Abstract
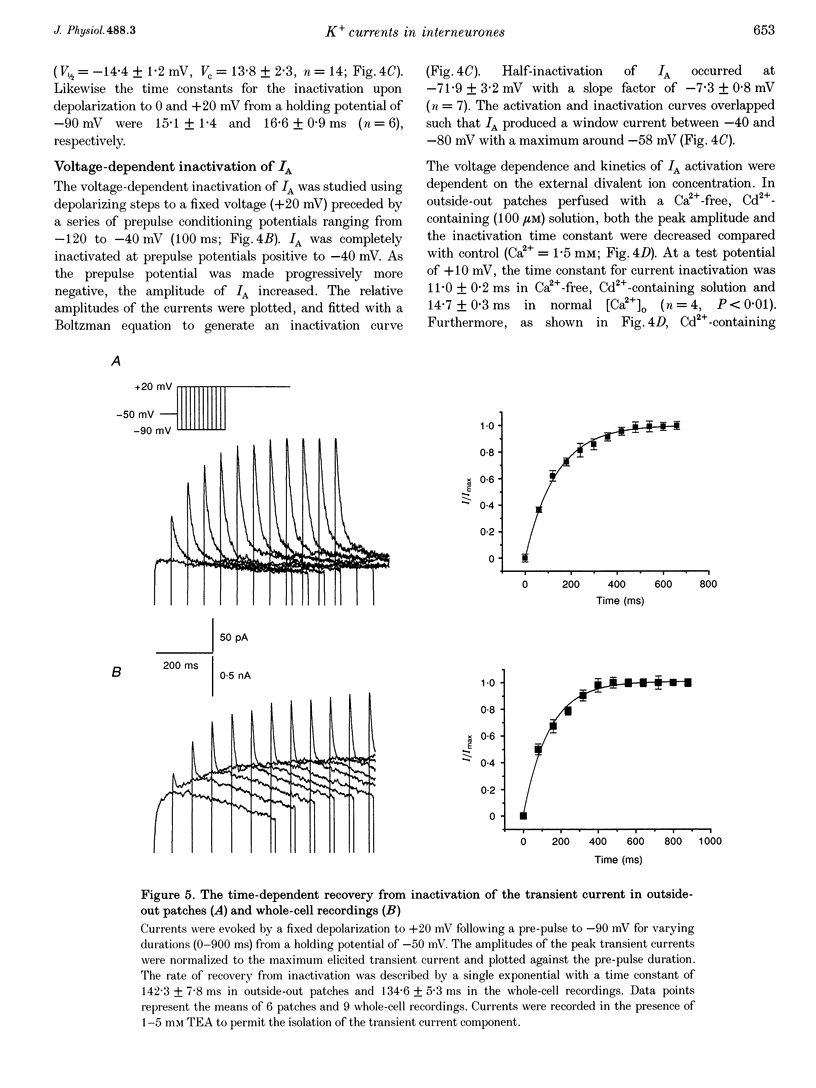
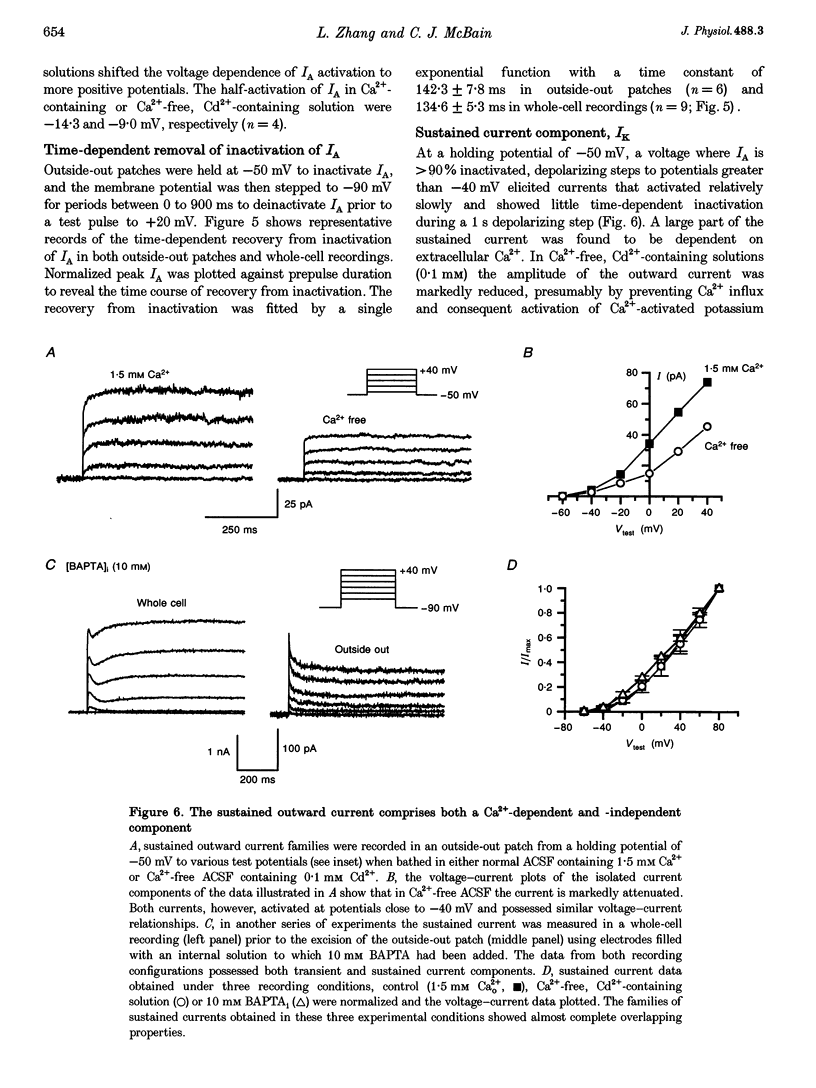
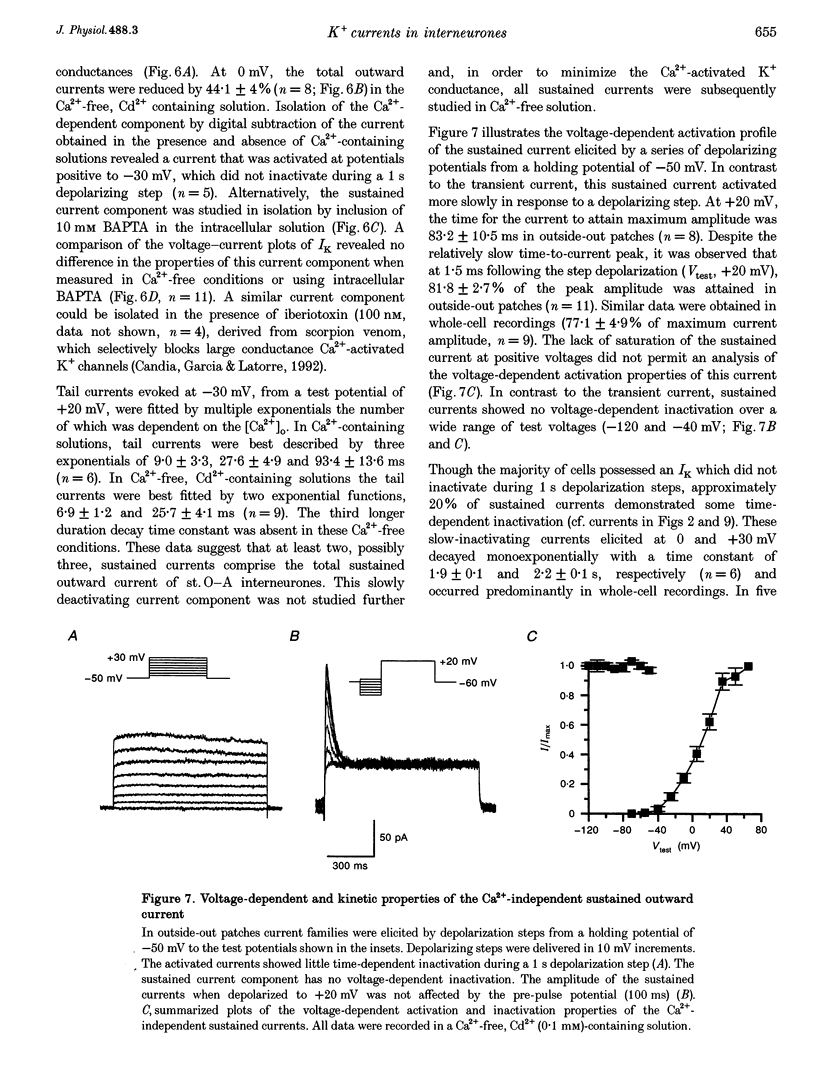
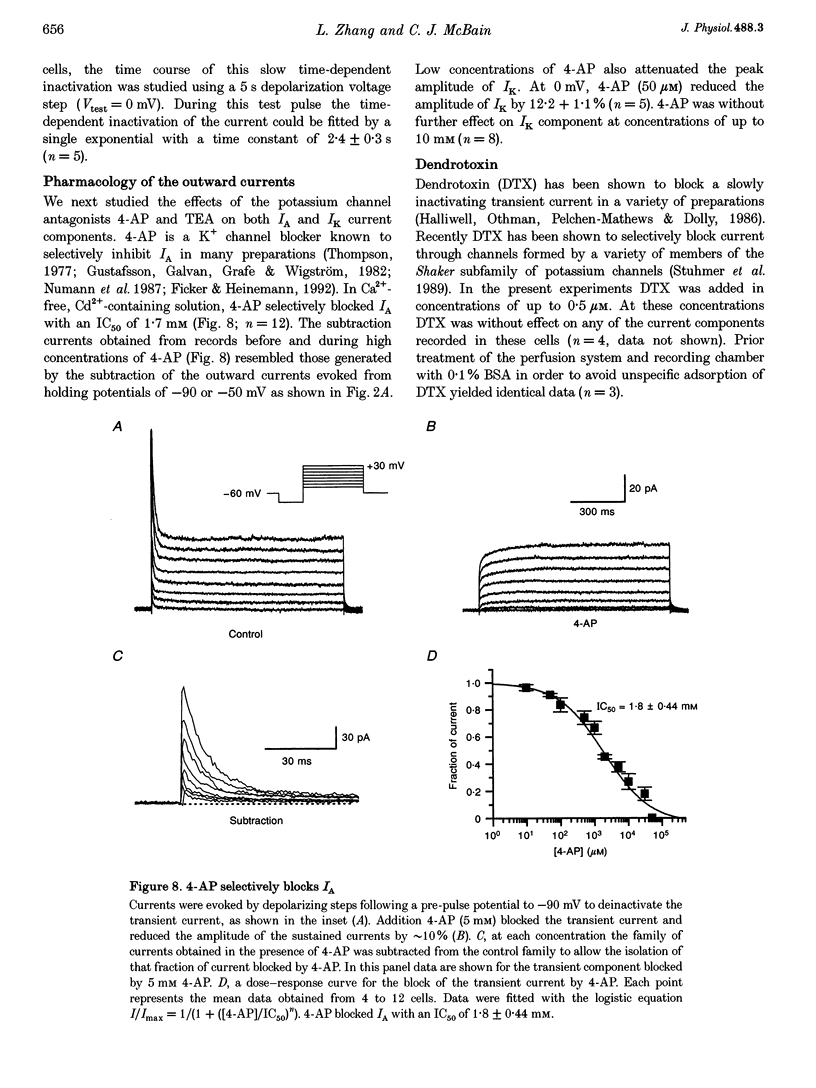
1. Voltage-activated K+ currents were recorded from visually identified inhibitory interneurones of the CA1 stratum oriens-alveus region in neonatal rat hippocampal slices using outside-out patch and whole-cell voltage clamp techniques. 2. Outward currents comprised both a transient and a sustained component when elicited from a holding potential of -90 mV. Tail current analysis of current reversal potentials showed that outward currents were carried by potassium ions. 3. The transient current, IA, was activated with a time to peak within 5 ms, inactivated with a time constant of approximately 15 ms at 0 mV and possessed half-activation at -14 mV. Half-inactivation of the transient current occurred at -71 mV. At -90 mV, the transient current recovered from inactivation with a time constant of 142 ms. 4. Activation of currents from a holding potential of -50 mV permitted isolation of the sustained current, IK. In Ca(2+)-free conditions the sustained current showed rapid activation, reaching about 80% of its maximum within 1.5 ms, and showed little inactivation during 1 s depolarizing steps. The majority of sustained outward currents showed no voltage-dependent inactivation. In approximately 20% of cells, a slow time-dependent inactivation of the sustained current was observed, suggesting the presence of a second type of sustained current in these cells. 5. A Ca(2+)-dependent K+ current comprised a significant portion of the total sustained current; this current was activated at voltages positive to -30 mV and showed no time-dependent inactivation over a 1 s depolarizing step. This current component was removed in Ca(2+)-free conditions or by iberiotoxin. 6. Low concentrations of 4-AP (50 microM) attenuated both the transient and sustained current components recorded in a Ca(2+)-free solution. Higher concentrations of 4-AP (< 10 mM) were without further effect on the sustained current but completely blocked the transient current with an IC50 of 1.8 mM. TEA blocked the sustained current with an IC50 of 7.9 mM without significantly reducing the transient current. Both current components were resistant to dendrotoxin (500 nM).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Candia S., Garcia M. L., Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca(2+)-activated K+ channel. Biophys J. 1992 Aug;63(2):583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Ficker E., Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol. 1992 Jan;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Nguyen A. N., Aiyar J., Hanson D. C., Mather R. J., Gutman G. A., Karmilowicz M. J., Auperin D. D., Chandy K. G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994 Jun;45(6):1227–1234. [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Othman I. B., Pelchen-Matthews A., Dolly J. O. Central action of dendrotoxin: selective reduction of a transient K conductance in hippocampus and binding to localized acceptors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):493–497. doi: 10.1073/pnas.83.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kues Wilfried A., Wunder Frank. Heterogeneous Expression Patterns of Mammalian Potassium Channel Genes in Developing and Adult Rat Brain. Eur J Neurosci. 1992;4(12):1296–1308. doi: 10.1111/j.1460-9568.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. J., DiChiara T. J., Kauer J. A. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J Neurosci. 1994 Jul;14(7):4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain C. J. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol. 1994 Dec;72(6):2853–2863. doi: 10.1152/jn.1994.72.6.2853. [DOI] [PubMed] [Google Scholar]
- McBain C., Dingledine R. Dual-component miniature excitatory synaptic currents in rat hippocampal CA3 pyramidal neurons. J Neurophysiol. 1992 Jul;68(1):16–27. doi: 10.1152/jn.1992.68.1.16. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Rettig J., Wunder F., Stocker M., Lichtinghagen R., Mastiaux F., Beckh S., Kues W., Pedarzani P., Schröter K. H., Ruppersberg J. P. Characterization of a Shaw-related potassium channel family in rat brain. EMBO J. 1992 Jul;11(7):2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A. Single voltage-dependent potassium channels in cultured rat hippocampal neurons. J Neurophysiol. 1986 Aug;56(2):481–493. doi: 10.1152/jn.1986.56.2.481. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sah P., Gibb A. J., Gage P. W. Potassium current activated by depolarization of dissociated neurons from adult guinea pig hippocampus. J Gen Physiol. 1988 Aug;92(2):263–278. doi: 10.1085/jgp.92.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serôdio P., Kentros C., Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol. 1994 Oct;72(4):1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder G., Harrison N. L. On the mechanism of modulation of transient outward current in cultured rat hippocampal neurons by di- and trivalent cations. J Neurophysiol. 1995 Jan;73(1):73–79. doi: 10.1152/jn.1995.73.1.73. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurbon D., Field A., Redman S. Electrotonic profiles of interneurons in stratum pyramidale of the CA1 region of rat hippocampus. J Neurophysiol. 1994 May;71(5):1948–1958. doi: 10.1152/jn.1994.71.5.1948. [DOI] [PubMed] [Google Scholar]
- Tsaur M. L., Sheng M., Lowenstein D. H., Jan Y. N., Jan L. Y. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992 Jun;8(6):1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E., Moreno H., Fruhling D., Kentros C., Rudy B. Cloning of ShIII (Shaw-like) cDNAs encoding a novel high-voltage-activating, TEA-sensitive, type-A K+ channel. Proc Biol Sci. 1992 Apr 22;248(1321):9–18. doi: 10.1098/rspb.1992.0036. [DOI] [PubMed] [Google Scholar]
- Weiser M., Vega-Saenz de Miera E., Kentros C., Moreno H., Franzen L., Hillman D., Baker H., Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994 Mar;14(3 Pt 1):949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbicz K. L., Weight F. F. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985 Apr;53(4):1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Zhang L., McBain C. J. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J Physiol. 1995 Nov 1;488(Pt 3):661–672. doi: 10.1113/jphysiol.1995.sp020998. [DOI] [PMC free article] [PubMed] [Google Scholar]


