the role of surface bound calcium. Biophys J. 1991 Mar;59(3):703–721. doi: 10.1016/S0006-3495(91)82284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Chen Q., van Breemen C. The superficial buffer barrier in venous smooth muscle: sarcoplasmic reticulum refilling and unloading. Br J Pharmacol. 1993 Jun;109(2):336–343. doi: 10.1111/j.1476-5381.1993.tb13575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. L., Rembold C. M. Cyclic nucleotide-dependent regulation of Mn2+ influx, [Ca2+]i, and arterial smooth muscle relaxation. Am J Physiol. 1992 Aug;263(2 Pt 1):C468–C473. doi: 10.1152/ajpcell.1992.263.2.C468. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C., Hoth M., Penner R. Multiple mechanisms of manganese-induced quenching of fura-2 fluorescence in rat mast cells. Pflugers Arch. 1993 May;423(3-4):225–231. doi: 10.1007/BF00374399. [DOI] [PubMed] [Google Scholar]
- Gilbert E. K., Weaver B. A., Rembold C. M. Depolarization decreases the [Ca2+]i sensitivity of myosin light-chain kinase in arterial smooth muscle: comparison of aequorin and fura 2 [Ca2+]i estimates. FASEB J. 1991 Aug;5(11):2593–2599. doi: 10.1096/fasebj.5.11.1868983. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Kargacin G., Fay F. S. Ca2+ movement in smooth muscle cells studied with one- and two-dimensional diffusion models. Biophys J. 1991 Nov;60(5):1088–1100. doi: 10.1016/S0006-3495(91)82145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990 Apr 20;248(4953):283–283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- McDaniel N. L., Rembold C. M., Richard H. M., Murphy R. A. Cyclic AMP relaxes swine arterial smooth muscle predominantly by decreasing cell Ca2+ concentration. J Physiol. 1991 Aug;439:147–160. doi: 10.1113/jphysiol.1991.sp018661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. D., Etter E. F., Philipson K. D., Carrington W. A., Fogarty K. E., Lifshitz L. M., Fay F. S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Kasai H., Hori M., Sato K., Ishihara H., Karaki H. Direct inhibition of chicken gizzard smooth muscle contractile apparatus by caffeine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Mar;341(3):262–267. doi: 10.1007/BF00169741. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Grégoire G., Mironneau C., Mironneau J. Noradrenaline-activated heparin-sensitive Ca2+ entry after depletion of intracellular Ca2+ store in portal vein smooth muscle cells. J Biol Chem. 1993 Feb 25;268(6):3866–3872. [PubMed] [Google Scholar]
- Rembold C. M. Desensitization of swine arterial smooth muscle to transplasmalemmal Ca2+ influx. J Physiol. 1989 Sep;416:273–290. doi: 10.1113/jphysiol.1989.sp017760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988 Sep;63(3):593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992 Jul 23;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Shuttleworth T. J. Receptor-activated calcium entry in exocrine cells does not occur via agonist-sensitive intracellular pools. Biochem J. 1990 Mar 15;266(3):719–726. doi: 10.1042/bj2660719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T. J., Thompson J. L. Effect of temperature on receptor-activated changes in [Ca2+]i and their determination using fluorescent probes. J Biol Chem. 1991 Jan 25;266(3):1410–1414. [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J. 1989 Sep;3(11):2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull J. T., Hsu L. C., Tansey M. G., Kamm K. E. Myosin light chain kinase phosphorylation in tracheal smooth muscle. J Biol Chem. 1990 Sep 25;265(27):16683–16690. [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto A., Arai H., Ogawa Y. Reassessment of Fura-2 and the ratio method for determination of intracellular Ca2+ concentrations. Cell Calcium. 1991 Jan;12(1):29–37. doi: 10.1016/0143-4160(91)90082-p. [DOI] [PubMed] [Google Scholar]
- Van Breemen C. Calcium requirement for activation of intact aortic smooth muscle. J Physiol. 1977 Nov;272(2):317–329. doi: 10.1113/jphysiol.1977.sp012046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Riper D. A., Weaver B. A., Stull J. T., Rembold C. M. Myosin light chain kinase phosphorylation in swine carotid artery contraction and relaxation. Am J Physiol. 1995 Jun;268(6 Pt 2):H2466–H2475. doi: 10.1152/ajpheart.1995.268.6.H2466. [DOI] [PubMed] [Google Scholar]
- Villa A., Podini P., Panzeri M. C., Söling H. D., Volpe P., Meldolesi J. The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J Cell Biol. 1993 Jun;121(5):1041–1051. doi: 10.1083/jcb.121.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle P., Nishikawa M., Yost D. A., Adelstein R. S. Increased phosphorylation of myosin light chain kinase after an increase in cyclic AMP in intact smooth muscle. Science. 1984 Mar 30;223(4643):1415–1417. doi: 10.1126/science.6322302. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Lukeman S., Leijten P., Yamamoto H., Loutzenhiser R. The role of superficial SR in modulating force development induced by Ca entry into arterial smooth muscle. J Cardiovasc Pharmacol. 1986;8 (Suppl 8):S111–S116. doi: 10.1097/00005344-198600088-00023. [DOI] [PubMed] [Google Scholar]


