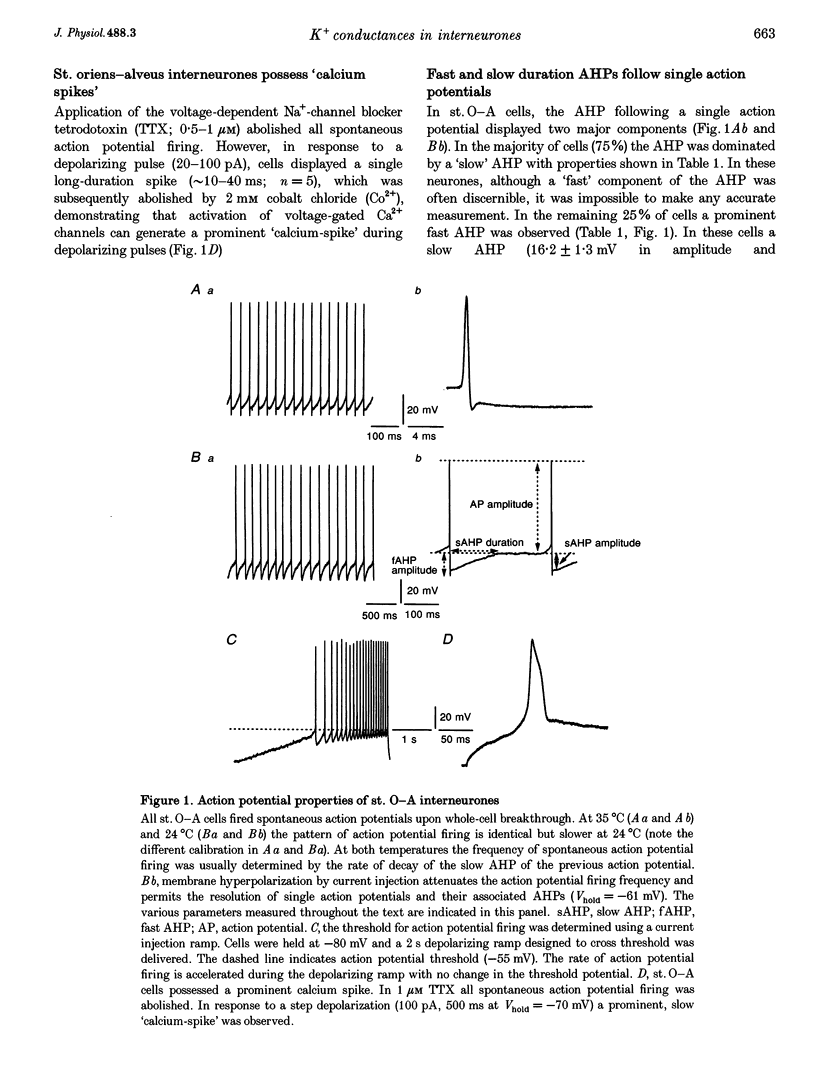
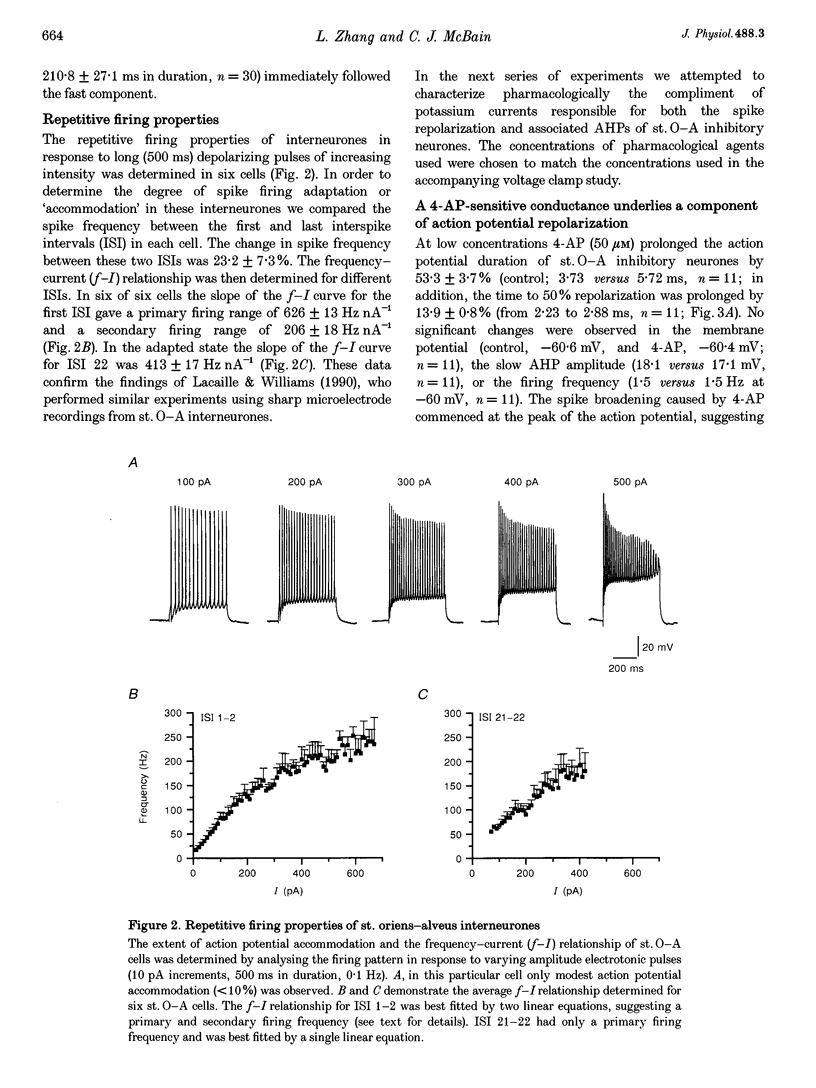
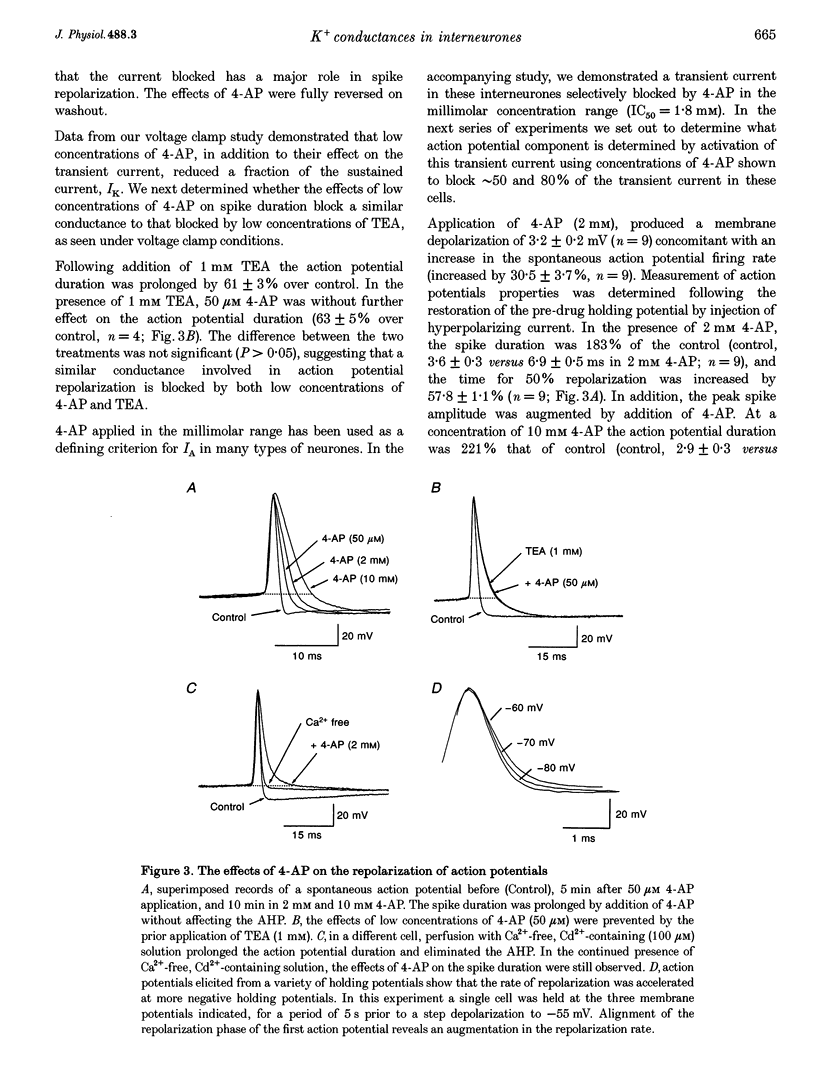
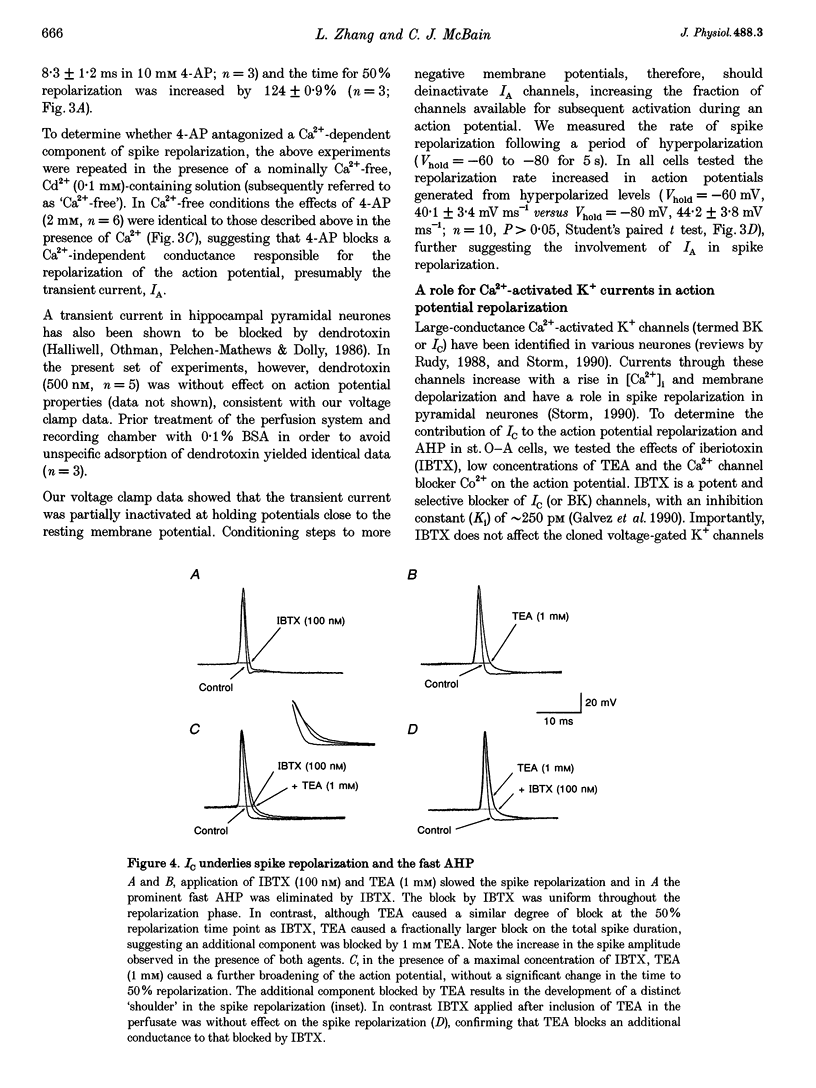
Abstract
1. The roles of multiple potassium conductances underlying action potential repolarization and after-hyperpolarization (AHP) in visually identified st. oriens-alveus (st. O-A) inhibitory interneurones of neonatal rat CA1 hippocampal slices were determined using whole-cell patch clamp techniques. 2. 4-Aminopyridine dose-dependently prolonged the action potential repolarization. The effects of 4-AP persisted in Ca(2+)-free conditions. Action potentials evoked from hyperpolarized potentials possessed an increased rate of repolarization. These data suggest an involvement of the rapidly activating transient current, IA, in spike repolarization. 3. Action potential duration was increased in the presence of Ca(2+)-free, Cd(2+)-containing solution, iberiotoxin or 1 mM TEA. The fast component of the AHP was attenuated by these agents suggesting that the Ca(2+)-activated K+ conductance, IC, underlies both the spike repolarization and fast AHP. 4. In Ca(2+)-free conditions, TEA (> 1 mM) dose-dependently prolonged the action potential duration by blocking a late conductance in action potential repolarization, suggesting a role for the sustained current, IK. 5. The slow AHP was attenuated by Ca(2+)-free medium, apamin or the Ca2+ chelator EGTA, suggesting a role for the Ca(2+)-activated K+ conductance, IAHP. 6. We conclude that action potential repolarization and AHP of st. O-A interneurones result from the activation of pharmacologically distinct, temporally overlapping potassium conductances. These findings are discussed with reference to the voltage clamp data presented in the preceding manuscript.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourque C. W., Brown D. A. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987 Nov 23;82(2):185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Buhl E. H., Han Z. S., Lörinczi Z., Stezhka V. V., Karnup S. V., Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J Neurophysiol. 1994 Apr;71(4):1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Gehlert D. R., Gackenheimer S. L. Comparison of the distribution of binding sites for the potassium channel ligands [125I]apamin, [125I]charybdotoxin and [125I]iodoglyburide in the rat brain. Neuroscience. 1993 Jan;52(1):191–205. doi: 10.1016/0306-4522(93)90192-i. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Othman I. B., Pelchen-Matthews A., Dolly J. O. Central action of dendrotoxin: selective reduction of a transient K conductance in hippocampus and binding to localized acceptors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):493–497. doi: 10.1073/pnas.83.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Mueller A. L., Kunkel D. D., Schwartzkroin P. A. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987 Jul;7(7):1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille J. C., Williams S. Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience. 1990;36(2):349–359. doi: 10.1016/0306-4522(90)90431-3. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A., Perkel D. J. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991 Jan;11(1):23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987 Aug;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch. 1987 Dec;410(6):614–622. doi: 10.1007/BF00581321. [DOI] [PubMed] [Google Scholar]
- McBain C. J. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol. 1994 Dec;72(6):2853–2863. doi: 10.1152/jn.1994.72.6.2853. [DOI] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P., Storm J. F. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993 Dec;11(6):1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989 Feb;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988 Nov 24;336(6197):379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Buzsáki G. Computer simulation of carbachol-driven rhythmic population oscillations in the CA3 region of the in vitro rat hippocampus. J Physiol. 1992;451:653–672. doi: 10.1113/jphysiol.1992.sp019184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Krnjević K. Apamin depresses selectively the after-hyperpolarization of cat spinal motoneurons. Neurosci Lett. 1987 Feb 10;74(1):58–62. doi: 10.1016/0304-3940(87)90051-6. [DOI] [PubMed] [Google Scholar]
- Zhang L., McBain C. J. Voltage-gated potassium currents in stratum oriens-alveus inhibitory neurones of the rat CA1 hippocampus. J Physiol. 1995 Nov 1;488(Pt 3):647–660. doi: 10.1113/jphysiol.1995.sp020997. [DOI] [PMC free article] [PubMed] [Google Scholar]



