Abstract
Brain insulin resistance has recently been described as a metabolic abnormality of brain glucose homeostasis that has been proven to downregulate insulin receptors, both in astrocytes and neurons, triggering a reduction in glucose uptake and glycogen synthesis. This condition may generate a mismatch between brain’s energy reserve and expenditure, ??mainly during high metabolic demand, which could be involved in the chronification of migraine and, in the long run, at least in certain subsets of patients, in the prodromic phase of Alzheimer’s disease, along a putative metabolic physiopathological continuum. Indeed, the persistent disruption of glucose homeostasis and energy supply to neurons may eventually impair protein folding, an energy-requiring process, promoting pathological changes in Alzheimer's disease, such as amyloid-β deposition and tau hyperphosphorylation. Hopefully, the “neuroenergetic hypothesis” presented herein will provide further insight on there being a conceivable metabolic bridge between chronic migraine and Alzheimer’s disease, elucidating novel potential targets for the prophylactic treatment of both diseases.
Keywords: metabolism, migraine, Alzheimer's disease, brain insulin resistance, glucose
Introduction
Migraine and Alzheimer's disease (AD) remain two major public health issues. Indeed, migraine and other primary headache disorders are the second leading causes of disability worldwide, according to The World Health Organization [1]. Notably, migraine is the leading cause of disability in the under-50s [2], affecting about 14% of the world's population [3]. Although migraine is generally an episodic disorder, it may evolve over time into a chronic condition, with an average annual progression rate of 3% [4]. AD is the most common form of dementia in developed countries and its prevalence is on the increase, due to population aging [5].
Indeed, the Global Burden of Disease 2019 Dementia Forecasting Collaborators estimated that the number of people with dementia would have increased from 57.4 million cases globally in 2019 to 152.8 million cases in 2050 worldwide [6]. Despite recent therapeutic advances [7], there are still unmet needs in migraine preventive treatment [8]. Likewise, halting the progression of AD currently remains a challenge [9]. Although the novel anti-amyloid antibody treatments have reached the objective of promoting amyloid-β (Aβ) clearance and slowing down AD progression over several months, their efficacy is only moderate. Indeed, there are still numerous hurdles to overcome to improve their long-term efficacy, safety and accessibility [10-12]. Therefore, research has focused on other pathophysiological factors that might play a role in the complex aetiology of AD, such as a decrease in the brain energy metabolism [13, 14].
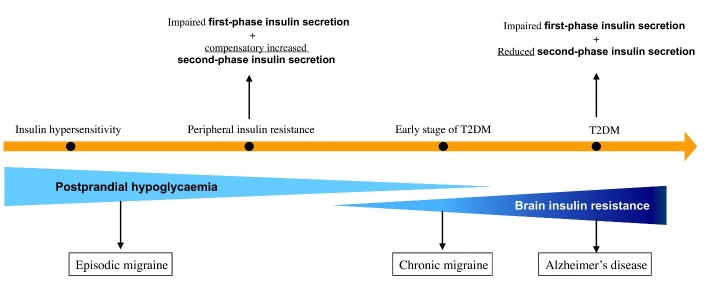
After having reviewed the current literature on the metabolic aspects of migraine pathophysiology, we proposed a "neuroenergetic hypothesis” of migraine [15] (Fig. 1).
Figure 1.
The “Neuroenergetic hypothesis”: a metabolic bridge between migraine and Alzheimer’s disease (AD). This illustrates the Neuroenergetic Hypothesis that we first described elsewhere [15]. Herein we focused on the “extended Neuroenergetic Hypothesis” as to there being a metabolic bridge between chronic migraine and AD. Conditions that are connected by a continuum of time and pathophysiology are marked by an orange arrow, i.e., insulin hypersensitivity, insulin resistance, early stage of T2DM and T2DM. Both insulin hypersensitivity and peripheral insulin resistance can lead to postprandial hypoglycaemia through different mechanisms. Postprandial hypoglycaemia has been identified as a major contributor to cerebral energy deficiency that underlies episodic migraine. The worsening of glucose metabolism, evidenced by the orange arrow, may extend to the brain over time, leading to brain insulin resistance. Brain insulin resistance generates a chronic mismatch between the energy reserve of the brain and functional expenditure, which is involved in the chronification of migraine and, in the long run, at least in certain subsets of patients, in the prodromic phase of AD, along a putative metabolic physiopathological continuum. T2DM, type 2 diabetes mellitus.
We assumed that an energy deficit (a mismatch between the brain’s energy reserve and workload), caused by an altered glucose and insulin metabolism in the brain, i.e., the condition of brain insulin resistance, may be a pivotal mechanism in the pathophysiology of migraine, promoting its chronification.
Background of the “Neuroenergetic hypothesis”
In 2017, Blonz first proposed a “Neuroenergetic hypothesis” for AD [13] which, interestingly, may overlap with some pathophysiological alterations in migraine. He hypothesized that the decreased availability of metabolizable energy resources in the central nervous system is a key factor in AD pathogenesis, mainly as a result of an age-related decline in the ability of glucose to cross the blood-brain barrier.
After which, Zulfiqar et al. [16] revised the "Neuroenergetic hypothesis" and proposed that AD may be underpinned by “a novel” pathophysiological mechanism. They reported that, in their opinion, cerebral glucose hypometabolism is an early event in AD, caused by a deficit in the support of neuronal physiological needs, mainly due to an imbalanced neuron-astrocyte lactate shuttle. This would imply that astrocytes play a key role in this revised "Neuroenergetic hypothesis" of AD pathophysiology [16]. This seems to be in line with what was previously reported by other authors in 2015, i.e., that astrocyte hypertrophy and lesions occur early in AD progression [17].
Therefore, the "Neuroenergetic hypothesis" seems to be an appealing theoretical frame, accounting for some brain metabolic abnormalities shared by migraine and AD and providing further insight into a putative metabolic bridge between chronic migraine (CM) and AD.
Migraine and Alzheimer’s disease may have overlapping pathophysiological mechanisms
This scoping review focuses on the fact that, as aforementioned, there are intriguing similarities between the pathophysiology of CM and AD: brain insulin resistance, impaired brain glucose metabolism, an alteration in brain mitochondrial bioenergetics and neuroinflammation. These are likely to be common pathophysiological alterations shared by these two pathological conditions and may well underlie the reduction in grey matter volume in specific areas, the disrupted default mode network connectivity observed at neuroimaging and the increased theta and delta activity evidenced on EEG in both diseases (Fig. 2).
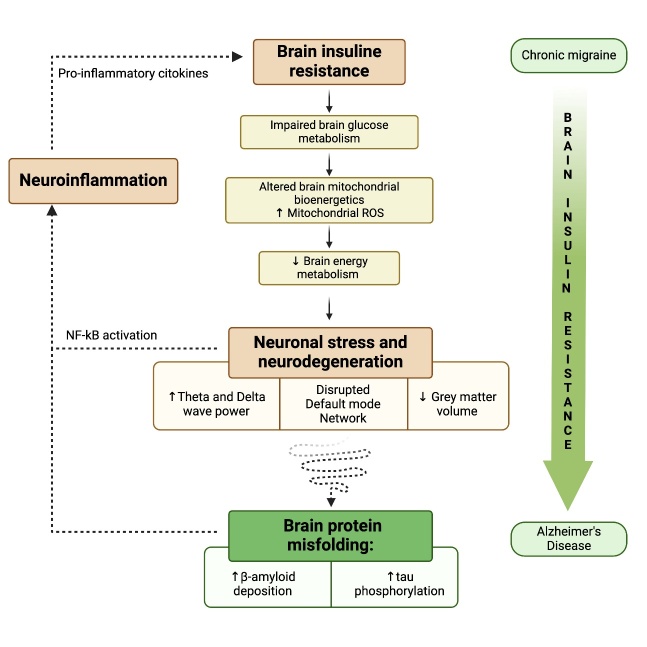
Figure 2.
From migraine to Alzheimer’s Disease (AD): the metabolic physiopathological continuum of the “Neuroenergetic hypothesis”. Brain insulin resistance is the metabolic alteration underlying the common pathophysiological alterations between chronic migraine (CM) and AD, i.e., impaired brain glucose metabolism and altered brain mitochondrial bioenergetics - leading to an overproduction of mitochondrial reactive oxygen species - which generate a reduction in the brain energy metabolism, leading to neuronal stress and subsequent neuronal degeneration, detectable as a reduction in grey matter volume, a disrupted default mode network connectivity and the increased theta and delta activity observed at EEG, shared by CM and AD. In the long run, brain insulin resistance and the related energy deficiency might favour the pathological changes involved in AD, promoting a shift towards the amyloidogenic cascade and increasing tau hyperphosphorylation. The shaded green arrow to the right of the figure illustrates that increased brain insulin resistance sustains the altered metabolic pathway and the progression from CM to AD.
Indeed, in the long-run, the persistent disruption of the glucose homeostasis may impair protein folding, which is an energy-requiring process essential for brain tissue turnover and functionality. This might be one of the mechanisms, promoting a shift towards the amyloidogenic pathway and tau phosphorylation, in the complex pathophysiology underlying AD pathological changes [18] (Fig. 2).
Therefore, in our hypothesis we propose that brain insulin resistance could be considered a “metabolic bridge” between CM and AD, in a sort of pathophysiological continuum. Recent epidemiological studies also support this hypothesis, as they report a positive association between migraine and the subsequent development of dementia, where migraine with aura in early life is associated with a two-fold increased risk of dementia and a four-fold increased risk of AD [19, 20].
The last paragraph discusses potential treatment options, targeting the mechanisms involved in such a pathophysiological hypothesis, these include: the traditional Mediterranean Diet, regular aerobic exercise and Mind-Body Interventions (MBI). Although the “Neuroenergetic Hypothesis” as a potential therapeutic target is still a major concern, these sustainable long-term options could significantly reduce the frequency and intensity of migraine attacks and, hopefully, also play a role in preventing or, at least, delaying, the onset of some types of dementias, in particular AD. Further clinical studies are required to prove this hypothesis.
Epidemiological evidence on a putative correlation between migraine and dementia
Although the question of the relationship between migraine and dementia remains controversial, in particular how migraine and AD may influence each other, there is an increasing body of evidence (Table 1) as to a positive association between these two conditions. Recent data from observational and investigational studies (Table 1) suggest that migraine - especially migraine with aura - may be a risk factor for dementia, mainly AD, with a weaker positive association also for vascular dementia (VaD).
Table 1.
Summary of the studies investigating the epidemiological relationship between migraine and dementia.
| First author | Year | Method | Country | Observations |
|---|---|---|---|---|
| Cermelli A [21] | 2023 | Systematic review and meta-analysis | Not applicable | Migraine was associated with both a moderate increased risk of all-cause dementia (OR = 1,26; p = 0,00; 95% CI: 1,13-1,40) as well as a moderate increased risk of Alzheimer's disease (AD) (OR = 2,00; p = 0,00; 95% CI: 1,46-2,75). |
| Qu H [22] | 2022 | Meta-Analysis of Cohort Studies | Not applicable | Types of dementia considered: all-cause dementia, AD, vascular dementia (VaD).A history of migraine is associated with a higher risk of dementia (OR = 1.32; 95% CI: 1.13-1.40; I2 = 75.6%, P < 0.001), but the risk is lower than that of non-migraine headache patients. |
| Wang L [23] | 2022 | Meta-Analysis of Cohort Studies | Not applicable | Pooled analysis showed that migraine was associated with increased risk of all-cause dementia (RR: 1.34, 95% CI: 1.13-1.59) and AD (RR: 2.49, 95% CI: 1.16-5.32). However, we did not find any association between migraine and risk of VaD (RR: 1.51, 95% CI: 0.77-2.96). |
| Kim SJ [24] | 2022 | Nationwide Retrospective Cohort Study | South Korea | Patients with migraine had a 1.18 (adjusted hazard ratio [aHR], 1.18; 95% CI, 1.12-1.24), 1.21 (aHR, 1.21; 95% CI, 1.10-1.32), and 1.18 (aHR, 1.18; 95% CI, 1.13-1.24) times higher risk of developing AD, VaD and all-cause dementia. |
| Hurh K [25] | 2022 | Nationwide Retrospective Cohort Study. | South Korea | Patients with migraine had a 1.30 (hazard ratio [HR], 1.30; 95% CI, 1.25-1.35), 1.29 (HR, 1.29; 95% CI, 1.23-1.35), 1.35 (HR, 1.35; 95% CI, 1.19-1.54), 1.36 (HR, 1.36; 95% CI, 1.00-1.83), and 1.30 (HR, 1.30; 95% CI, 1.17-1.45) times higher risk of developing all-cause dementia, AD, VaD, mixed or other specified dementias, and unspecified dementia than their matched controls, respectively. |
| Gu L [26] | 2022 | Meta-Analysis | Not applicable | The study showed no significant association between migraine without aura and risk of dementia with a random effects model (OR/RR = 1.03, 95% CI 0.89 to 1.19, I2 = 0.0%, p = 0.453). The study showed significant associations between migraine and risk of VaD (OR/RR = 1.84, 95% CI 1.18 to 2.88, I2 = 0.0%, p = 0.423) and AD (OR/RR = 2.60, 95% CI 1.51 to 4.48, I2 = 43.8%, p = 0.169) with random effects models. |
| Lee HJ [27] | 2021 | Nationwide Retrospective Cohort Study | South Korea | Patients with migraine had a significantly higher incidence of AD (adjusted HR = 1.31, 95% CI, 1.08-1.58), but not VaD, than those without migraine. |
| Islamoska S [19] | 2020 | Longitudinal population-based register study.62578 individuals, 10857 with migraine.Follow-up 6.9 years. | Denmark | Types of dementia considered: unspecified dementia, AD, VaD, frontotemporal dementia, and Lewy body dementia. They were not evaluated individually.207 individuals with migraine developed dementia. Individuals without aura had a 19% higher rate of dementia, and individuals with aura had a two-fold higher rate of dementia, compared with individuals without migraine. |
| Kostev K [28] | 2019 | Retrospective cohort study. 7454 individuals from 67 general practices inthe UK | United Kingdom | Types of dementia considered: VaD, AD, unspecified dementia.They observed - only in women - a positive significant association between migraine diagnoses and all-cause dementia (hazard-ratio [HR] = 1.65) as well as AD (HR = 2.27). |
| Morton RE [20] | 2019 | Prospective cohort study. 679 community-dwelling participants 65+ years, follow up 5 years. | Canada | A history of migraines was significantly associated with both all-cause dementia (odds ratio [OR]=2.97; 95% confidence interval [CI]=1.25-6.61) and AD (OR=4.22; 95% CI=1.59-10.42). Although no significant association was found between migraine and vascular dementia. |
| Lee SY [29] | 2019 | Retrospective cohort study. 11438 dementia participants, 45752 controls | Korea | Dementia was defined as diagnoses of AD or dementia in AD.7.7% of patients in the dementia group and 6.3% of those in the control group had a history of migraine.The crude and adjusted odds ratios for migraine with dementia was 1.22 and 1.13, respectively. |
| Hagen K[30] | 2014 | A prospective population-based study. 51,383 participants from the Nord-Trøndelag Health Study | Norway | There was a significant interaction between age and any headache regarding VaD (p < 0.0001): In subsequent analyses stratifying by age, any headache increased the risk of VaD more among individuals 75 years of age at baseline (HR ¼ 3.0; 95% CI 1.3-6.7, p ¼ 0.007) than among those.They observed a positive association between VaD diagnoses and all types of migraine (hazard-ratio [HR] = 2.9; 95% confidence interval [CI]= 1.3-6.6). This was more marked for migraine on 15 days/month (HR = 9.1 (2.2-40.1), p = 0.003) than for nonmigrainous headache on 15 days/month (HR = 3.2 (1.0-10.3), p = 0.057).No association was found between headache and AD. However, the association between AD and migraine has not been investigated. |
| Chuang CS [31] | 2013 | Retrospective cohort study.Data from the National Health Insurance Research database in Taiwan | Taiwan | Types of dementia considered: AD, senile dementia, dementia in conditions classified elsewhere (e.g., dementia of the Alzheimer's type). They were not evaluated individually.After adjusting the covariates, migraine patients had a 1.33-fold higher risk of developing dementia, compared with individuals without migraine. Young adults have a higher association between migraine and dementia than older adults. |
This association may arise from the well-known vascular comorbidity of migraine. However, it is still a question of debate as to whether migraine should be considered a true “vascular disease” or if the comorbidity between migraine and cerebrovascular disease may have underlying shared risk factors or pathophysiological mechanisms [32, 33].
Three meta-analyses on the association between migraine and dementia were published in 2022. Qu et al. reported that a history of migraine is associated with a higher risk of all-cause dementia, but the risk is lower than that of non-migraine headache patients [22]. Wang et al. demonstrated that migraine was associated with an increased risk of all-cause dementia, especially AD [23]. Gu et al. observed significant associations between migraine with aura and risk of VaD and AD and that the association was stronger for AD [26].
In the same year, two Nationwide Retrospective Cohort Studies, performed in South Korea, also reported that patients with migraine run a higher risk of subsequently developing dementia, in particular, VaD [24, 25]. Moreover, in 2023 a systematic review and meta-analysis reported that migraine was associated with both a 26% increased risk of all-cause dementia as well as a two-fold increased risk of AD [21]. Therefore, although the epidemiological evidence linking migraine with a higher risk of dementia and AD is compelling, further studies are still required to understand the nature of this association and whether this implies a direct causative relationship or shared risk factors.
Our scoping review fits into this framework, as it proposes a pathophysiological hypothesis that may explain at least some of the mechanisms involved in this association, which still remain partly unknown.
Insulin resistance and the brain
Insulin resistance is commonly characterized as a decreased sensitivity of bodily tissues to the action of insulin [34]. It may be defined as a subnormal physiological response of target tissue to insulin stimulation [35]. Similarly, brain insulin resistance is the inability of brain cells to respond to insulin [36]. Systemic and cerebral insulin resistance may have a strong correlation, in as much as systemic insulin resistance, in patients with type 2 diabetes (T2DM), may lead to brain insulin resistance and brain dysfunction, whereas aberrant insulin signalling in the brain may have systemic repercussions that affect metabolic regulation [37-39]. Although the question of the relationship between brain and peripheral insulin resistance is currently under debate. The two conditions are not always interlinked, and it remains to be confirmed whether peripheral and central insulin resistance are able to exist independently [40]. Moreover, at present, there is no internationally accepted criteria for the identification of a neurophysiological or neuroimaging response as a marker of brain insulin resistance [41]. The methods to investigate brain insulin resistance have been reviewed elsewhere [40].
Various mechanisms may underlie the diminished response to insulin, such as the downregulation of insulin receptors, the inability of insulin receptors to bind insulin and/or the aberrant activation of the insulin signalling cascade [37]. At a cellular level, this abnormality may manifest itself as an impaired neurotransmitter release, altered receptor regulation in neurons and glial cells and/or a dysfunction in the processes that are most directly related to insulin metabolism and glucose homeostasis, such as glucose uptake in neurons and inflammatory responses to insulin [42, 43]. The role of insulin resistance in the context of the “Neuroenergetic hypothesis” in migraine pathophysiology and chronification was recently assessed in a review by Del Moro et al. [15].Growing evidence suggests that insulin resistance is a pivotal pathophysiological mechanism also in AD, which is emerging as ‘‘type 3 diabetes’’, in agreement with Steen et al.’s hypothesis [39]. Other authors [18, 44, 45] further developed this hypothesis, reporting that oxidative stress, impaired glucose metabolism and tau hyperphosphorylation and Aβ deposition were all linked to perturbation in insulin/insulin-like growth factor signaling.
Indeed, anti-diabetic drugs, such as metformin, intranasal insulin, incretins, SGLT2 inhibitors, PPAR-γ agonists and DPP4 inhibitors are now being investigated in the context of AD treatment and prevention. Most of these drugs have provided some promising results in clinical trials; however, additional research is required to confirm their therapeutic potential [46, 47].
To date, no randomized controlled trial (RCT) that evaluates the safety and efficacy of anti-diabetes drugs in the treatment of chronic migraine has been published.
Decreased brain glucose metabolism
The results of positron emission tomography (PET) studies and voxel-based statistical parametric mapping analysis of (18)F-fluorodeoxyglucose-PET report functional neuroimaging evidence of a decreased cerebral glucose metabolism in migraine patients, especially in CM [48-50].
Notably, certain brain regions in AD (Table 2) were identified as being particularly vulnerable to hypometabolism [79, 80], a reduced glucose metabolic rate [60, 62], a reduced cortical thickness, volume loss [64], atrophy [43, 78] and amyloid deposition [87]. These areas were named AD-vulnerable brain regions [64, 71, 81, 87], and include the parietal cortex, the posterior cingulate cortex, the temporal gyrus, the temporal pole, the medial temporal lobe (parahippocampal gyrus, hippocampus, amygdala, entorhinal cortex), the prefrontal cortex and the superior and middle frontal gyrus (Table 2).
Table 2.
Comparison between brain areas affected by reduced glucose metabolism, volume and energy metabolism in insulin resistance (IR), Alzheimer’s disease (AD-vulnerable brain regions) and migraine (migraine-vulnerable brain regions).
| Brain areas | Reduced regional cerebral glucose metabolism in subjects with insulin resistance | Reduced regional cerebral glucose metabolism in migraine subjects | Reduced regional cerebral energy metabolismin migraine subjects *** | Reduced regional cerebral volume in migraine subjects | Alzheimer’s disease - vulnerable brain regions |
|---|---|---|---|---|---|
| The insular lobe | Insular lobe: CM [50]Insular cortex: EM [48], CM [49] | Insular lobe: † [51], [52]Insular cortex: *CM [53] | |||
| The parietal lobe | Parietal lobe: [54] lateralParietal cortex: †††† [55] leftBrodmann areas 7 and 40: [56] | Parietal cortex: CM [49] | ↓ PCr/Pi ¯ [57]↓ [Mg+2] ¯ [58]↓ PCr/Pi ¯ [59] | Parietal lobe: CM [53]Parietal operculum: † CM [51] left | Parietal lobe: [60-62]Parietal cortex: [43]Parieto-temporal cortex: [63]Inferior parietal cortex: [64] |
| The anterior cingulate cortex | Anterior cingulate cortex: CM [49], [48] | Anterior cingulate cortex: ** EM e CM [65], † CM [51], CM [66], [67] right, [52] | |||
| The posterior cingulate cortex | Posterior cingulate cortex: [56] | Posterior cingulate cortex: EM [48] | Posterior cingulate cortex: [60-64, 68] | ||
| The temporal lobe | Temporal lobe: [54]Middle temporal cortex: †††† [55] leftTemporal/angular gyri (Brodmann area 39): [56] | Inferior temporal, temporal pole, right-banks superior temporal sulcus: CM [50] | ↓ PCr/Pi ¯ [57]↓ [Mg+2 ] ¯ [58]↓ PCr/Pi ¯ [59] | Temporal pole, superior temporal lobe: CM [66] Left superior temporal gyrus, right fusiform gyrus, right middle temporal gyrus: CM [69]Superior temporal sulcus left, inferior temporal gyrus left : CM [69] | Temporal lobe: [43, 60]Temporal cortex: [64, 68] lateral, [70]Inferior temporal gyrus: [71]Temporopolar cortex: [64] |
| The prefrontal cortex | Prefrontal cortex:[54]Anterior and inferior prefrontal cortices (Brodmann areas 10, 45, 47): [56] | Prefrontal cortex: EM [48] leftPars triangularis (Brodmann areas 44): CM [50] Orbitofrontal cortex (Brodmann area 10, 11 and 47)****: CM [49]Orbitofrontal (Brodman area 47)††: CM [50] left | Inferior frontal gyri (Brodmann areas 44, 45, 47)*****:CM [51]†, EM [72], [67]Dorsolateral prefrontal cortex (Brodmann areas 46 and 9): EM [65, 73]Pars triangularis (Brodmann areas 44): CM [66]Lateral orbital frontal cortex (Brodman area 47)††: CM [69, 74, 75] left, ††† CM [76]Medial orbital frontal gyrus: CM [66] | Prefrontal Cortex [43]Inferior frontal cortex (Brodmann areas 44, 45, 47)*****: [64] | |
| The frontal lobe | Superior frontal gyrus: †††† [55] right Middle frontal gyrus: †††† [55] | Superior frontal gyrus: CM [50] leftFrontal pole: CM [50] right Precentral gyrus: CM [50] right | ↓ PCr/Pi ¯ [57]↓ Pi/Tp ¯ [57]↓ [Mg+2] ¯ [58]↓PCr/Pi ¯ [59] | Medial frontal lobes: ††† CM [76]Superior frontal gyrus: CM [75]Middle frontal gyrus: [67], CM [53] caudal Precentral gyrus: CM [53, 69, 72], [67] rightRight frontal pole: CM [74],††† [76] | Frontal lobes: [60, 61, 63]Superior frontal gyrus: [68]Middle frontal gyrus: [68, 71] |
| The hippocampus | Hippocampus: [55] | Hippocampus: CM [65, 77] leftEntorhinal cortex: CM [66]Parahippocampal gyrus: [67] leftParahippocampus:EM [72] | Hippocampus: [43, 60, 62, 64, 68, 78-81]Entorhinal cortex:[64, 68, 81]Parahippocampal gyrus:[68] | ||
| The amygdala | Amygdala: [55] | Amygdala: † [51, 82] left | Amygdala: [61, 68, 78, 81] | ||
| The occipital lobe | ↓ PCr/Pi ¯, ↓ [Mg+2 ] ¯ [82] |
| EM | episodic migraine |
| CM | chronic migraine |
| PCr | phosphocreatine |
| Pi | inorganic phosphate |
| TP | total phosphorus signal |
| PCr/Pi, PCr/ATP | a reduced ratio indicates energy deficit |
| ATP | adenosine triphosphate |
| In bold | brain areas affected by reduced glucose metabolism and shared by insulin resistance, migraine and AD subjects. |
| * | Lai KL et al enrolled patients with CM without medication overuse headache, major depression or prior preventive treatment. |
| ** | A higher headache frequency was associated with smaller grey matter volume in the anterior cingulate cortex and hippocampus in EM and CM |
| *** | According to current literature, most studies have chosen the occipital cortex as the region of interest, as aura, most commonly with visual symptoms, is attributed to this area in patients suffering from this type of migraine [83] |
| **** | The orbitofrontal cortex includes the Brodmann areas 10, 11 and 47 [84] |
| ***** | The inferior frontal gyrus includes the Brodmann areas 44, 45 and 47 [85] |
| † | In comparing the brains of CM patients with EM patients, Valfrè et al reported that CM patients had significant grey matter reductions in these areas. |
| †† | The lateral orbital gyrus includes the Brodmann area 47 [86] Mackey, Sott; Petrides, Michael (2006). "Chapter 2: The orbitofrontal cortex: sulcal and gyral morphology and architecture". In Zald, David H.; Rauch, Scott (eds.). The Orbitofrontal Cortex. New York: Oxford University Press. p. 34 |
| ††† | Chronic migraine patients had smaller frontal regions than episodic migraine patients. |
| †††† | This study on young women with Polycystic Ovary Syndrome reported a direct association between mild insulin resistance and brain glucose hypometabolism, which was independent of overweight or obesity. |
Neuroimaging studies on migraine also revealed structural and functional changes in certain brain regions of sufferers - therefore, in our opinion, these could be named, “migraine-vulnerable brain regions”, also suggesting an association between attack frequency and the degree of abnormalities [52].
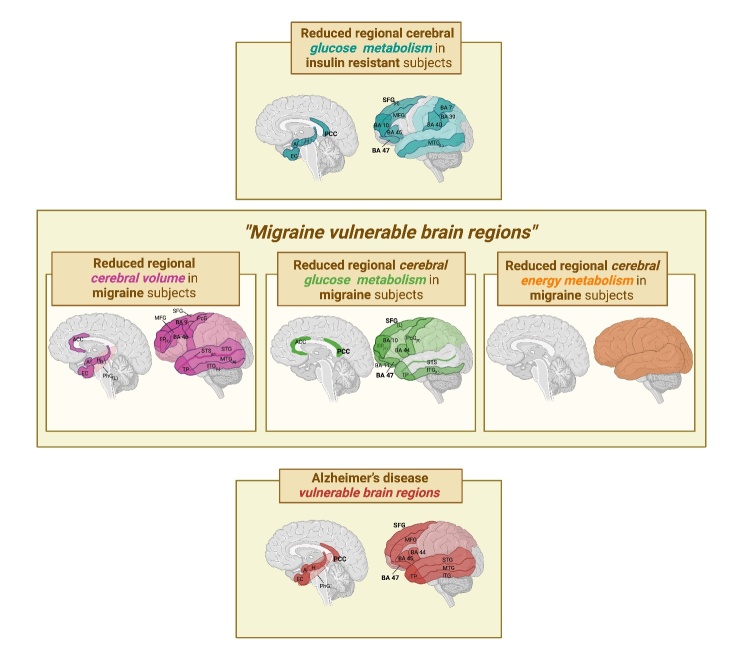
Interestingly, several “migraine-vulnerable brain regions” match the AD-vulnerable brain regions (Fig. 3, Table 2). Noteworthy is the fact that the superior frontal gyrus, the posterior cingulate cortex and the Brodmann area 47, part of the prefrontal cortex [85], are affected by glucose hypometabolism in insulin resistance, migraine and AD subjects (Fig. 3, Table 2), suggesting a shared metabolic alteration between the three conditions. Moreover, CM and AD subjects share several brain areas affected by volume loss; they include the medial temporal lobe (parahippocampal gyrus, hippocampus, amygdala, entorhinal cortex), the superior and middle frontal gyrus, the inferior frontal cortex (i.e., the Brodmann areas 44, 45, 47 [85]), the parietal cortex, the temporal gyrus and the temporal pole (Fig. 3, Table 2). Indeed, the energy deficit, most likely promoted by insulin resistance, may lead to neuronal dysfunction and, over time, neurodegeneration and lobe atrophy [88].
Figure 3.
Graphic representation of brain areas affected by reduced glucose metabolism, volume and energy metabolism in insulin resistance, migraine (i.e., “migraine-vulnerable brain regions”) and Alzheimer’s disease (i.e., AD-vulnerable brain regions). Insulin resistance, migraine and AD subjects share three brain areas (bold) affected by reduced glucose metabolism (i.e., the superior frontal gyrus, the posterior cingulate cortex and the Brodmann area 47). Chronic migraine and AD subjects share several brain areas affected by volume loss; they include the medial temporal lobe (parahippocampal gyrus, hippocampus, amygdala, entorhinal cortex), the superior and middle frontal gyrus, the inferior frontal cortex (i.e., the Brodmann areas 44, 45, 47), the parietal cortex, the temporal gyrus and the temporal pole. (L), left; (R), right; A, amygdala; H, hippocampus; PCC, posterior cingulate cortex; BA, Brodmann area; SFG, superior frontal gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; ACC, anterior cingulate cortex; EC, enthorinal cortex; PhG, parahippocampal gyrus; FP, frontal pole; PcG, precentral gyrus; SFG, superior frontal gyrus; TP, temporal pole; STG, superior temporal gyrus; ITG, inferior temporal gyrus; STS, superior temporal sulcus.
Indeed, our “Neuroenergetic Hypothesis” proposes that brain insulin resistance, glucose hypometabolism, and energy deficit are related pivotal factors that contribute to the neuronal stress involved in migraine attack chronification, and, subsequently, if they persist over time, in the prodromal stage of AD, at least in some subgroups of patients, along a pathophysiological continuum (Fig. 2).
This is supported by other evidence:
-
a.
The glucose transporter type 4 (GLUT4), which is insulin-sensitive [15], is expressed by neurons and astrocytes in some brain regions which are particularly responsive to insulin and related to memory, learning, emotional and cognitive functions; these include the hippocampus, amygdala and a vast area of the cerebral cortex (i.e., posterior cingulate cortex, temporo-parietal cortex, frontal and prefrontal cortex) [37, 89-92]. This suggests that the insulin signalling pathway may play a key role in glucose utilization in these areas [90]. Noteworthy is the fact that all these areas are affected in insulin resistance, migraine and AD (Fig. 3, Table 2).
-
b.
A decreased GLUT4 expression has been reported in the membrane fraction of the frontal cortex in rats affected by depression and obesity [92], diseases known to have insulin resistance among their comorbidities [93].
-
c.
Activation of GLUT4 by insulin is thought to improve glucose flux into neurons during periods of high metabolic demand, such as during learning or other cognitive tasks [94-96]. If this increased glucose demand is not satisfied, in CM sufferers partly due to brain insulin resistance, and if the brain is unable to effectively utilize ketone bodies [97], as should occur during fasting or carbohydrate restriction, then this would lead to an energy deficit, which would, in turn, trigger a migraine attack [15]. Arnold et colleagues also observed that changes in insulin levels might affect neuronal glucose uptake and metabolism via GLUT4 translocation in response to insulin-IRS1-AKT signalling in the brain regions crucial for cognitive and emotional function [37].
-
d.
A reduced cerebral glucose metabolism and lower ATP concentration in AD-vulnerable regions are associated with the severity of peripheral insulin resistance and cognitive impairment [43]. This finding also supports our hypothesis that prolonged peripheral insulin resistance in subjects with chronic migraine leads to a reduced regional cerebral glucose metabolism, which may eventually favour neurodegeneration and the development of AD (Fig. 1).
Moreover, the data from a study carried out on young normal weight women with mild insulin resistance (suffering from polycystic ovary syndrome (PCOS)) strengthens the hypothesis that insulin resistance is, in itself, a primary cause of cerebral glucose hypometabolism. Indeed, a direct association was reported between mild insulin resistance and brain glucose hypometabolism, whatever the degree of overweight or obesity [55]. The same authors observed that women with PCOS had a pattern of reduced regional cerebral glucose metabolism, similar to that observed in the early stages of AD [55]. It was reported that brain regions with low cerebral metabolic glucose rates, e.g., the frontal and parietal cortex show volume reduction [55].
Alterations in brain mitochondrial bioenergetics
The mitochondrial oxidative phosphorylation system produces most cell energy [98]. Alterations in cellular energy metabolites concentration, such as phosphocreatine (PCr), phosphate (Pi), adenosine diphosphate (ADP), adenosine triphosphate (ATP) and cytosolic free magnesium (Mg2+) suggest abnormal mitochondrial function [59, 70, 80, 99]. Indeed, a reduced PCr/Pi and PCr/ATP ratio indicates an energy deficit. Phosphorus (31P)-magnetic resonance spectroscopy (MRS) can provide information on these metabolites from specific brain regions of interest [83].
As early as 1989 [57, 59, 100, 101], 31P-MRS studies were performed in a variety of migraine subtypes, during either the ictal or the interictal period, mostly focused on the occipital lobe as it was considered the region of interest [82, 83]. These studies [57, 59, 100, 101] were carried out to assess oxidative phosphorylation, i.e., the process responsible for generating 90% of the brain's energy and demonstrated that cerebral cortical energy metabolism is abnormal in migraine. Indeed, the lowest energy metabolites concentrations, compared to controls, were detected in the brain of migraineurs, mostly those with aura, and were associated with reduced glucose metabolism in certain areas such as in the parietal lobe, temporal, occipital and frontal lobe, especially in subjects with CM (studies collected in Table 2).
There is further evidence that altered mitochondrial bioenergetics play a role in migraine pathophysiology [97]. Indeed, neuroimaging studies have shown that ATP and “mitochondrial phosphorylation potential” are decreased in the brain of migraineurs without aura interictally, compared to controls [82]. Moreover, the lowest ATP concentrations were detected in the patients who were most severely affected by migraine [97].
Noteworthy is the fact that some experimental studies on energy metabolism reported that AD and migraine share common brain areas which suffer from reduced glucose metabolism, that also show impaired energy metabolism [43, 70] (Table 2).
In AD, these areas are the temporal, parietal, frontal cortex, and the hippocampus [43, 70, 102]. In migraine, they are temporal, parietal, frontal and occipital lobes (Table 2).
Whilst no genetic association has been reported between mitochondrial DNA in AD and migraine [103]. A large population-based cohort study in Norway reported that mitochondrial genetic variation did not play a major role in migraine pathophysiology [104]. Therefore, based on current evidence, this would be an acquired metabolic disorder of the brain which may be related to insulin resistance.
Growing evidence, mainly from in vivo experiments, reported that an altered mitochondrial bioenergetics in the brain occurred in association with brain insulin resistance, with an overproduction of mitochondrial reactive oxygen species, along with mitochondrial depolarization and swelling, and that these two events could lead to the development of cognitive decline and AD [105] (Fig. 2), so that AD is now emerging as type III diabetes mellitus [18].
Neuroinflammation
Neuroinflammation is considered to be an adaptive response triggered by noxious agents, such as infection, injury and/or tissue stress. It plays a significant role in the pathophysiology of various central nervous system diseases, including migraine and AD [106-110]. There is evidence that inflammation may play a pathophysiological role both before and after the neuronal stress involved in a migraine attack [111, 112].
Firstly, inflammation hinders insulin action. The presence of pro-inflammatory cytokines - tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) - leads to a decrease in GLUT4 concentration [113-116]. Moreover, it was reported that nuclear factor-κB (NF-κB) regulates neuroinflammation by increasing oxidative damage and insulin resistance, in experimental diabetic neuropathy [117].
This evidence is in agreement with our “Neuroenergetic hypothesis” [15], which proposes that inflammation may well play a pivotal role in migraine pathophysiology by downregulating GLUT4, increasing brain insulin resistance and, in turn, leading to a reduction in cerebral glucose metabolism and inducing neuronal stress
Indeed, neuronal stress [118] and neurodegeneration, driven by protein misfolding [119], may lead to a neuroinflammatory response in a vicious circle. As early as 2013, Karatas et al. [118] reported, in a migraine animal model, a previously unknown signaling pathway between “stressed neurons” (according to our hypothesis, neurons affected by energy deficiency) and trigeminal afferents during cortical spreading depression (CSD), the presumed cause of migraine aura and headache. CSD is able to trigger NF-κB activation in astrocytes which may link neuronal stress to inflammatory response. Suppression of this cascade by Pannexin-1 channel inhibitor abolishes CSD-induced trigeminovascular activation, dural mast cell degranulation and headache [118].
Therefore, the inflammatory response may occur in parallel with a migraine attack and could play an important role in migraine chronification through trigeminal sensitization, most likely triggered by the release of inflammatory cytokines [111].
Clinical evidence also supports that inflammation plays a role in migraine, as reported by Hagen et al., who carried out a population-based follow-up study on the correlation between high-sensitivity C-reactive protein (hs-CRP) at baseline and the risk of developing migraine 11 years later. They reported that the group with the highest hs-CRP levels had nearly a three-fold higher risk of chronic migraine [120]. In another large-scale population-based study, elevated hs-CRP was associated with headache ≥ 7 days/month, which was particularly evident for those who had migraine with aura [121]. The results of further studies support that migraineurs have higher CRP levels than controls [122, 123].
A recent review on 47 studies analysed cytokines via different mediums and reported persistent alteration in inflammatory regulation in the interictal period in migraine [124].
Interictally, migraine patients have higher interleukin (IL)-1β, IL-6, TNF-α, IL-8, IL-12p70 and CCL3 [125-128] and lower IL-10 levels [125, 128]. Although there are contrasting results, most studies reported a rise in IL-1β, IL-6, and TNF-α [129-132] in the ictal phase.
However, the role of cytokines in migraine is still a question of debate, due to a lack of standardization [124]. Infact, as circadian rhythm influences proinflammatory cytokines levels [133], it makes their determination more challenging.
Sarchielli et al. carried out a study on migraine patients during attacks through serial analysis of internal jugular venous blood samples. They observed a transitory rise in sICAM-1, TNF-α, IL-6 and IL-8 levels and NF-κB activity, along with a transient drop in IκBα expression [134-136], in the first two hours after catheter insertion. The rise in IL-8 lasted until the 4th hour; there was an up-regulation of iNOS from the 4th through the 6th hour which decreased at the end of the attack [134, 136].
Similarly to that observed in migraine, there is a strong link between insulin resistance and neuroinflammation in the pathophysiology of AD [109, 137, 138]. Notably, there is a growing body of research that identifies oxidative stress and neuroinflammation as an early event in the pathogenesis of mild cognitive impairment (MCI) and AD [80].
Chronic low-grade inflammation is associated with poor cognitive performance in the elderly [139]. According to data from recent longitudinal studies, elevated IL-6 levels significantly increase the risk of cognitive decline [140]. Furthermore, a recent meta-analysis of 13 studies reported that high CRP is associated with a higher risk of progression from normal cognition to dementia [141]. Other authors have shown that patients with MCI and AD have higher levels of IL-1β, IL-6 and TNF-α compared to controls [140]. Indeed, some pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12, which are produced peripherally, are able to cross the blood-brain barrier [142, 143]. Their subsequent activation, through the receptor binding, could hinder the insulin effects and promote the disease progression of AD [143].
An important factor in the development of insulin resistance is TNF-α [144]. TNF-α impairs insulin signalling by phosphorylating insulin receptor substrate-1 (IRS-1) and Protein Phosphatase-1 serine [145], therefore acting as an inhibitor of the insulin receptor and phosphatidylinositol-3 kinase signaling [146]. TNF-α also reduces the expression of GLUT4 [116].
Monozygotic twin studies found that 60% of the variation in the production capacity of TNF-α is related to genetic variability [147]. Individuals with the TNF-308G>A polymorphism were classified as high producers [148]. A meta-analysis of 6,682 migraineurs reported that TNF-308G>A polymorphism may be a genetic susceptibility factor for migraine among non-Caucasians [149]. The same polymorphism may be a significant risk factor for AD in East Asians [150]. Therefore, both migraine and AD seem to share a common polymorphism which predisposes the carriers to higher levels of TNF-α, inflammation and most likely insulin resistance.
S100B is a brain-specific protein, also referred to as the “C-reactive protein of the brain” [151], produced mainly by astrocytes [151]. S100B stimulates the activity of fructose-1,6-bisphosphate aldolase and of phosphoglucomutase and is able to regulate energy metabolism [151]. Its effects seem to be dose-dependent; at low concentrations it is neuroprotective, whilst at high concentrations it’s able to promote inflammatory activity and to induce apoptosis [152, 153].
Data from in vitro experiments reported that the release of S100B by astrocytes can be induced by glucose deprivation [154]. Indeed, recent evidence suggests that S100B is a multifacet pathogenic factor in various neurological disorders, sharing common pathogenic processes that can reasonably be attributed to neuroinflammation [155].
During a migraine attack, sufferers have elevated S100B serum levels [156-159], a marker of glial damage. Moreover, S100B was reported to be significantly elevated during the interictal period [157, 159]. Interestingly, high S100B levels induce glycogen synthase kinase 3beta - dependent hyperphosphorylation of the tau protein which is a hallmark of AD [160]. In mouse models, S100B overexpression exacerbates amyloidosis, accelerating disease progression [161].
Post-mortem studies of AD brains demonstrated a correlation between S100B astrocytic expression and dystrophic neurites in amyloid plaques [162]. S100B was also increased in the temporal lobe, where there is a concentration of neurite plaques in AD patients [163].
Moreover, S100B levels in cerebrospinal fluid, together with other AD biomarkers, such as Aβ and phosphorylated tau, have recently been shown to have an inverse correlation with gray matter volumes and glucose metabolism in key AD-related regions [164, 165]. Interestingly, gene polymorphisms upregulating S100B expression were shown to be associated with an increase in AD risk [165].
However, the precise role neuroinflammation and mitochondrial dysfunction play as contributing factors, in the complex interplay with brain insulin resistance, in both migraine chronification and AD pathologic cascade and clinical progression, is still a question of debate.
Alterations at neuroimaging and neurophysiological studies: similarities between chronic migraine and Alzheimer’s disease
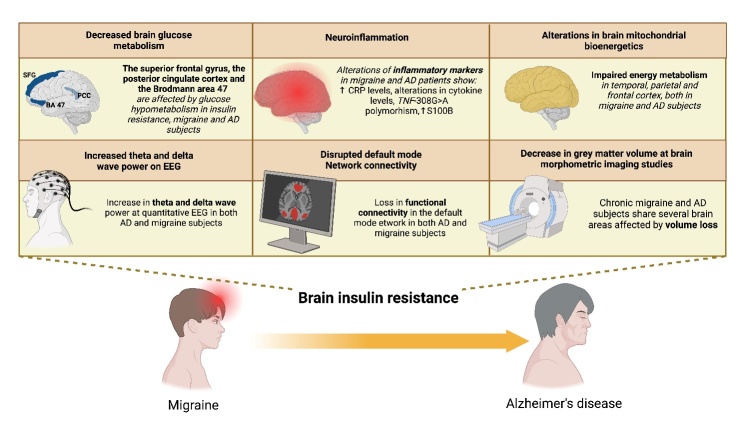
Interesting similarities have been described by neuroimaging and neurophysiological studies between CM and AD (Table 2), which are reported to be related to the aforementioned common pathophysiological alterations of glucose homeostasis and energy supply to neurons, shared by these two pathological conditions (Fig. 4). Although we are aware that how these changes can be interpreted clinically and implemented into disease management has not yet been defined.
Figure 4.
The intriguing similarities between the pathophysiology of chronic migraine and Alzheimer’s disease (AD). Impaired brain glucose metabolism, an alteration in brain mitochondrial bioenergetics and neuroinflammation are common pathophysiological alterations shared by these two pathological conditions that may underlie the reduction in grey matter volume in specific areas, the disrupted default mode network connectivity observed at neuroimaging and the increased theta and delta activity evidenced on EEG in both diseases. Brain insulin resistance may be the pivotal factor linking chronic migraine with AD. PCC, posterior cingulate cortex; SFG, superior frontal gyrus; BA, Brodmann area; AD, Alzheimer’s disease; EEG, electroencephalogram; CRP, C-reactive protein.
Decrease in grey matter volume described by brain morphometric imaging studies
In a previous review [15], it was evidenced that insulin resistance is a common factor in several diseases (i.e., CM, major depression, chronic back pain, PCOS, fibromyalgia, osteoarthritis, obesity and T2DM), which are characterized by a reduction in grey matter volume in specific areas: most of these regions (i.e., the frontal/temporoparietal cortex, prefrontal cortex and hippocampus) are affected by volume loss and atrophy also in AD (Table 2), according to magnetic resonance imaging in mice and humans [61, 64, 87].
A correlation analysis revealed that headache frequency was negatively correlated with the volume of the right frontal pole, right lateral orbital gyrus, and medial frontal lobes [76]. Another study [51] reported that there was a focal grey matter decrease in the bilateral anterior cingulate cortex, left amygdala, left parietal operculum, left middle frontal gyrus and inferior frontal gyrus, and bilateral insula in migraineurs compared to controls (Table 2). There was also a statistically significant correlation between a grey matter reduction in the anterior cingulate cortex and the frequency of migraine attacks, in line with the concept that migraine may be a progressive disorder [51].
Schwedt et al. observed that reductions in the regional volume, cortical surface area, and cortical thickness of specific brain regions in the frontotemporal area may distinguish patients with CM from both healthy individuals and patients with episodic migraine with an accuracy of 86% and 84% respectively. Nevertheless, the precision in distinguishing episodic migraine patients from controls was at 67%. This also suggests the existence of a progressive brain structural and metabolic alteration [66].
As previously reported [15], the grey matter volume reduction impacts brain regions that are specifically involved in higher cognitive and affective activities, such as memory, regulation of affective states, emotion, awareness of bodily states and cognitive processing [76, 166-170]. These brain regions are characterized by the expression of insulin-sensitive glucose transporters [37, 171] that optimize the glucose influx into astrocytes and neurons during the aforementioned metabolic high-demand tasks.
As a reduced glucose uptake and glycogen synthesis in astrocytes would impair the neuronal function, it is reasonable to hypothesize that a persistent and progressive brain metabolic alteration (brain insulin resistance and the related disruption of energy supply) would trigger neurodegeneration, altering a signaling cascade and promoting the AD pathologic changes and amyloid-β (Aβ) deposition [44].
Disrupted default mode network connectivity
The default mode network (DMN), the largest network of functionally correlated brain areas, highly active during rest [172], is crucial for higher cognitive processes, such as memory and executive function. Subjects with dementia show a loss in functional connectivity in the DMN, including the AD-vulnerable brain regions: posterior cingulate cortex, prefrontal cortex, lateral temporal cortex, and the hippocampus [43]. Infact, the AD-vulnerable brain regions are the first cortical areas to show decreased glucose metabolism in AD patients [43]. Interestingly, persons with T2DM and obese individuals also show diminished functional connectivity within this network. Implying there is an extensive overlap between brain regions affected by AD, T2DM and obesity, especially in regions belonging to the default mode network [43].
Noteworthy is the fact that alterations in the DMN are also detectable in migraine. Indeed, Trufanov et al. demonstrated that patients with CM could be characterized by specific dysfunctional interactions between the DMN and other networks, in the resting state [173].
Russo et al. suggested that DMN abnormalities could represent a prognostic imaging biomarker capable of identifying the migraine patients who are more inclined to migraine chronification [174]. Coppola et al. observed that CM patients had significantly reduced functional connectivity between the DMN than healthy controls [175].
Less functional connectivity and/or lower frequency fluctuations within regions of the DMN have been reported in migraineurs [176, 177]. Kullmann et al. observed that the disruption of functional connectivity and reduced cerebral glucose metabolism in the DMN regions, which are coincident with the AD-vulnerable brain regions, is related to the severity of peripheral insulin resistance and cognitive impairment [43]. This seems to provide further evidence in support of our hypothesis that insulin resistance plays a major role in the pathophysiology of CM and AD (Fig. 2).
Increased theta and delta wave power on EEG
The electrical activity in the brain represents the metabolic state of the neurons and can be investigated and measured by electroencephalography (EEG). An association between hypoglycaemia and changes in the EEG was demonstrated [178], as the EEG is highly sensitive to hypoglycaemic states [179, 180]. There is evidence that hypoglycaemia caused by insulin administration is accompanied by an increase in delta and theta activity [171, 180, 181].
An increase in theta and delta activity was also observed in the topographic EEG mapping of dementia subjects and those with insulin-dependent diabetes mellitus, even when their blood glucose levels were not very low (50-60 mg/dl) [180]. Several older quantitative EEG (qEEG) studies reported increased delta [182, 183] or theta power [182-185] in migraineurs. On the whole, migraineurs had increased relative theta power in all cortical regions and increased delta activity in the painful fronto-central region than controls [186]. Moreover, headache intensity correlated positively with EEG global delta power [186].
Noteworthy is the fact that interictal relative theta power at topographic EEG mapping was higher in migraineurs in the frontocentral regions [186], parieto-occipital regions [184] and temporal regions [183]. Several AD studies have demonstrated that qEEG measurements are able to identify a dysfunction in neuronal (synaptic) activity, in its topographical distribution and synchronization. Infact, generalized EEG slowing, reduced global synchronization and anteriorization of neuronal generators of fast-frequency resting-state EEG activity have been reported in patients along the AD continuum [187]. There is also a strong correlation with qEEG measurements and surrogate markers of AD neuropathology [187].This is supported by a study carried out on amyloid-positive AD patients, where the increase in relative theta power and decrease in relative beta power was reported to be indirect measures of (Aβ-mediated) hyperactivity of pyramidal cells and/or interneuron dysfunction along a pathological continuum [188].
Another study on the baseline EEGs of 18 AD patients used quantitative spectral analysis to investigate the relationship between EEG abnormalities and medial temporal lobe atrophy. There was a statistically significant increase in the power of theta waves in the centro-temporal area in the severe atrophy group. These results suggest that qEEG abnormalities are correlated with the medial temporal lobe atrophy [189]. On the basis of these findings, it is reasonable to hypothesize that a reduced cerebral glucose metabolism is one of the main factors underlying the increase in theta and delta wave power at qEEG in both AD and migraine.
The brain energy deficit as a driving factor of alzheimer's disease amyloidogenic cascade and protein misfolding
Nowadays, AD is considered to be one of the “protein misfolding diseases”, characterized by protein accumulation, mainly extracellular plaques and intracellular neurofibrillary tangles, which contain the pathological hallmarks of AD, i.e., abnormal Aβ and hyperphosphorylated tau, respectively [190]. As is widely known, aging is the main risk factor for AD. Aging may be linked to AD, at least to some extent, by a perturbed energy metabolism driven by conditions strongly associated with it: insulin resistance, loss of mitochondrial function and a low-grade systemic inflammation [191].
Considering the high energy requirements of basic metabolic processes in neurons, such as protein biosynthesis and folding, it is reasonable that an altered brain glucose metabolism, exacerbated by the time-dependent functional decline, may impair, at least in certain subsets of patients, the proper protein folding and synaptic integrity, leading to neurodegeneration [192] (Figure 2). Indeed, growing evidence supports the hypothesis that insulin resistance and reduced brain glucose metabolism may promote a “shift” towards the amyloidogenic pathway and tau phosphorylation, in the complex interplay of AD pathophysiology [14, 193, 194].
Noteworthy is the fact that there is a close epidemiological relationship between AD and T2DM (81% of cases of AD sufferers had either T2DM or impaired fasting glucose [195]) and that there are striking similarities in the protein misfolding and insulin signalling in both of these diseases, which could be due to the key role that insulin resistance plays in both AD and T2DM [18, 45].
Actually, it has been postulated that insulin resistance is the missing link between brain neuronal loss and pancreatic β-cell loss in both diseases, to the extent that some authors, as aforementioned, have called AD “diabetes of the brain” or “type 3 diabetes” [18]. Notably, the hypothesis of an insulin-mediated AD pathology was first conceived by Steen et al. and subsequently developed by other authors [18, 37, 39, 45]. There is recent evidence that insulin has a role in proteostasis, influencing Aβ clearance and tau phosphorylation [14, 18, 44, 193], and that it plays a remarkable, putative role in the development of AD pathological markers [18, 44, 45].
Glycogen synthase kinase-3 remains unphosphorylated and activated in the presence of a reduced insulin stimulation, leading to an impaired glucose metabolism and tau hyperphosphorylation in various metabolic disorders, including AD [196]. In fact, overactivation of glycogen synthase kinase-3 is a common finding in the brains of neurodegenerative patients [196]. Furthermore, β-N-acetylglucosamine (GlcNAc)-mediated O-GlcNAcylation has been demonstrated to regulate tau phosphorylation [197]. Interestingly, Liu et al. observed that impaired glucose metabolism downregulates O-GlcNAcylation, consequently leading to tau hyperphosphorylation in an animal model [197]. Other authors demonstrated not only that metabolic stress induces the phosphorylation of endogenous tau but also, remarkably, that tau phosphorylation is reversible upon restoration of the metabolic homeostasis in cell models as well as in a physiological hypometabolic model in vivo [192, 198].
As is widely known, if neuronal glucose metabolism is impaired, as in the case of brain insulin resistance, the oxidative phosphorylation in neurons will be reduced, leading to a decreased ATP production [15]. Interestingly, early research indicated that the inhibition of oxidative phosphorylation causes Aβ precursor protein (βAPP) to transform itself into Aβ, as βAPP can only be partially inserted into synaptic membranes [199]. A series of similar studies strengthened the hypothesis that an abnormal accumulation of Aβ is triggered by oxidative energy metabolism disturbances, which may “switch” the βAPP metabolism towards the amyloidogenic cascade [44, 45, 200-203].
Moreover, acetylcholine deficiency, long recognized as an early functional abnormality in AD, has also been linked to insulin resistance [44, 204]. In fact, acetylcholine transferase, involved in acetylcholine synthesis, is expressed in insulin and insulin-like growth factor-1 receptor-positive cortical neurons [205] and insulin resistance has been linked to a decreased acetylcholine transferase expression and consequently reduced acetylcholine levels in AD [206]. Notably, Aβ oligomers bind to hippocampal neurons and displace insulin receptors from the plasma membrane interrupting normal insulin signaling [207, 208]. Other studies elucidated the detrimental effect of Aβ on mitochondrial metabolism [209] and its potential to induce neuroinflammation [119], putatively triggering a vicious circle, where the metabolic disruption and neurodegeneration enhance each other. Glucose metabolism is also necessary for autophagy, which is responsible for the clearance of folded proteins in the cell so its dysfunction may lead to Aβ aggregation and tauopathy [210].
In summary, a growing body of research clearly indicates that brain regional hypometabolism, which occurs in certain AD brain regions (Figure 3, Table 2) and can be caused by insulin resistance, may hamper a proper proteostasis and facilitate neurodegeneration [192, 198]. Indeed, in agreement with our “Neuroenergetic hypothesis”, aberrant insulin signaling, and energy deficit may well be predisposing metabolic conditions for both the main pathological changes in AD, the Aβ deposition and tau hyperphosphorylation [18, 44, 45, 205] (Figure 2).
Summary
In summary, considering that:
-
–
Recent meta-analyses demonstrated that migraine is associated with increased risk of all-cause dementia, but in particular that of AD [21-23, 26].
-
–
Altered insulin signalling and glucose homeostasis are frequently observed in both migraine and AD [15, 37, 195].
-
–
Brain insulin resistance is a pathophysiological mechanism widely described in T2DM and AD [18, 37, 39], and has been hypothesized in CM [15].
-
–
An increasing body of evidence supports the hypothesis that brain insulin resistance, reduced cerebral glucose metabolism and the consequent energy deficit may promote a “shift” towards the amyloidogenic pathway and tau phosphorylation [14, 193, 194].
-
–
There are intriguing similarities between the pathophysiology of CM and AD: brain insulin resistance, an impaired brain glucose metabolism, alterations in brain mitochondrial bioenergetics, and neuroinflammation. These seem to be common pathophysiological alterations, underlying a grey matter volume reduction in specific brain areas, a disrupted default mode network connectivity on neuroimaging, and an increased theta and delta activity on EEG, which are shared by these two pathological conditions, i.e., CM and AD.
Based on this evidence, we would like to propose an “extended neuroenergetic hypothesis” (illustrated in Fig. 1 and 2) where brain insulin resistance may be a metabolic bridge that links CM to AD along a pathophysiological continuum.
Potential Treatments targeting the mechanisms highlighted by the “Neuroenergetic hypothesis”
Although there are significant gaps in the current research in understanding how the mechanisms highlighted at a molecular level can be effectively targeted by new therapies in the clinical practice, we suggest that the aforementioned metabolic abnormalities involving glucose homeostasis, energy deficit and neuroinflammation in the frame of the “Neuroenergetic hypothesis”, may well become appealing targets for preventive therapeutic approaches to both migraine and, possibly, AD, i.e., diet, aerobic exercise and mind-body interventions.
Diet
An optimal dietary pattern should be able to reduce systemic inflammation [211, 212], exclude high glycemic index foods, be sustainable at long-term and have no adverse effects. The traditional Mediterranean diet is a dietary pattern that meets all four of these requirements [213-215]. In fact, there is evidence supporting [216-218] that diets similar to the traditional Mediterranean one, i.e., the Healthy Eating Plate and the Dietary Approaches to Stop Hypertension (DASH), are efficacious in reducing the frequency and intensity of migraine and its associated disability. Moreover, RCTs, meta-analyses and systematic reviews that, over the past decade, have evaluated the role diet plays in the treatment and prevention of depression [219-221] and dementia [222-227] have suggested that a higher adherence to the Mediterranean diet or similar ones (the DASH, the Healthy Nordic diet and Mediterranean-DASH Intervention for Neurodegenerative Delay) is associated with higher remissions and a lower incidence of depression, slower cognitive decline and a reduction in the risk of developing dementia. Interestingly, a 3-months RCT on the DASH diet in migraine patients reported a decrease in migraine frequency and severity [228]. Accordingly, in another 3-month RCT, a very low-glycemic index diet proved to be as effective as standard pharmacological treatment in migraine prophylaxis [229]. Moreover, a recent 3-year two-arm RCT [230], assessed the effect of a Mediterranean-DASH Intervention for Neurodegenerative Delay and a Mild Caloric Restriction Intervention. Both interventions led to improved overall cognition. Indeed, caloric restriction is a known modulator of insulin signaling in the peripheral tissues and seems to preserve brain energy metabolism during the aging process [231].
Long-term RCTs promoting a Mediterranean diet may be useful to clarify whether improved adherence to such preventive approach may be beneficial in preventing AD or delaying the onset of AD pathological changes and dementia. Some case reports and prospective studies have demonstrated the efficacy of the ketogenic diet for episodic and chronic migraine [232]. This diet mimics, to some extent, the state of fasting and promotes hepatic production of an alternative to glucose as an energy substrate for the brain [97]. This would contribute to the restoration of brain excitability and metabolism and counteracting neuroinflammation in migraine [232].
Moreover, Di Lorenzo et al. investigated the effects of one-month ketogenic diet had on 18 migraneurs. The resulting data demonstrated that not only was there a decrease in the frequency and duration of headache attacks, but also a normalization of some parameters of evoked potentials in response to visual and somatosensory stimuli [233]. Accordingly, Caprio et al., reported on a two month very low-calorie ketogenic diet and demonstrated that it effectively reduced monthly migraine days [234]. Interestingly, it was also proven that the ketogenic diet was able to reduce insulin-resistance in other diseases, like T2DM and PCOS [235, 236].
However, although recent data indicate the possibility of good compliance and an improved quality of life over a span of one year, the safety of a ketogenic diet has not yet been fully assessed in long-term trials [214] and to date, the ketogenic diet does not meet two of the four aforementioned criteria, i.e., the long-term safety and sustainability.
Nevertheless, long interventional trials on the Mediterranean diet such as the Predimed and Cardioprev trial, reported a good 5-year adherence [237, 238]. Moreover, dietary change seemed feasible also in over 70-years olds [239]. However, further studies are needed to support the efficacy and feasibility of a “metabolic” strategy for AD prevention, particularly over the long-term.
Exercise and Mind-Body interventions
Regular exercise and mind-body interventions are supported by a growing body of evidence as being effective prophylaxis interventions for migraine, AD and age-related cognitive decline. Cross-sectional and population-based studies reported that low physical activity is associated with a higher prevalence of migraine [240]. Regular moderate aerobic physical exercise (>40 min, 3 times per week) seems effective in reducing both the severity and frequency of migraine attacks [241, 242]. Meta-analyses in literature evidence that the frequency, intensity and duration of migraine pain are improved by both strength training and high-intensity aerobic exercise [243, 244]. This effect could be attributed to improved glucose tolerance and increased mitochondrial biogenesis [245, 246]. Two other meta-analyses [247, 248] of prospective studies on physical activity reported that it reduces the risk of dementia and AD in a dose-response fashion [247, 248]. The protective effect observed by these authors may be due to an enhanced hippocampus volume in the more elderly adults, which was induced by physical activity and the ability exercise has to counteract age-related brain volume deterioration [249-251]. A recent clinical study reported that a sedentary lifestyle led to obesity and brain insulin resistance, and that exercise could reverse this metabolic abnormality [46]. This highlights brain insulin resistance as a plausible therapeutic target for the prevention of cognitive decline and dementia due to AD.
Interestingly, according to a recent meta-analysis on MCI patients, mind-body interventions exert a stronger effect on cognitive gain than does exercise alone [252]. Furthermore, in older adults with MCI, mind-body interventions were reported to improve cognitive and everyday function, memory, resilience and mindfulness [253].
In 2021, Wells et al. reported that mindfulness-based stress reduction lessened disability and enhanced the quality of life, well-being and self-efficacy in migraine sufferers. Mindfulness-based stress reduction also mitigated pain catastrophizing and depression and, importantly, lead to a reduction in experimentally induced pain, suggesting a fundamental shift in pain perception and processing [254]. These transversal results are in agreement with research which emphasizes how mind-body interventions can downregulate the expression of pro-inflammatory genes (e.g. NF-κB) [255-259]. Meaning that mind-body interventions could be cost-effective and empowering interventions to target multiple diseases that have an inflammatory basis [260].
Meditation is one of the most common and popular mind-body practices [261].
A 2018 meta-analysis of ten RCTs and 315 migraine patients reported that mindfulness meditation lessens pain intensity [262]. Individuals who engage in long-term meditation practices have numerous neurological benefits. It seems that meditators have an increased cortical thickness, reduced age-related white matter connectivity [263] and atrophy, particularly in the hippocampus, frontal, temporal brain regions and the amygdala [264-267]. This has led to speculation that meditators' brains are less affected by the aging process [268]. An interesting study on a small sample of 6 expert elderly meditators, compared to 67 elder controls, reported that meditators had a higher glucose metabolism than did the at-rest controls in aging-sensitive regions, such as the ventromedial prefrontal and anterior cingulate cortex bilaterally, the right insula, temporoparietal junction and posterior cingulate cortex. It was also reported that expert meditators have more preservation of grey matter volume than controls [269]. Another functional neuroimaging study carried out a network-based analysis of anatomical pathways and observed that the meditators had a stronger connectivity than controls between four areas in the left hemisphere pertaining to the somato-motor, dorsal attention, subcortical and visual networks [270].
Overall, these findings imply that exercise and mind-body interventions are potentially valid, cost-effective and user-friendly ways to counteract age-related disruptions in glucose metabolism ??and reduce the risk of both migraine chronification and cognitive impairment. Ultimately, the multicenter randomized-controlled FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) trial demonstrated that a multimodal intervention, encompassing nutrition, physical activity and cognitive training has the potential to be cost-effective in preventing dementia in the long-term [271].
Although the results at an individual level are rather modest, the societal benefits can be substantial, because of the potentially large target population [271]. This model is now being tested globally [272], by the World-Wide FINGERS Network, and the preliminary results seem to support the hypothesis that the above-mentioned preventive interventions might be beneficial in preventing cognitive decline. However, further clinical studies are mandatory to prove the efficacy of these approaches in reducing the risk of AD.
CONCLUSIONS
This review highlights a potential missing link between migraine and AD: brain insulin resistance. This is the “core” of our “neuroenergetic hypothesis” and could be a pivotal pathophysiological feature, shared by CM and AD (Fig. 1 and 2).
We describe intriguing similarities between migraine and AD pathophysiology, i.e., a lower cerebral glucose metabolism, alterations in brain mitochondrial bioenergetics and neuroinflammation, all of which most likely underlie the reduction in gray matter volume in specific areas, a disrupted DMN connectivity and the increased theta and delta waves observed at EEG in both migraine and AD (Figure 4). All of these alterations, shared by migraine and AD, could be related, at least partly, to brain insulin resistance, which is bidirectionally related to mitochondrial functional alterations and neuroinflammation in a complex interplay. This prompts us to advocate that brain insulin resistance could be considered a “metabolic bridge” between CM and AD, along a pathophysiological continuum (Figures 1 and 2). Indeed, in the long run, brain insulin resistance and the related energy deficiency might favour the pathological changes characteristic of AD, promoting a shift towards the amyloidogenic cascade and enhancing tau hyperphosphorylation. ??Although further studies are required to support this novel “Neuroenergetic hypothesis” as a bridge linking migraine to AD, hopefully it may elucidate new targets for innovative preventive treatment of these two leading neurological causes of disability worldwide.
Further research
Further investigations and new approaches are needed for the prophylaxis and treatment of both migraine and AD that, despite extensive efforts, continue to be major causes of disability worldwide. We deem that further research, aimed at completing the puzzle of the “Neuroenergetic hypothesis”, herein presented, should focus on improving our research strategies to investigate brain insulin resistance in the general population. Indeed, this will improve our understanding of how brain insulin resistance relates to chronic migraine and AD.
Furthermore, it would also allow us to clarify whether improving brain insulin resistance could lead to a decrease in headache attacks in chronic migraineurs in a real-world setting. Moreover, it could shed more light on whether improving brain insulin resistance could have any clinically relevant impact on the prevention, or slowing, of AD clinical progression, as has been frequently reported in pre-clinical research [46].
Our hypothesis suggests that treating altered glucose metabolism in episodic migraine sufferers with effective strategies might be a beneficial to prevent the clinical progression to chronic migraine and, maybe, in the long-term, also to reduce the risk of developing AD, although more clinical studies are required to demonstrate this hypothesis and to clarify this still controversial issue [138].
Another welcome line of research, could focus on clarifying the exact mechanisms that could effectively therapeutically target brain insulin resistance at a molecular level, aimed at achieving an indirect reduction of the protein misfolding and/or an improvement in clinical outcomes [47]. It would be also helpful if anti-diabetes drugs, investigated in the context of AD treatment, were also assessed for the treatment of chronic migraine [47]. We are of the opinion that, in the future, long-term RCTs should be carried out to evaluate the cost-effectiveness of lifestyle multimodal interventions (i.e. diet, aerobic exercise and mind-body interventions) as a prophylactic strategy for migraine and AD, as well as RCTs to further evaluate the efficacy and safety of anti-diabetic drugs in the prophylaxis and treatment of chronic migraine and AD [47].
Acknowledgments
The authors thank Barbara Wade, Contract Professor at Turin University, for her linguistic advice and her precious help in bibliographic research. We gratefully acknowledge Milena Simeoni, co-founder of LUMEN International Foundation, this article would not have been possible without her input. Figures 2, 3 and 4 were created with BioRender.com. I wish to dedicate this work to my father, Aldo Del Moro, a loving father who recently passed over.
Funding Statement
The authors thank Barbara Wade, Contract Professor at Turin University, for her linguistic advice and her precious help in bibliographic research. We gratefully acknowledge Milena Simeoni, co-founder of LUMEN International Foundation, this article would not have been possible without her input. Figures 2, 3 and 4 were created with BioRender.com. I wish to dedicate this work to my father, Aldo Del Moro, a loving father who recently passed over.
Contributions
LDM and EP conceived the presented idea and wrote the draft of the manuscript. LDM, ER and EP researched evidence and wrote the manuscript. ER made a substantial contribution to the revision of the text for the content and edited the manuscript before submission. LDM and EP designed the figures. All the authors contributed to the final manuscript and approved it.
Competing interests
The authors declare that they have no competing interests.
References
- [1].GBD 2015. Disease and Injury Incidence and Prevalence Collaborators (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388:1545-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z (2018). Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain, 19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, 390:1211-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scher AI, Stewart WF, Ricci JA, Lipton RB (2003). Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain, 106:81-89. [DOI] [PubMed] [Google Scholar]
- [5].Wolters FJ, Chibnik LB, Waziry R, Anderson R, Berr C, Beiser A, et al. (2020). Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology, 95:e519-e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].GBD 2019. Dementia Forecasting Collaborators (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health, 7:e105-e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang X, Chen Y, Song J, You C (2021). Efficacy and Safety of Monoclonal Antibody Against Calcitonin Gene-Related Peptide or Its Receptor for Migraine: A Systematic Review and Network Meta-analysis. Front Pharmacol, 12:649143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bentivegna E, Galastri S, Onan D, Martelletti P (2024). Unmet Needs in the Acute Treatment of Migraine. Adv Ther, 41:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van der Flier WM, de Vugt ME, Smets EMA, Blom M, Teunissen CE (2023). Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia. Nat Aging, 3:494-505. [DOI] [PubMed] [Google Scholar]
- [10].Loeffler DA (2023). Antibody-Mediated Clearance of Brain Amyloid-β: Mechanisms of Action, Effects of Natural and Monoclonal Anti-Aβ Antibodies, and Downstream Effects. J Alzheimers Dis Rep, 7:873-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Buccellato FR, D’Anca M, Tartaglia GM, Del Fabbro M, Scarpini E, Galimberti D (2023). Treatment of Alzheimer’s Disease: Beyond Symptomatic Therapies. Int [J] Mol Sci. doi: 10.3390/ijms241813900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Perneczky R, Jessen F, Grimmer T, Levin J, Flöel A, Peters O, et al. (2023). Anti-amyloid antibody therapies in Alzheimer’s disease. Brain, 146:842-849. [DOI] [PubMed] [Google Scholar]
- [13].Blonz ER (2017). Alzheimer’s Disease as the Product of a Progressive Energy Deficiency Syndrome in the Central Nervous System: The Neuroenergetic Hypothesis. J Alzheimers Dis, 60:1223-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kellar D, Craft S (2020). Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol, 19:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Del Moro L, Rota E, Pirovano E, Rainero I (2022). Migraine, Brain Glucose Metabolism and the “Neuroenergetic” Hypothesis: A Scoping Review. J Pain, 23:1294-1317. [DOI] [PubMed] [Google Scholar]
- [16].Zulfiqar S, Garg P, Nieweg K (2019). Contribution of astrocytes to metabolic dysfunction in the Alzheimer’s disease brain. Biol Chem, 400:1113-1127. [DOI] [PubMed] [Google Scholar]
- [17].Garwood CJ, Ratcliffe LE, Morgan SV, Simpson JE, Owens H, Vazquez-Villaseñor I, et al. (2015). Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain, 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Van Giau V (2020). Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int [J] Mol Sci. doi: 10.3390/ijms21093165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Islamoska S, Hansen ÅM, Wang H-X, Garde AH, Andersen PK, Garde E, et al. (2020). Mid- to late-life migraine diagnoses and risk of dementia: a national register-based follow-up study. J Headache Pain, 21:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morton RE, St John PD, Tyas SL (2019). Migraine and the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia: A prospective cohort study in community-dwelling older adults. Int J Geriatr Psychiatry, 34:1667-1676. [DOI] [PubMed] [Google Scholar]
- [21].Cermelli A, Roveta F, Giorgis L, Boschi S, Grassini A, Ferrandes F, et al. (2023). Is headache a risk factor for dementia? A systematic review and meta-analysis. Neurol Sci. doi: 10.1007/s10072-023-07069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Qu H, Yang S, Yao Z, Sun X, Chen H (2022). Association of Headache Disorders and the Risk of Dementia: Meta-Analysis of Cohort Studies. Front Aging Neurosci, 14:804341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang L, Wu J-C, Wang F-Y, Chen X, Wang Y (2022). Meta-analysis of association between migraine and risk of dementia. Acta Neurol Scand, 145:87-93. [DOI] [PubMed] [Google Scholar]
- [24].Kim S-J, Park SM, Cho H-J, Park JW (2022). Primary headaches increase the risk of dementias: An 8-year nationwide cohort study. PLoS One, 17:e0273220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hurh K, Jeong SH, Kim SH, Jang S-Y, Park E-C, Jang S-I (2022). Increased risk of all-cause, Alzheimer’s, and vascular dementia in adults with migraine in Korea: a population-based cohort study. J Headache Pain, 23:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gu L, Wang Y, Shu H (2022). Association between migraine and cognitive impairment. J Headache Pain, 23:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee H-J, Yu H, Gil Myeong S, Park K, Kim D-K (2021). Mid- and Late-Life Migraine Is Associated with an Increased Risk of All-Cause Dementia and Alzheimer’s Disease, but Not Vascular Dementia: A Nationwide Retrospective Cohort Study. [J] Pers Med. doi: 10.3390/jpm11100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kostev K, Bohlken J, Jacob L (2019). Association Between Migraine Headaches and Dementia in More than 7,400 Patients Followed in General Practices in the United Kingdom. J Alzheimers Dis, 71:353-360. [DOI] [PubMed] [Google Scholar]
- [29].Lee S-Y, Lim J-S, Oh DJ, Kong IG, Choi HG (2019). Increased risk of neurodegenerative dementia in women with migraines: A nested case-control study using a national sample cohort. Medicine, 98:e14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hagen K, Stordal E, Linde M, Steiner TJ, Zwart J-A, Stovner LJ (2014). Headache as a risk factor for dementia: a prospective population-based study. Cephalalgia, 34:327-335. [DOI] [PubMed] [Google Scholar]
- [31].Chuang C-S, Lin C-L, Lin M-C, Sung F-C, Kao C-H (2013). Migraine and risk of dementia: a nationwide retrospective cohort study. Neuroepidemiology, 41:139-145. [DOI] [PubMed] [Google Scholar]
- [32].Guidetti D, Rota E, Morelli N, Immovilli P (2014). Migraine and stroke: “vascular” comorbidity. Front Neurol, 5:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Altamura C, Corbelli I, de Tommaso M, Di Lorenzo C, Di Lorenzo G, Di Renzo A, et al. (2021). Pathophysiological Bases of Comorbidity in Migraine. Front Hum Neurosci, 15:640574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bagdade JD (1968). Bagdade JD: Basal insulin and obesity. Lancet, 2:630-631. [DOI] [PubMed] [Google Scholar]
- [35].Freeman AM, Acevedo LA, Pennings N Insulin Resistance. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- [36].Mielke JG, Taghibiglou C, Liu L, Zhang Y, Jia Z, Adeli K, et al. (2005). A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem, 93:1568-1578. [DOI] [PubMed] [Google Scholar]
- [37].Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang H-Y, Ahima RS, et al. (2018). Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol, 14:168-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Salkovic-Petrisic M, Hoyer S (2007). Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J Neural Transm Suppl, 217-233. [DOI] [PubMed] [Google Scholar]
- [39].Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis, 7:63-80. [DOI] [PubMed] [Google Scholar]
- [40].Rhea EM, Banks WA, Raber J (2022). Insulin Resistance in Peripheral Tissues and the Brain: A Tale of Two Sites. Biomedicines. doi: 10.3390/biomedicines10071582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kellar D, Craft S (2020). Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol, 19:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koepsell H (2020). Glucose transporters in brain in health and disease. Pflugers Arch, 472:1299-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring H-U (2016). Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev, 96:1169-1209. [DOI] [PubMed] [Google Scholar]
- [44].Ahmed S, Mahmood Z, Zahid S (2015). Linking insulin with Alzheimer’s disease: emergence as type III diabetes. Neurol Sci, 36:1763-1769. [DOI] [PubMed] [Google Scholar]
- [45].Yoon JH, Hwang J, Son SU, Choi J, You S-W, Park H, et al. (2023). How Can Insulin Resistance Cause Alzheimer’s Disease? Int J Mol Sci. doi: 10.3390/ijms24043506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rhea EM, Leclerc M, Yassine HN, Capuano AW, Tong H, Petyuk VA, et al. (2023). State of the Science on Brain Insulin Resistance and Cognitive Decline Due to Alzheimer’s Disease. Aging Dis. doi: 10.14336/AD.2023.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Adem MA, Decourt B, Sabbagh MN (2024). Pharmacological Approaches Using Diabetic Drugs Repurposed for Alzheimer’s Disease. Biomedicines. doi: 10.3390/biomedicines12010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kim JH, Kim S, Suh S-I, Koh S-B, Park K-W, Oh K (2010). Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia, 30:53-61. [DOI] [PubMed] [Google Scholar]
- [49].Mathew NT (2011). Pathophysiology of chronic migraine and mode of action of preventive medications. Headache, 51 Suppl 2:84-92. [DOI] [PubMed] [Google Scholar]
- [50].Torres-Ferrus M, Pareto D, Gallardo VJ, Cuberas-Borrós G, Alpuente A, Caronna E, et al. (2021). Cortical metabolic and structural differences in patients with chronic migraine. An exploratory 18FDG-PET and MRI study. J Headache Pain, 22:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Valfrè W, Rainero I, Bergui M, Pinessi L (2008). Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache, 48:109-117. [DOI] [PubMed] [Google Scholar]
- [52].Ashina S, Bentivegna E, Martelletti P, Eikermann-Haerter K (2021). Structural and Functional Brain Changes in Migraine. Pain Ther, 10:211-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lai K-L, Niddam DM, Fuh J-L, Chen W-T, Wu J-C, Wang S-J (2020). Cortical morphological changes in chronic migraine in a Taiwanese cohort: Surface- and voxel-based analyses. Cephalalgia, 40:575-585. [DOI] [PubMed] [Google Scholar]
- [54].Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, et al. (2015). Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol, 72:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Castellano C-A, Baillargeon J-P, Nugent S, Tremblay S, Fortier M, Imbeault H, et al. (2015). Regional Brain Glucose Hypometabolism in Young Women with Polycystic Ovary Syndrome: Possible Link to Mild Insulin Resistance. PLoS One, 10:e0144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S (2011). Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol, 68:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Welch KM, Levine SR, D’Andrea G, Schultz LR, Helpern JA (1989). Preliminary observations on brain energy metabolism in migraine studied by in vivo phosphorus 31 NMR spectroscopy. Neurology, 39:538-541. [DOI] [PubMed] [Google Scholar]
- [58].Ramadan NM, Halvorson H, Vande-Linde A, Levine SR, Helpern JA, Welch KM (1989). Low brain magnesium in migraine. Headache, 29:590-593. [DOI] [PubMed] [Google Scholar]
- [59].Schulz UG, Blamire AM, Davies P, Styles P, Rothwell PM (2009). Normal cortical energy metabolism in migrainous stroke: A 31P-MR spectroscopy study. Stroke, 40:3740-3744. [DOI] [PubMed] [Google Scholar]
- [60].Du Y-H, Yang R-Y, Wang Q, Wang L-Y, Liang L-C, Zhu L, et al. (2021). Bibliometric Analysis Study on the Mechanisms of Brain Energy Metabolism Disorders in Alzheimer’s Disease From 2000 to 2020. Front Neurol, 12:670220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Aberathne I, Kulasiri D, Samarasinghe S (2023). Detection of Alzheimer’s disease onset using MRI and PET neuroimaging: longitudinal data analysis and machine learning. Neural Regeneration Res, 18:2134-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, et al. (2006). Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging, 27:1604-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mosconi L (2005). Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging, 32:486-510. [DOI] [PubMed] [Google Scholar]
- [64].Sabuncu MR, Desikan RS, Sepulcre J, Yeo BTT, Liu H, Schmansky NJ, et al. (2011). The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch Neurol, 68:1040-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yu Y, Zhao H, Dai L, Su Y, Wang X, Chen C, et al. (2021). Headache frequency associates with brain microstructure changes in patients with migraine without aura. Brain Imaging Behav, 15:60-67. [DOI] [PubMed] [Google Scholar]
- [66].Schwedt TJ, Chong CD, Wu T, Gaw N, Fu Y, Li J (2015). Accurate Classification of Chronic Migraine via Brain Magnetic Resonance Imaging. Headache, 55:762-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jia Z, Yu S (2017). Grey matter alterations in migraine: A systematic review and meta-analysis. Neuroimage Clin, 14:130-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jett S, Dyke JP, Boneu Yepez C, Zarate C, Carlton C, Schelbaum E, et al. (2023). Effects of sex and APOE ε4 genotype on brain mitochondrial high-energy phosphates in midlife individuals at risk for Alzheimer’s disease: A 31Phosphorus MR spectroscopy study. PLoS One, 18:e0281302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Planchuelo-Gómez Á, García-Azorín D, Guerrero ÁL, Rodríguez M, Aja-Fernández S, de Luis-García R (2020). Gray Matter Structural Alterations in Chronic and Episodic Migraine: A Morphometric Magnetic Resonance Imaging Study. Pain Med, 21:2997-3011. [DOI] [PubMed] [Google Scholar]
- [70].Das N, Ren J, Spence J, Chapman SB (2021). Phosphate Brain Energy Metabolism and Cognition in Alzheimer’s Disease: A Spectroscopy Study Using Whole-Brain Volume-Coil 31Phosphorus Magnetic Resonance Spectroscopy at 7Tesla. Front Neurosci, 15:641739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dienel GA (2019). Metabolomic Assays of Postmortem Brain Extracts: Pitfalls in Extrapolation of Concentrations of Glucose and Amino Acids to Metabolic Dysregulation In Vivo in Neurological Diseases. Neurochem Res, 44:2239-2260. [DOI] [PubMed] [Google Scholar]
- [72].Al Qawasmeh M, Ahmed YB, Al-Bzour AN, Al-Majali GN, Alzghoul SM, Al-Khalili AA, et al. (2022). Meta-analytical evidence of functional and structural abnormalities associated with pain processing in migraine patients: An activation likelihood estimation. Medicine, 101:e31206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB (2017). Structural Gray Matter Alterations in Chronic Migraine: Implications for a Progressive Disease? Headache, 57:400-416. [DOI] [PubMed] [Google Scholar]
- [74].Naguib LE, Abdel Azim GS, Abdellatif MA (2021). A volumetric magnetic resonance imaging study in migraine. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery, 57:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Navarro-González R, García-Azorín D, Guerrero-Peral ÁL, Planchuelo-Gómez Á, Aja-Fernández S, de Luis-García R (2023). Increased MRI-based Brain Age in chronic migraine patients. J Headache Pain, 24:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chen X-Y, Chen Z-Y, Dong Z, Liu M-Q, Yu S-Y (2020). Regional volume changes of the brain in migraine chronification. Neural Regeneration Res, 15:1701-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Xu P, Chen A, Li Y, Xing X, Lu H (2019). Medial prefrontal cortex in neurological diseases. Physiol Genomics, 51:432-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].DeTure MA, Dickson DW (2019). The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener, 14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Song T, Song X, Zhu C, Patrick R, Skurla M, Santangelo I, et al. (2021). Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res Rev, 72:101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Song T, Song X, Zhu C, Du F (2021). Magnetic Resonance Spectroscopy in Mild Cognitive Impairment and Alzheimer’s Disease: Systematic Review and Meta-Analysis. Biol Psychiatry, 89:S154. [Google Scholar]
- [81].Pozueta J, Lefort R, Shelanski ML (2013). Synaptic changes in Alzheimer’s disease and its models. Neuroscience, 251:51-65. [DOI] [PubMed] [Google Scholar]
- [82].Reyngoudt H, Paemeleire K, Descamps B, De Deene Y, Achten E (2011). 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia, 31:1243-1253. [DOI] [PubMed] [Google Scholar]
- [83].Nikolova S, Schwedt TJ (2022). Magnetic resonance spectroscopy studies in migraine. Neurobiol Pain, 12:100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kringelbach ML (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci, 6:691-702. [DOI] [PubMed] [Google Scholar]
- [85].Guenther FH, Tourville JA, Bohland JW (2015). Speech Production. In: Toga AW, editor Brain Mapping. Waltham: Academic Press, 435-444. [Google Scholar]
- [86].Petrides M, Mackey S (2006). The orbitofrontal cortex: sulcal and gyral morphology and architecture. [Google Scholar]
- [87].Zhou Y-N, Jiang L, Zhang Y, Zhou C-N, Yang H, He Q, et al. (2023). Anti-LINGO-1 antibody protects neurons and synapses in the medial prefrontal cortex of APP/PS1 transgenic mice. Neurosci Res, 193:28-40. [DOI] [PubMed] [Google Scholar]
- [88].Sas K, Párdutz A, Toldi J, Vécsei L (2010). Dementia, stroke and migraine--some common pathological mechanisms. J Neurol Sci, 299:55-65. [DOI] [PubMed] [Google Scholar]
- [89].Sankar R, Thamotharan S, Shin D, Moley KH, Devaskar SU (2002). Insulin-responsive glucose transporters-GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res, 107:157-165. [DOI] [PubMed] [Google Scholar]
- [90].Hamer JA, Testani D, Mansur RB, Lee Y, Subramaniapillai M, McIntyre RS (2019). Brain insulin resistance: A treatment target for cognitive impairment and anhedonia in depression. Exp Neurol, 315:1-8. [DOI] [PubMed] [Google Scholar]
- [91].Hölzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, et al. (2011). Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res, 191:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Głombik K, Detka J, Góralska J, Kurek A, Solnica B, Budziszewska B (2020). Brain Metabolic Alterations in Rats Showing Depression-Like and Obesity Phenotypes. Neurotox Res, 37:406-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Akbaraly TN, Kivimäki M, Brunner EJ, Chandola T, Marmot MG, Singh-Manoux A, et al. (2009). Association between metabolic syndrome and depressive symptoms in middle-aged adults: results from the Whitehall II study. Diabetes Care, 32:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].McNay EC, Fries TM, Gold PE (2000). Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A, 97:2881-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].McNay EC, Gold PE (2002). Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behav Cogn Neurosci Rev, 1:264-280. [DOI] [PubMed] [Google Scholar]
- [96].Pearson-Leary J, McNay EC (2016). Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory. J Neurosci, 36:11851-11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gross EC, Lisicki M, Fischer D, Sándor PS, Schoenen J (2019). The metabolic face of migraine - from pathophysiology to treatment. Nat Rev Neurol, 15:627-643. [DOI] [PubMed] [Google Scholar]
- [98].Reinecke F, Smeitink JAM, van der Westhuizen FH (2009). OXPHOS gene expression and control in mitochondrial disorders. Biochim Biophys Acta, 1792:1113-1121. [DOI] [PubMed] [Google Scholar]
- [99].Iotti S, Malucelli E Free magnesium concentration in the human brain. University of Adelaide Press; 2011. [PubMed] [Google Scholar]
- [100].Schulz UG, Blamire AM, Corkill RG, Davies P, Styles P, Rothwell PM (2007). Association between cortical metabolite levels and clinical manifestations of migrainous aura: an MR-spectroscopy study. Brain, 130:3102-3110. [DOI] [PubMed] [Google Scholar]
- [101].Ramadan NM, Halvorson H, Vande-Linde A, Levine SR, Helpern JA, Welch KM (1989). Low brain magnesium in migraine. Headache, 29:416-419. [DOI] [PubMed] [Google Scholar]
- [102].Brown GG, Levine SR, Gorell JM, Pettegrew JW, Gdowski JW, Bueri JA, et al. (1989). In vivo 31P NMR profiles of Alzheimer’s disease and multiple subcortical infarct dementia. Neurology, 39:1423-1427. [DOI] [PubMed] [Google Scholar]
- [103].Fachal L, Mosquera-Miguel A, Pastor P, Ortega-Cubero S, Lorenzo E, Oterino-Durán A, et al. (2015). No evidence of association between common European mitochondrial DNA variants in Alzheimer, Parkinson, and migraine in the Spanish population. Am J Med Genet B Neuropsychiatr Genet, 168B:54-65. [DOI] [PubMed] [Google Scholar]
- [104].Børte S, Zwart J-A, Skogholt AH, Gabrielsen ME, Thomas LF, Fritsche LG, et al. (2020). Mitochondrial genome-wide association study of migraine - the HUNT Study. Cephalalgia, 40:625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sripetchwandee J, Chattipakorn N, Chattipakorn SC (2018). Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Front Endocrinol, 9:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lénárt N, Brough D, Dénes Á (2016). Inflammasomes link vascular disease with neuroinflammation and brain disorders. J Cereb Blood Flow Metab, 36:1668-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Conti P, D’Ovidio C, Conti C, Gallenga CE, Lauritano D, Caraffa A, et al. (2019). Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur J Pharmacol, 844:87-94. [DOI] [PubMed] [Google Scholar]
- [108].Craft S (2012). Alzheimer disease: Insulin resistance and AD--extending the translational path. Nat Rev Neurol, 8:360-362. [DOI] [PubMed] [Google Scholar]
- [109].Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, et al. (2015). Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediators Inflamm, 2015:105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kursun O, Yemisci M, van den Maagdenberg AMJM, Karatas H (2021). Migraine and neuroinflammation: the inflammasome perspective. J Headache Pain, 22:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Edvinsson L, Haanes KA, Warfvinge K (2019). Does inflammation have a role in migraine? Nat Rev Neurol, 15:483-490. [DOI] [PubMed] [Google Scholar]
- [112].Biscetti L, Cresta E, Cupini LM, Calabresi P, Sarchielli P (2023). The putative role of neuroinflammation in the complex pathophysiology of migraine: From bench to bedside. Neurobiol Dis, 180:106072. [DOI] [PubMed] [Google Scholar]
- [113].Halse R, Pearson SL, McCormack JG, Yeaman SJ, Taylor R (2001). Effects of tumor necrosis factor-alpha on insulin action in cultured human muscle cells. Diabetes, 50:1102-1109. [DOI] [PubMed] [Google Scholar]
- [114].Hotamisligil GS (1999). The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med, 245:621-625. [DOI] [PubMed] [Google Scholar]
- [115].Rotter V, Nagaev I, Smith U (2003). Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem, 278:45777-45784. [DOI] [PubMed] [Google Scholar]
- [116].Stephens JM, Lee J, Pilch PF (1997). Tumor Necrosis Factor-α-induced Insulin Resistance in 3T3-L1 Adipocytes Is Accompanied by a Loss of Insulin Receptor Substrate-1 and GLUT4 Expression without a Loss of Insulin Receptor-mediated Signal Transduction*. J Biol Chem, 272:971-976. [DOI] [PubMed] [Google Scholar]
- [117].Negi G, Kumar A, Sharma SS (2011). Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J Pineal Res, 50:124-131. [DOI] [PubMed] [Google Scholar]
- [118].Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Koçak E, Sen ZD, et al. (2013). Spreading depression triggers headache by activating neuronal Panx1 channels. Science, 339:1092-1095. [DOI] [PubMed] [Google Scholar]
- [119].Parajuli B, Sonobe Y, Horiuchi H, Takeuchi H, Mizuno T, Suzumura A (2013). Oligomeric amyloid β induces IL-1β processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis, 4:e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hagen K, Stovner LJ, Zwart J-A (2020). High sensitivity C-reactive protein and risk of migraine in a 11-year follow-up with data from the Nord-Trøndelag health surveys 2006-2008 and 2017-2019. J Headache Pain, 21:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hagen K, Stovner LJ, Nilsen KB, Kristoffersen ES, Winsvold BS (2019). The impact of C-reactive protein levels on headache frequency in the HUNT study 2006-2008. BMC Neurol, 19:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lippi G, Mattiuzzi C, Cervellin G (2014). C-reactive protein and migraine. Facts or speculations? Clin Chem Lab Med, 52:1265-1272. [DOI] [PubMed] [Google Scholar]
- [123].Geng C, Yang Z, Xu P, Zhang H (2022). Aberrations in peripheral inflammatory cytokine levels in migraine: A systematic review and meta-analysis. J Clin Neurosci, 98:213-218. [DOI] [PubMed] [Google Scholar]
- [124].Thuraiaiyah J, Erritzøe-Jervild M, Al-Khazali HM, Schytz HW, Younis S (2022). The role of cytokines in migraine: A systematic review. Cephalalgia, 42:1565-1588. [DOI] [PubMed] [Google Scholar]
- [125].Uzar E, Evliyaoglu O, Yucel Y, Ugur Cevik M, Acar A, Guzel I, et al. (2011). Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur Rev Med Pharmacol Sci, 15:1111-1116. [PubMed] [Google Scholar]
- [126].Duarte H, Teixeira AL, Rocha NP, Domingues RB (2015). Increased interictal serum levels of CXCL8/IL-8 and CCL3/MIP-1α in migraine. Neurol Sci, 36:203-208. [DOI] [PubMed] [Google Scholar]
- [127].Boćkowski L, Sobaniec W, Zelazowska-Rutkowska B (2009). Proinflammatory plasma cytokines in children with migraine. Pediatr Neurol, 41:17-21. [DOI] [PubMed] [Google Scholar]
- [128].Oliveira AB, Bachi ALL, Ribeiro RT, Mello MT, Tufik S, Peres MFP (2017). Unbalanced plasma TNF-α and IL-12/IL-10 profile in women with migraine is associated with psychological and physiological outcomes. J Neuroimmunol, 313:138-144. [DOI] [PubMed] [Google Scholar]
- [129].Yücel M, Kotan D, Gurol Çiftçi G, Çiftçi IH, Cikriklar HI (2016). Serum levels of endocan, claudin-5 and cytokines in migraine. Eur Rev Med Pharmacol Sci, 20:930-936. [PubMed] [Google Scholar]
- [130].Wang F, He Q, Ren Z, Li F, Chen W, Lin X, et al. (2015). Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol Sci, 36:535-540. [DOI] [PubMed] [Google Scholar]
- [131].Perini F, D’Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, et al. (2005). Plasma cytokine levels in migraineurs and controls. Headache, 45:926-931. [DOI] [PubMed] [Google Scholar]
- [132].Armağan HH, Karaman K, Yilmaz DY (2020). Antioxidant and cytokine levels in plasma of patients with attack and non-attack periods. Journal of Cellular Neuroscience and Oxidative Stress, 12:914-921. [Google Scholar]
- [133].Scheff JD, Calvano SE, Lowry SF, Androulakis IP (2010). Modeling the influence of circadian rhythms on the acute inflammatory response. J Theor Biol, 264:1068-1076. [DOI] [PubMed] [Google Scholar]
- [134].Sarchielli P, Floridi A, Mancini ML, Rossi C, Coppola F, Baldi A, et al. (2006). NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia, 26:1071-1079. [DOI] [PubMed] [Google Scholar]
- [135].Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, et al. (2006). Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache, 46:200-207. [DOI] [PubMed] [Google Scholar]
- [136].Sarchielli P, Alberti A, Vaianella L, Pierguidi L, Floridi A, Mazzotta G, et al. (2004). Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache, 44:961-968. [DOI] [PubMed] [Google Scholar]
- [137].de la Monte SM (2012). Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res, 9:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Vinuesa A, Pomilio C, Gregosa A, Bentivegna M, Presa J, Bellotto M, et al. (2021). Inflammation and Insulin Resistance as Risk Factors and Potential Therapeutic Targets for Alzheimer’s Disease. Front Neurosci, 15:653651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, et al. (2012). The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age, 34:1295-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Leonardo S, Fregni F (2023). Association of inflammation and cognition in the elderly: A systematic review and meta-analysis. Front Aging Neurosci, 15:1069439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Long S, Chen Y, Meng Y, Yang Z, Wei M, Li T, et al. (2023). Peripheral high levels of CRP predict progression from normal cognition to dementia: A systematic review and meta-analysis. J Clin Neurosci, 107:54-63. [DOI] [PubMed] [Google Scholar]
- [142].Magaki S, Mueller C, Dickson C, Kirsch W (2007). Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol, 42:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Akhtar A, Sah SP (2020). Insulin signaling pathway and related molecules: Role in neurodegeneration and Alzheimer’s disease. Neurochem Int, 135:104707. [DOI] [PubMed] [Google Scholar]
- [144].Akash MSH, Rehman K, Liaqat A (2018). Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem, 119:105-110. [DOI] [PubMed] [Google Scholar]
- [145].Borst SE (2004). The role of TNF-alpha in insulin resistance. Endocrine, 23:177-182. [DOI] [PubMed] [Google Scholar]
- [146].Fasshauer M, Paschke R (2003). Regulation of adipocytokines and insulin resistance. Diabetologia, 46:1594-1603. [DOI] [PubMed] [Google Scholar]
- [147].Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, et al. (1997). Genetic influence on cytokine production and fatal meningococcal disease. Lancet, 349:170-173. [DOI] [PubMed] [Google Scholar]
- [148].Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW (1997). Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proceedings of the National Academy of Sciences, 94:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chen M, Tang W, Hou L, Liu R, Dong Z, Han X, et al. (2015). Tumor Necrosis Factor (TNF) -308G>A, Nitric Oxide Synthase 3 (NOS3) +894G>T Polymorphisms and Migraine Risk: A Meta-Analysis. PLoS One, 10:e0129372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lee YH, Choi SJ, Ji JD, Song GG (2015). Association between TNF-α promoter -308 A/G polymorphism and Alzheimer’s disease: a meta-analysis. Neurol Sci, 36:825-832. [DOI] [PubMed] [Google Scholar]
- [151].Sen J, Belli A (2007). S100B in neuropathologic states: the CRP of the brain? J Neurosci Res, 85:1373-1380. [DOI] [PubMed] [Google Scholar]
- [152].Rothermundt M, Peters M, Prehn JHM, Arolt V (2003). S100B in brain damage and neurodegeneration. Microsc Res Tech, 60:614-632. [DOI] [PubMed] [Google Scholar]
- [153].Sorci G, Bianchi R, Riuzzi F, Tubaro C, Arcuri C, Giambanco I, et al. (2010). S100B Protein, A Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc Psychiatry Neurol. doi: 10.1155/2010/656481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Steiner J, Bernstein H-G, Bogerts B, Gos T, Richter-Landsberg C, Wunderlich MT, et al. (2008). S100B is expressed in, and released from, OLN-93 oligodendrocytes: Influence of serum and glucose deprivation. Neuroscience, 154:496-503. [DOI] [PubMed] [Google Scholar]
- [155].Michetti F, Clementi ME, Di Liddo R, Valeriani F, Ria F, Rende M, et al. (2023). The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int [J] Mol Sci. doi: 10.3390/ijms24119605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Teepker M, Munk K, Mylius V, Haag A, Möller JC, Oertel WH, et al. (2009). Serum concentrations of s100b and NSE in migraine. Headache, 49:245-252. [DOI] [PubMed] [Google Scholar]
- [157].Yilmaz N, Karaali K, Ozdem S, Turkay M, Unal A, Dora B (2011). Elevated S100B and neuron specific enolase levels in patients with migraine-without aura: evidence for neurodegeneration? Cell Mol Neurobiol, 31:579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Papandreou O, Soldatou A, Tsitsika A, Kariyannis C, Papandreou T, Zachariadi A, et al. (2005). Serum S100beta protein in children with acute recurrent headache: a potentially useful marker for migraine. Headache, 45:1313-1316. [DOI] [PubMed] [Google Scholar]
- [159].Chu C, Zhong R, Cai M, Li N, Lin W (2022). Elevated Blood S100B Levels in Patients With Migraine: A Systematic Review and Meta-Analysis. Front Neurol, 13:914051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Esposito G, Scuderi C, Lu J, Savani C, De Filippis D, Iuvone T, et al. (2008). S100B induces tau protein hyperphosphorylation via Dickopff-1 up-regulation and disrupts the Wnt pathway in human neural stem cells. J Cell Mol Med, 12:914-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mori T, Koyama N, Arendash GW, Horikoshi-Sakuraba Y, Tan J, Town T (2010). Overexpression of human S100B exacerbates cerebral amyloidosis and gliosis in the Tg2576 mouse model of Alzheimer’s disease. Glia, 58:300-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Mrak RE, Sheng JG, Griffin WS (1996). Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease. J Neuropathol Exp Neurol, 55:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Marshak DR, Pesce SA, Stanley LC, Griffin WS (1992). Increased S100 beta neurotrophic activity in Alzheimer’s disease temporal lobe. Neurobiol Aging, 13:1-7. [DOI] [PubMed] [Google Scholar]
- [164].Peskind ER, Griffin WS, Akama KT, Raskind MA, Van Eldik LJ (2001). Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer’s disease. Neurochem Int, 39:409-413. [DOI] [PubMed] [Google Scholar]
- [165].Salvadó G, Shekari M, Falcon C, Operto G, Milà-Alomà M, Sánchez-Benavides G, et al. (2022). Brain alterations in the early Alzheimer’s continuum with amyloid-β, tau, glial and neurodegeneration CSF markers. Brain Commun, 4:fcac134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Strotzer M (2009). One century of brain mapping using Brodmann areas. Klin Neuroradiol, 19:179-186. [DOI] [PubMed] [Google Scholar]
- [167].Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, et al. (2017). Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry, 22:1455-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev, 37:2529-2553. [DOI] [PubMed] [Google Scholar]
- [169].Critchley HD, Mathias CJ, Dolan RJ (2001). Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci, 4:207-212. [DOI] [PubMed] [Google Scholar]
- [170].Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci, 16:55-61. [DOI] [PubMed] [Google Scholar]
- [171].Jensen VFH, Bøgh IB, Lykkesfeldt J (2014). Effect of insulin-induced hypoglycaemia on the central nervous system: evidence from experimental studies. J Neuroendocrinol, 26:123-150. [DOI] [PubMed] [Google Scholar]
- [172].Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, et al. (2019). An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun Biol, 2:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Trufanov A, Markin K, Frunza D, Litvinenko I, Odinak M (2020). Alterations in internetwork functional connectivity in patients with chronic migraine within the boundaries of the Triple Network Model. Neurol Clin Neurosci, 8:289-297. [Google Scholar]
- [174].Russo A, Silvestro M, Trojsi F, Bisecco A, De Micco R, Caiazzo G, et al. (2020). Cognitive Networks Disarrangement in Patients With Migraine Predicts Cutaneous Allodynia. Headache, 60:1228-1243. [DOI] [PubMed] [Google Scholar]
- [175].Coppola G, Di Renzo A, Petolicchio B, Tinelli E, Di Lorenzo C, Parisi V, et al. (2019). Aberrant interactions of cortical networks in chronic migraine: A resting-state fMRI study. Neurology, 92:e2550-e2558. [DOI] [PubMed] [Google Scholar]
- [176].Chong CD, Schwedt TJ, Hougaard A (2019). Brain functional connectivity in headache disorders: A narrative review of MRI investigations. J Cereb Blood Flow Metab, 39:650-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hu S, Hao Z, Li M, Zhao M, Wen J, Gao Y, et al. (2023). Resting-state abnormalities in functional connectivity of the default mode network in migraine: A meta-analysis. Front Neurosci, 17:1136790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Blaabjerg L, Juhl CB (2016). Hypoglycemia-Induced Changes in the Electroencephalogram: An Overview. J Diabetes Sci Technol, 10:1259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bjørgaas M, Sand T, Vik T, Jorde R (1998). Quantitative EEG during controlled hypoglycaemia in diabetic and non-diabetic children. Diabet Med, 15:30-37. [DOI] [PubMed] [Google Scholar]
- [180].Tribl G, Howorka K, Heger G, Anderer P, Thoma H, Zeitlhofer J (1996). EEG topography during insulin-induced hypoglycemia in patients with insulin-dependent diabetes mellitus. Eur Neurol, 36:303-309. [DOI] [PubMed] [Google Scholar]
- [181].Hallschmid M, Schultes B, Marshall L, Mölle M, Kern W, Bredthauer J, et al. (2004). Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes, 53:2202-2208. [DOI] [PubMed] [Google Scholar]
- [182].Genco S, de Tommaso M, Prudenzano AM, Savarese M, Puca FM (1994). EEG features in juvenile migraine: topographic analysis of spontaneous and visual evoked brain electrical activity: a comparison with adult migraine. Cephalalgia, 14:41-6; discussion 4. [DOI] [PubMed] [Google Scholar]
- [183].Facchetti D, Marsile C, Faggi L, Donati E, Kokodoko A, Poloni M (1990). Cerebral mapping in subjects suffering from migraine with aura. Cephalalgia, 10:279-284. [DOI] [PubMed] [Google Scholar]
- [184].Lia C, Carenini L, Degioz C, Bottachi E (1995). Computerized EEG analysis in migraine patients. Ital J Neurol Sci, 16:249-254. [DOI] [PubMed] [Google Scholar]
- [185].Hughes JR, Robbins LD (1990). Brain mapping in migraine. Clin Electroencephalogr, 21:14-24. [DOI] [PubMed] [Google Scholar]
- [186].Bjørk MH, Stovner LJ, Engstrøm M, Stjern M, Hagen K, Sand T (2009). Interictal quantitative EEG in migraine: a blinded controlled study. J Headache Pain, 10:331-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Smailovic U, Jelic V (2019). Neurophysiological Markers of Alzheimer’s Disease: Quantitative EEG Approach. Neurol Ther, 8:37-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Scheijbeler EP, de Haan W, Stam CJ, Twisk JWR, Gouw AA (2023). Longitudinal resting-state EEG in amyloid-positive patients along the Alzheimer’s disease continuum: considerations for clinical trials. Alzheimers Res Ther, 15:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Lee S-J, Park M-H, Park S-S, Ahn J-Y, Heo J-H (2015). Quantitative EEG and medial temporal lobe atrophy in Alzheimer’s dementia: Preliminary study. Ann Indian Acad Neurol, 18:10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Polanco JC, Li C, Bodea L-G, Martinez-Marmol R, Meunier FA, Götz J (2018). Amyloid-β and tau complexity - towards improved biomarkers and targeted therapies. Nat Rev Neurol, 14:22-39. [DOI] [PubMed] [Google Scholar]
- [191].Xia X, Jiang Q, McDermott J, Han J-DJ (2018). Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell, 17:e12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].de Ceballos ML, Köfalvi A (2017). Boosting brain glucose metabolism to fight neurodegeneration? Oncotarget, 8:14273-14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Gupta S, Singh V, Ganesh S, Singhal NK, Sandhir R (2022). siRNA Mediated GSK3β Knockdown Targets Insulin Signaling Pathway and Rescues Alzheimer’s Disease Pathology: Evidence from In Vitro and In Vivo Studies. ACS Appl Mater Interfaces, 14:69-93. [DOI] [PubMed] [Google Scholar]
- [194].Li L, Hölscher C (2007). Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev, 56:384-402. [DOI] [PubMed] [Google Scholar]
- [195].Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC (2004). Increased risk of type 2 diabetes in Alzheimer disease. Diabetes, 53:474-481. [DOI] [PubMed] [Google Scholar]
- [196].Wang L, Li J, Di L-J (2022). Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med Res Rev, 42:946-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong C-X (2004). O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci U S A, 101:10804-10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].van der Harg JM, Nölle A, Zwart R, Boerema AS, van Haastert ES, Strijkstra AM, et al. (2014). The unfolded protein response mediates reversible tau phosphorylation induced by metabolic stress. Cell Death Dis, 5:e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Meier-Ruge WA, Bertoni-Freddari C (1997). Pathogenesis of decreased glucose turnover and oxidative phosphorylation in ischemic and trauma-induced dementia of the Alzheimer type. Ann N Y Acad Sci, 826:229-241. [DOI] [PubMed] [Google Scholar]
- [200].Folch J, Ettcheto M, Busquets O, Sánchez-López E, Castro-Torres RD, Verdaguer E, et al. (2018). The Implication of the Brain Insulin Receptor in Late Onset Alzheimer’s Disease Dementia. Pharmaceuticals. doi: 10.3390/ph11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Hoyer S (1994). Age as risk factor for sporadic dementia of the Alzheimer type? Ann N Y Acad Sci, 719:248-256. [DOI] [PubMed] [Google Scholar]
- [202].Meier-Ruge W, Bertoni-Freddari C, Iwangoff P (1994). Changes in brain glucose metabolism as a key to the pathogenesis of Alzheimer’s disease. Gerontology, 40:246-252. [DOI] [PubMed] [Google Scholar]
- [203].Meier-Ruge W, Bertoni-Freddari C (1996). The significance of glucose turnover in the brain in the pathogenetic mechanisms of Alzheimer’s disease. Rev Neurosci, 7:1-19. [DOI] [PubMed] [Google Scholar]
- [204].Schiöth HB, Craft S, Brooks SJ, Frey WH 2nd, Benedict C (2012). Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol, 46:4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB (2011). Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol, 71:365-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].de la Monte SM, Chen GJ, Rivera E, Wands JR (2003). Neuronal thread protein regulation and interaction with microtubule-associated proteins in SH-Sy5y neuronal cells. Cell Mol Life Sci, 60:2679-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Zhao W-Q, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, et al. (2008). Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J, 22:246-260. [DOI] [PubMed] [Google Scholar]
- [208].Liu X, Teng Z, Cui C, Wang R, Liu M, Zhang Y (2014). Amyloid beta-derived diffusible ligands (ADDLs) induce abnormal expression of insulin receptors in rat hippocampal neurons. J Mol Neurosci, 52:124-130. [DOI] [PubMed] [Google Scholar]
- [209].Chornenkyy Y, Wang W-X, Wei A, Nelson PT (2019). Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol, 29:3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Dice JF (2007). Chaperone-mediated autophagy. Autophagy, 3:295-299. [DOI] [PubMed] [Google Scholar]
- [211].Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M, School of Advanced Studies of the European Headache Federation (EHF-SAS) (2019). Association of diet and headache. J Headache Pain, 20:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Scheiber A, Mank V Anti-Inflammatory Diets. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- [213].Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D (2015). A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open, 5:e008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Ludwig DS, Willett WC, Volek JS, Neuhouser ML (2018). Dietary fat: From foe to friend? Science, 362:764-770. [DOI] [PubMed] [Google Scholar]
- [215].Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. (2019). Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet, 393:447-492. [DOI] [PubMed] [Google Scholar]
- [216].Altamura C, Cecchi G, Bravo M, Brunelli N, Laudisio A, Caprio PD, et al. (2020). The Healthy Eating Plate Advice for Migraine Prevention: An Interventional Study. Nutrients. doi: 10.3390/nu12061579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Hajjarzadeh S, Mahdavi R, Shalilahmadi D, Nikniaz Z (2020). The association of dietary patterns with migraine attack frequency in migrainous women. Nutr Neurosci, 23:724-730. [DOI] [PubMed] [Google Scholar]
- [218].Mirzababaei A, Khorsha F, Togha M, Yekaninejad MS, Okhovat AA, Mirzaei K (2020). Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr Neurosci, 23:335-342. [DOI] [PubMed] [Google Scholar]
- [219].Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. (2017). A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial). BMC Med, 15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N (2013). Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann Neurol, 74:580-591. [DOI] [PubMed] [Google Scholar]
- [221].Sánchez-Villegas A, Martínez-González MA, Estruch R, Salas-Salvadó J, Corella D, Covas MI, et al. (2013). Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Med, 11:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Kivipelto M, Mangialasche F, Ngandu T (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol, 14:653-666. [DOI] [PubMed] [Google Scholar]
- [223].Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, et al. (2013). The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement, 9:657-665. [DOI] [PubMed] [Google Scholar]
- [224].Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, et al. (2013). Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology, 24:479-489. [DOI] [PubMed] [Google Scholar]
- [225].Männikkö R, Komulainen P, Schwab U, Heikkilä HM, Savonen K, Hassinen M, et al. (2015). The Nordic diet and cognition--The DR’s EXTRA Study. Br J Nutr, 114:231-239. [DOI] [PubMed] [Google Scholar]
- [226].Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, et al. (2013). Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry, 84:1318-1325. [DOI] [PubMed] [Google Scholar]
- [227].Kheirouri S, Alizadeh M (2022). MIND diet and cognitive performance in older adults: a systematic review. Crit Rev Food Sci Nutr, 62:8059-8077. [DOI] [PubMed] [Google Scholar]
- [228].Arab A, Khorvash F, Kazemi M, Heidari Z, Askari G (2022). Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on clinical, quality of life and mental health outcomes in women with migraine: a randomised controlled trial. Br J Nutr, 128:1535-1544. [DOI] [PubMed] [Google Scholar]
- [229].Evcili G, Utku U, Öğün MN, Özdemir G (2018). Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri, 30:8-11. [DOI] [PubMed] [Google Scholar]
- [230].Barnes Lisa L., Dhana Klodian, Liu Xiaoran, Carey Vincent J., Ventrelle Jennifer Johnson Kathleen,et al. (2023). Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N Engl J Med, 389:602-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Guo J, Bakshi V, Lin A-L (2015). Early Shifts of Brain Metabolism by Caloric Restriction Preserve White Matter Integrity and Long-Term Memory in Aging Mice. Front Aging Neurosci, 7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Barbanti P, Fofi L, Aurilia C, Egeo G, Caprio M (2017). Ketogenic diet in migraine: rationale, findings and perspectives. Neurol Sci, 38:111-115. [DOI] [PubMed] [Google Scholar]
- [233].Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Evangelista M, Sirianni G, et al. (2016). Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain, 17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Caprio M, Moriconi E, Camajani E, Feraco A, Marzolla V, Vitiello L, et al. (2023). Very-low-calorie ketogenic diet vs hypocaloric balanced diet in the prevention of high-frequency episodic migraine: the EMIKETO randomized, controlled trial. J Transl Med, 21:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. (2020). Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes, 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M (2020). Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med, 18:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Quintana-Navarro GM, Alcala-Diaz JF, Lopez-Moreno J, Perez-Corral I, Leon-Acuña A, Torres-Peña JD, et al. (2020). Long-term dietary adherence and changes in dietary intake in coronary patients after intervention with a Mediterranean diet or a low-fat diet: the CORDIOPREV randomized trial. Eur J Nutr, 59:2099-2110. [DOI] [PubMed] [Google Scholar]
- [238].Cano-Ibáñez N, Quintana-Navarro GM, Alcala-Diaz JF, Rangel-Zuñiga OA, Camargo A, Yubero-Serrano EM, et al. (2022). Long-term effect of a dietary intervention with two-healthy dietary approaches on food intake and nutrient density in coronary patients: results from the CORDIOPREV trial. Eur J Nutr, 61:3019-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Casas R, Ribó-Coll M, Ros E, Fitó M, Lamuela-Raventos R-M, Salas-Salvadó J, et al. (2022). Change to a healthy diet in people over 70 years old: the PREDIMED experience. Eur J Nutr, 61:1429-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Song T-J, Chu MK (2021). Exercise in Treatment of Migraine Including Chronic Migraine. Curr Pain Headache Rep, 25:14. [DOI] [PubMed] [Google Scholar]
- [241].Irby MB, Bond DS, Lipton RB, Nicklas B, Houle TT, Penzien DB (2016). Aerobic Exercise for Reducing Migraine Burden: Mechanisms, Markers, and Models of Change Processes. Headache, 56:357-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Machado-Oliveira L, da Silva Gauto YO, de Santana Neto FJ, da Silva MG, Germano-Soares AH, Diniz PRB (2020). Effects of Different Exercise Intensities on Headache: A Systematic Review. Am J Phys Med Rehabil, 99:390-396. [DOI] [PubMed] [Google Scholar]
- [243].La Touche R, Fernández Pérez JJ, Proy Acosta A, González Campodónico L, Martínez García S, Adraos Juárez D, et al. (2020). Is aerobic exercise helpful in patients with migraine? A systematic review and meta-analysis. Scand J Med Sci Sports, 30:965-982. [DOI] [PubMed] [Google Scholar]
- [244].Woldeamanuel YW, Oliveira ABD (2022). What is the efficacy of aerobic exercise versus strength training in the treatment of migraine? A systematic review and network meta-analysis of clinical trials. J Headache Pain, 23:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Holloszy JO (2011). Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol, 1:921-940. [DOI] [PubMed] [Google Scholar]
- [246].Vukovich MD, Arciero PJ, Kohrt WM, Racette SB, Hansen PA, Holloszy JO (1996). Changes in insulin action and GLUT-4 with 6 days of inactivity in endurance runners. J Appl Physiol, 80:240-244. [DOI] [PubMed] [Google Scholar]
- [247].Iso-Markku P, Kujala UM, Knittle K, Polet J, Vuoksimaa E, Waller K (2022). Physical activity as a protective factor for dementia and Alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case-control studies. Br J Sports Med, 56:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Xu W, Wang HF, Wan Y, Tan C-C, Yu J-T, Tan L (2017). Leisure time physical activity and dementia risk: a dose-response meta-analysis of prospective studies. BMJ Open, 7:e014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus, 19:1030-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A, 108:3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Niemann C, Godde B, Voelcker-Rehage C (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front Aging Neurosci, 6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Biazus-Sehn LF, Schuch FB, Firth J, Stigger F de S (2020). Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch Gerontol Geriatr, 89:104048. [DOI] [PubMed] [Google Scholar]
- [253].Farhang M, Miranda-Castillo C, Rubio M, Furtado G (2019). Impact of mind-body interventions in older adults with mild cognitive impairment: a systematic review. Int Psychogeriatr, 31:643-666. [DOI] [PubMed] [Google Scholar]
- [254].Wells RE, O’Connell N, Pierce CR, Estave P, Penzien DB, Loder E, et al. (2021). Effectiveness of Mindfulness Meditation vs Headache Education for Adults With Migraine: A Randomized Clinical Trial. JAMA Intern Med, 181:317-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Bhasin MK, Dusek JA, Chang B-H, Joseph MG, Denninger JW, Fricchione GL, et al. (2013). Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One, 8:e62817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Buric I, Farias M, Jong J, Mee C, Brazil IA (2017). What Is the Molecular Signature of Mind-Body Interventions? A Systematic Review of Gene Expression Changes Induced by Meditation and Related Practices. Front Immunol, 8:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Innes KE, Bourguignon C, Taylor AG (2005). Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: a systematic review. J Am Board Fam Pract, 18:491-519. [DOI] [PubMed] [Google Scholar]
- [258].Ong W-Y, Stohler CS, Herr DR (2019). Role of the Prefrontal Cortex in Pain Processing. Mol Neurobiol, 56:1137-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Moreno JJ (2024). Modulation of inflammatory response and pain by mind-body therapies as meditation. Brain Behavior and Immunity Integrative, 5:100036. [Google Scholar]
- [260].Pretty J, Barton J (2020). Nature-Based Interventions and Mind-Body Interventions: Saving Public Health Costs Whilst Increasing Life Satisfaction and Happiness. Int [J] Environ Res Public Health. doi: 10.3390/ijerph17217769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Cramer H, Hall H, Leach M, Frawley J, Zhang Y, Leung B, et al. (2016). Prevalence, patterns, and predictors of meditation use among US adults: A nationally representative survey. Sci Rep, 6:36760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Gu Q, Hou J-C, Fang X-M (2018). Mindfulness Meditation for Primary Headache Pain: A Meta-Analysis. Chin Med J, 131:829-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Laneri D, Schuster V, Dietsche B, Jansen A, Ott U, Sommer J (2015). Effects of Long-Term Mindfulness Meditation on Brain’s White Matter Microstructure and its Aging. Front Aging Neurosci, 7:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Luders E, Cherbuin N, Kurth F (2014). Forever Young(er): potential age-defying effects of long-term meditation on gray matter atrophy. Front Psychol, 5:1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Luders E, Jain FA, Kurth F (2021). Diminished Age-Related Decline of the Amygdala in Long-Term Meditation Practitioners. Psychosom Med, 83:650-654. [DOI] [PubMed] [Google Scholar]
- [266].Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. (2005). Meditation experience is associated with increased cortical thickness. Neuroreport, 16:1893-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Kurth F, Cherbuin N, Luders E (2015). Reduced age-related degeneration of the hippocampal subiculum in long-term meditators. Psychiatry Res, 232:214-218. [DOI] [PubMed] [Google Scholar]
- [268].Kurth F, Cherbuin N, Luders E (2017). Promising Links between Meditation and Reduced (Brain) Aging: An Attempt to Bridge Some Gaps between the Alleged Fountain of Youth and the Youth of the Field. Front Psychol, 8:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Chételat G, Mézenge F, Tomadesso C, Landeau B, Arenaza-Urquijo E, Rauchs G, et al. (2017). Reduced age-associated brain changes in expert meditators: a multimodal neuroimaging pilot study. Sci Rep, 7:10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].De Filippi E, Escrichs A, Càmara E, Garrido C, Marins T, Sánchez-Fibla M, et al. (2022). Meditation-induced effects on whole-brain structural and effective connectivity. Brain Struct Funct, 227:2087-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Ngandu T, Lehtisalo J, Korkki S, Solomon A, Coley N, Antikainen R, et al. (2022). The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimers Dement, 18:1325-1334. [DOI] [PubMed] [Google Scholar]
- [272].Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. (2020). World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement, 16:1078-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]