Abstract
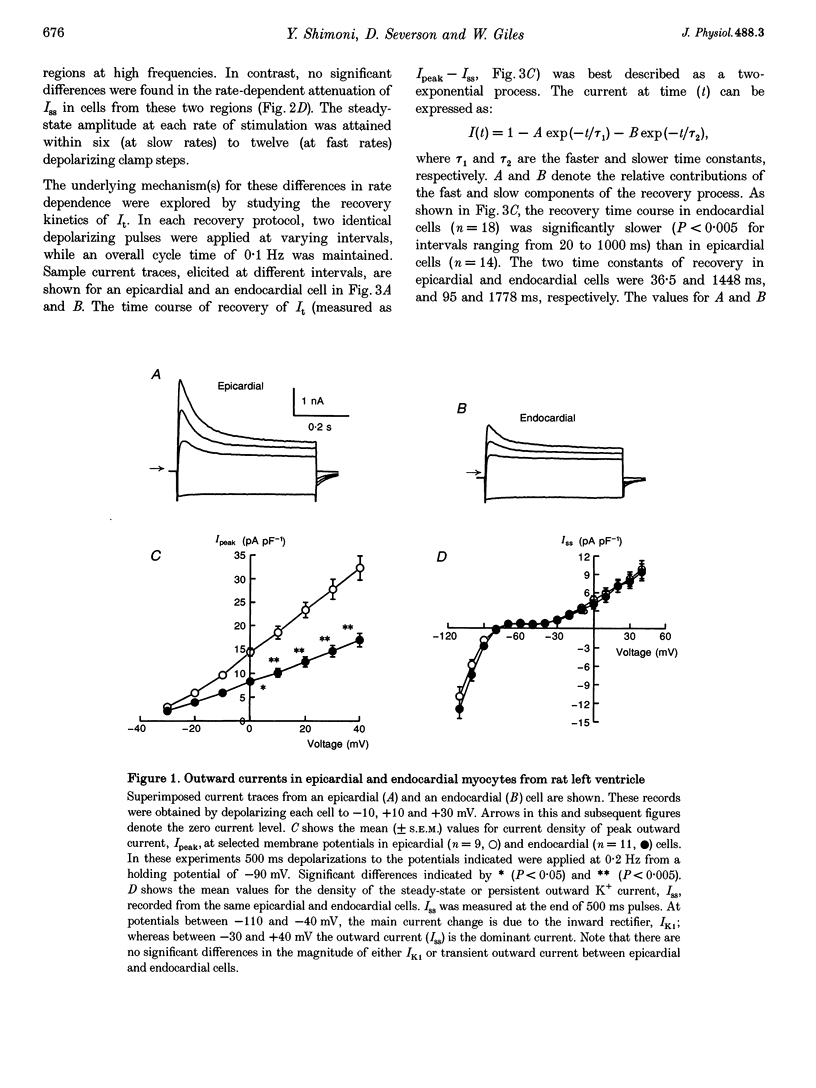
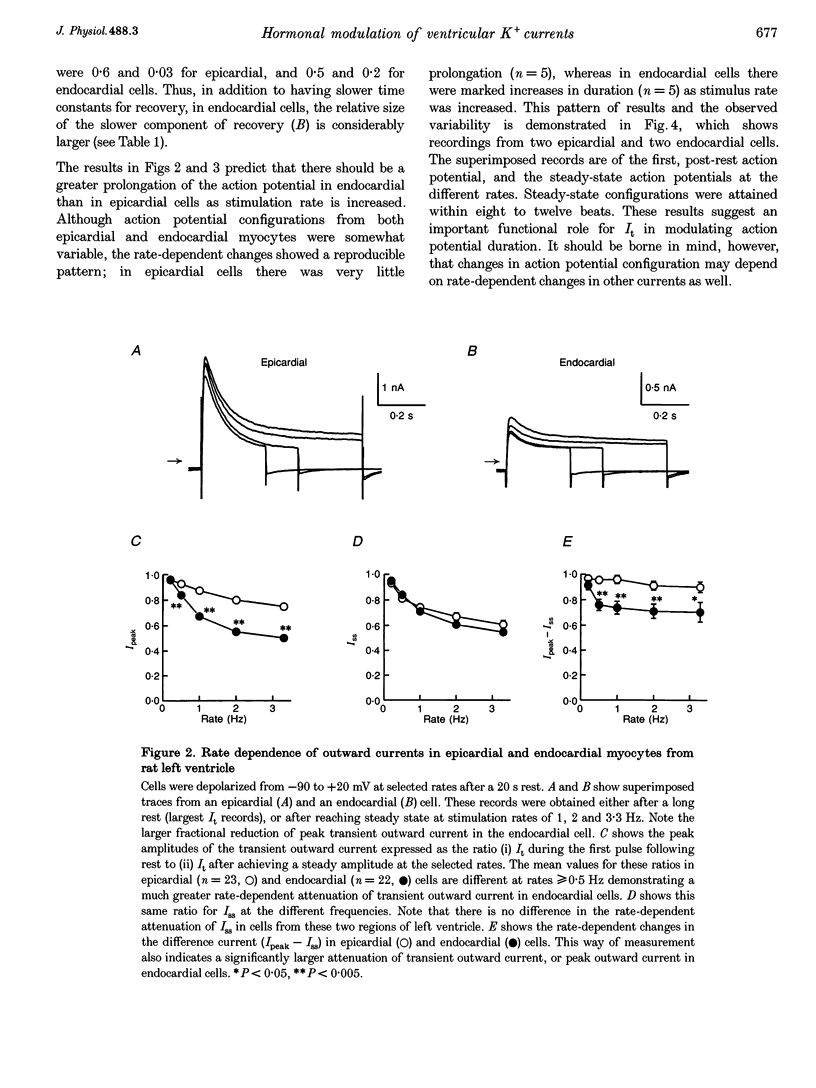
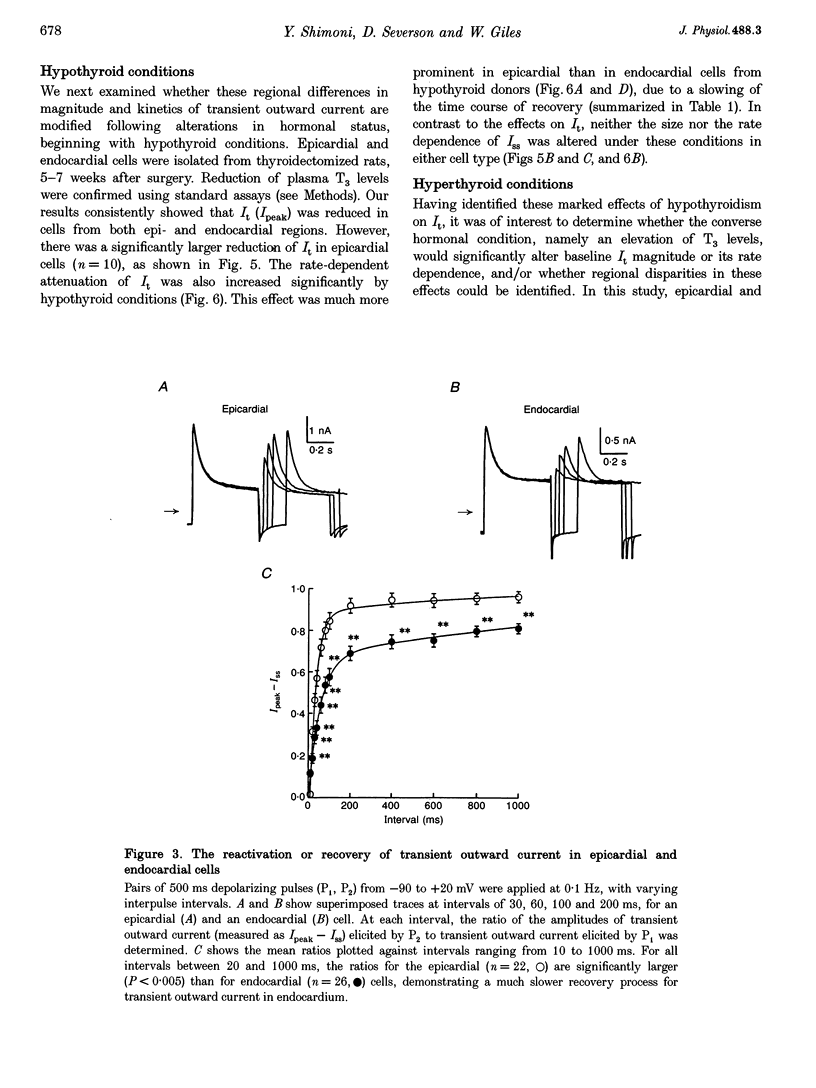
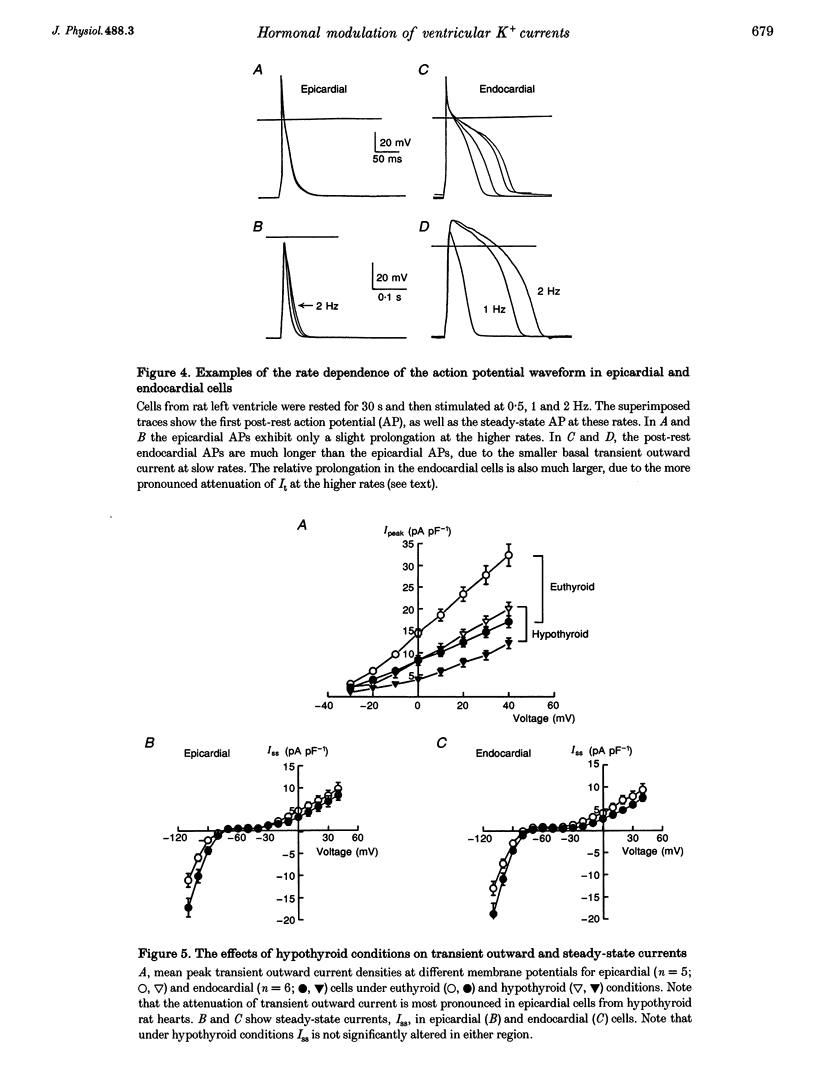
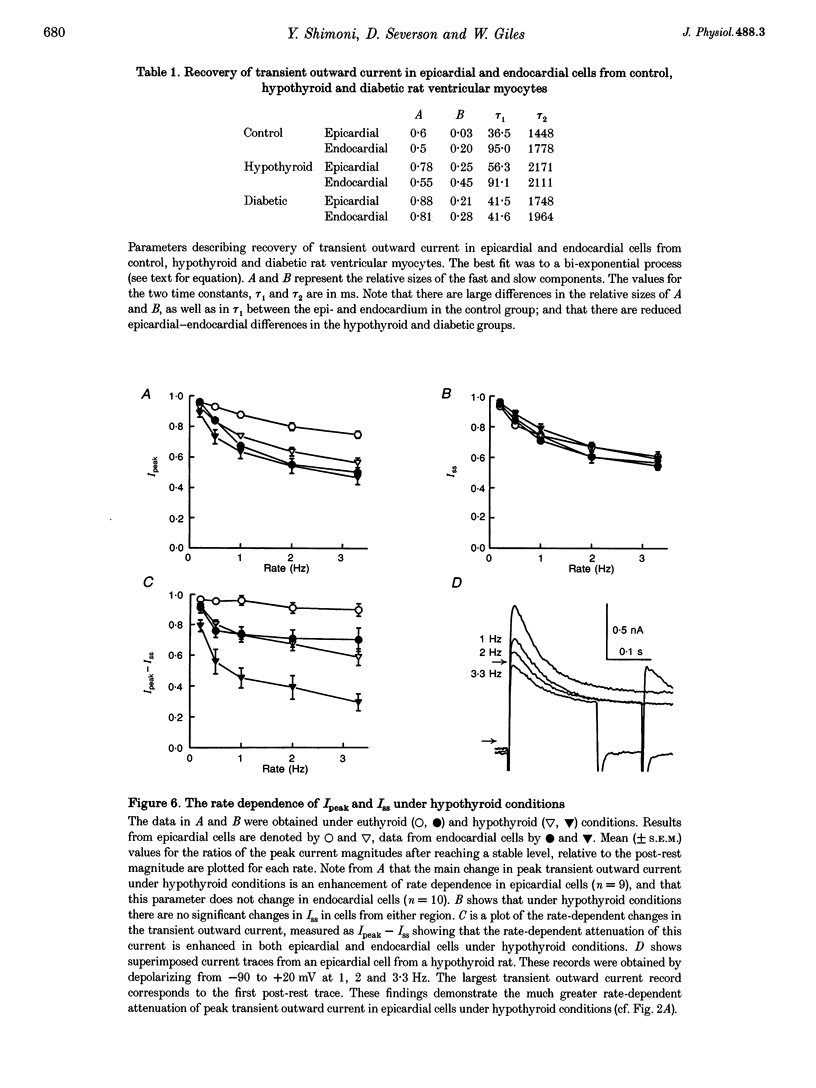
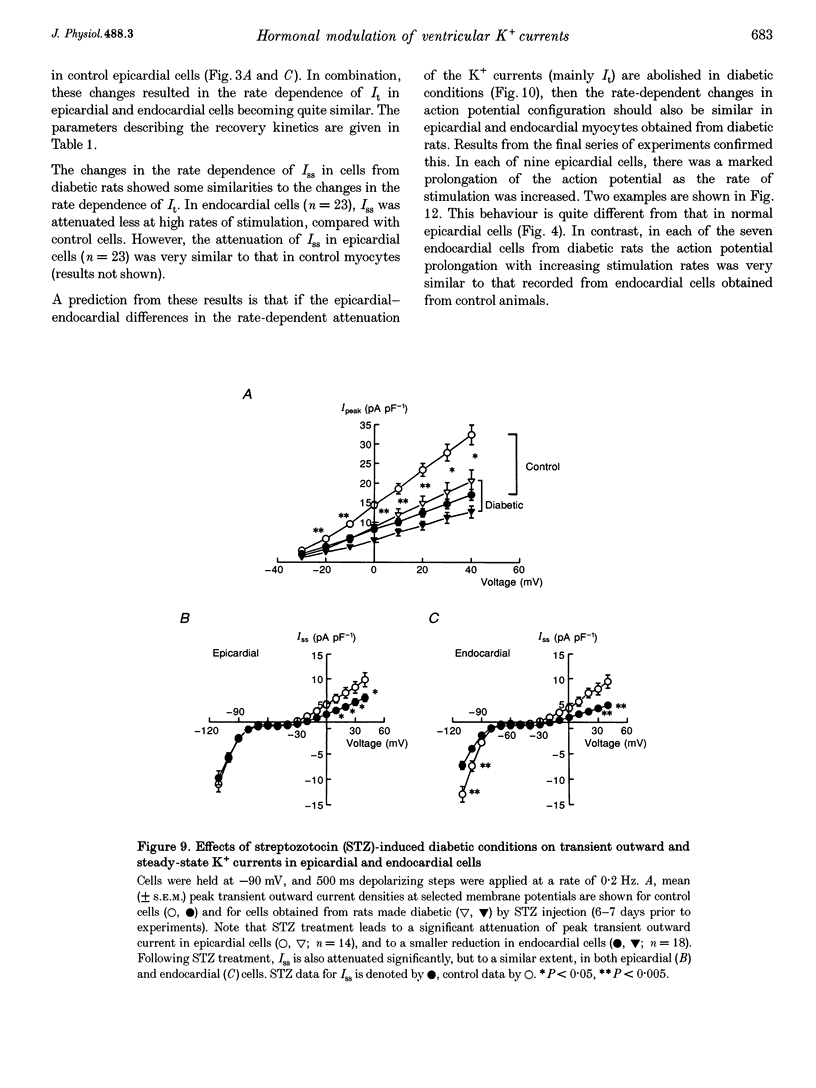
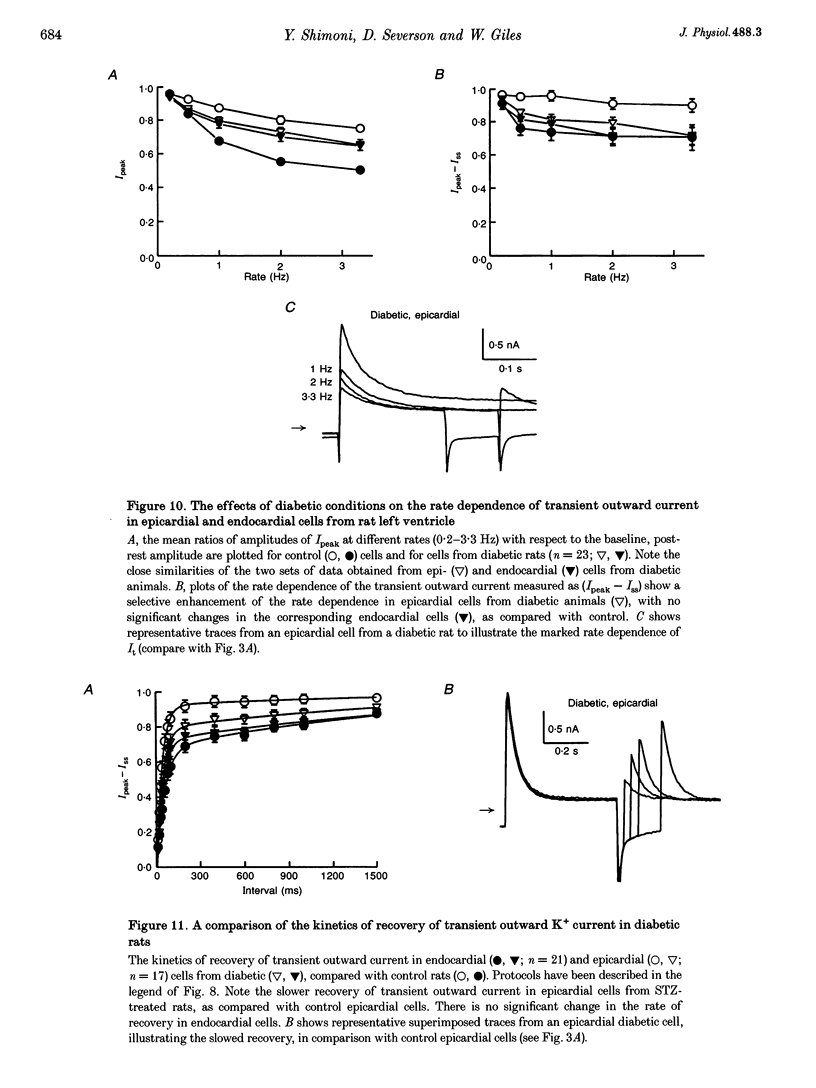
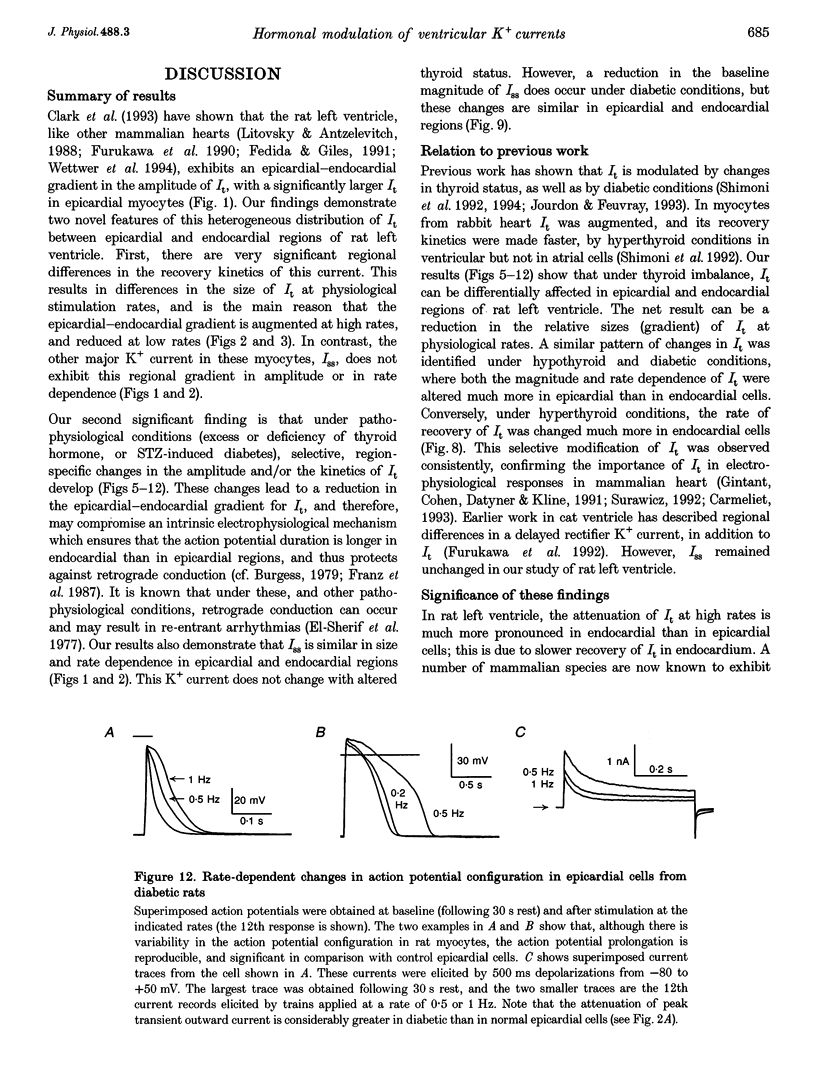
1. The rate dependence and recovery kinetics of the Ca(2+)-independent transient (I(t)) and steady-state or 'pedestal' (Iss) outward potassium (K+) currents were studied in single myocytes isolated from epicardial and endocardial regions of rat left ventricles. The whole-cell, suction microelectrode method was used to measure baseline (fully reactivated) I(t), as well as its rate-dependent attenuation. Results from a group of control animals were compared with data from three other groups having an experimentally altered hormonal status. 2. I(t) was significantly smaller in endocardial cells than in epicardial cells, in part due to a very large difference in the recovery kinetics of this current in endocardial cells. This was reflected in a pronounced rate-dependent prolongation of endocardial action potentials. In contrast, the non-inactivating 'pedestal' current, Iss, was very similar in magnitude and showed comparable rate dependence in cells from both epicardium and endocardium. 3. Changing the thyroid status had selective, differential actions on the amplitude and rate dependence of It in epicardial and endocardial cells. Under hypothyroid conditions there was a more pronounced reduction of baseline I(t) in epicardial than in endocardial cells. Moreover, a slowing of the recovery kinetics in epicardial cells resulted in an enhanced attenuation of this current at high rates. Changing thyroid status had no effect on the magnitude or rate dependence of Iss in cells from either region of the left ventricle. 4. Following establishment of hyperthyroid conditions, there was no significant change in I(t) magnitude at baseline. However, when compared with control data, the recovery of I(t) was considerably faster in endocardial cells, and marginally faster in epicardial cells. 5. Streptozotocin-induced diabetic conditions resulted in a much greater attenuation of I(t) in epicardial cells than in endocardial cells. Epicardial action potentials in these conditions showed prominent rate-dependent prolongation. Iss was reduced to a similar extent in cells from these two regions. 6. Our findings demonstrate that altered hormonal status can selectively change the amplitude and kinetics of It in the epi- and endocardium of rat left ventricle. These changes can reduce the epicardial-endocardial gradients in the magnitude and recovery kinetics of It and hence diminish the intrinsic differences in both action potential duration and refractoriness.
Full text
PDF


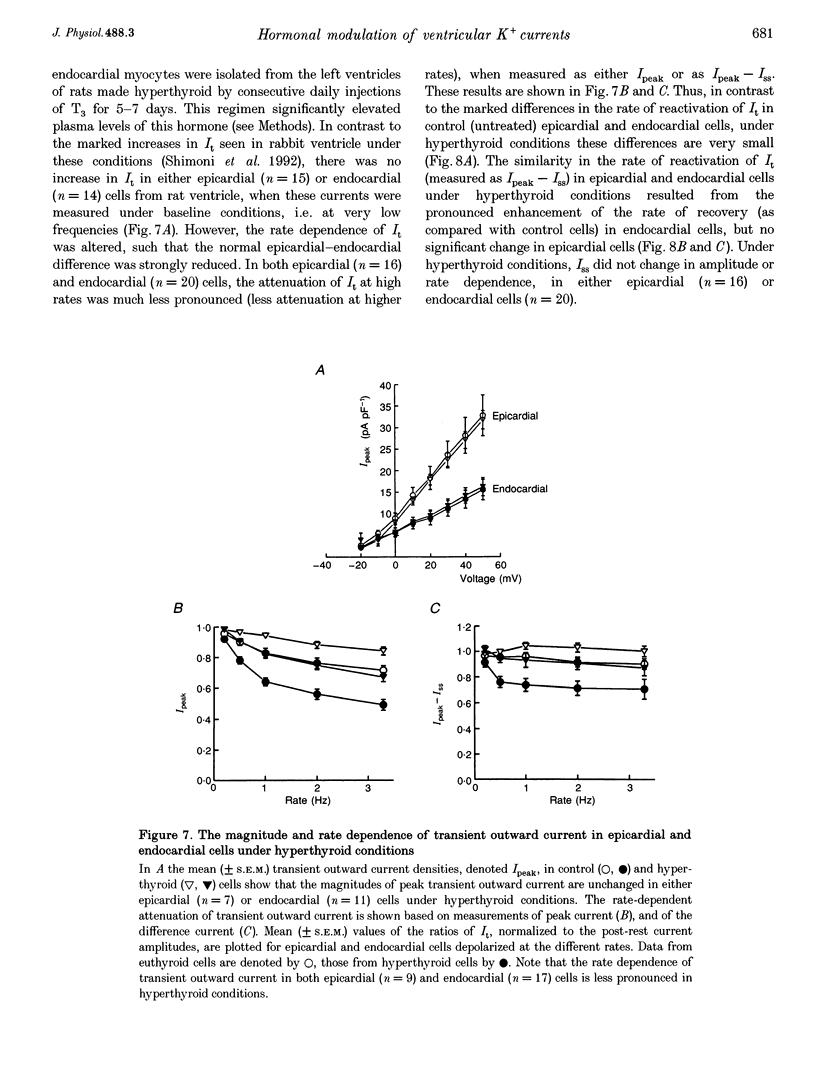
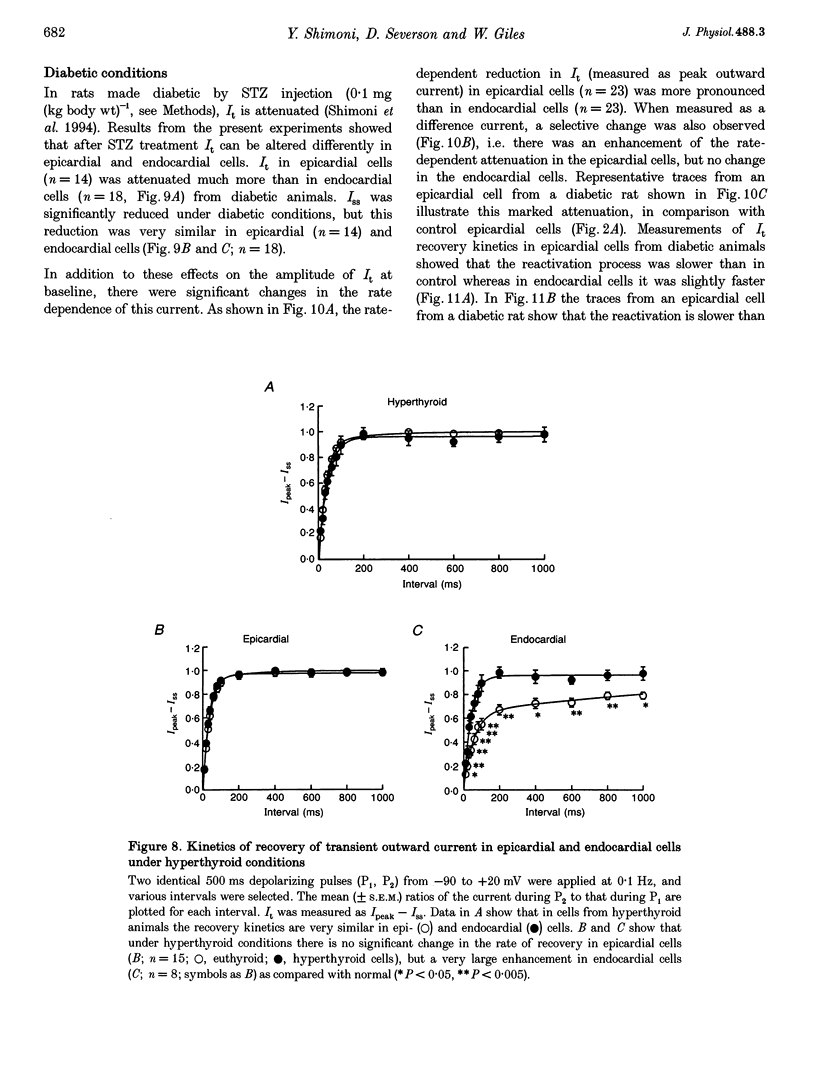



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airaksinen K. E. Electrocardiogram of young diabetic subjects. Ann Clin Res. 1985;17(4):135–138. [PubMed] [Google Scholar]
- Antzelevitch C., Sicouri S., Litovsky S. H., Lukas A., Krishnan S. C., Di Diego J. M., Gintant G. A., Liu D. W. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991 Dec;69(6):1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Apkon M., Nerbonne J. M. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991 May;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993 Aug;73(2):379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Bouchard R. A., Clark R. B., Giles W. R. Role of sodium-calcium exchange in activation of contraction in rat ventricle. J Physiol. 1993 Dec;472:391–413. doi: 10.1113/jphysiol.1993.sp019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Burgess M. J. Relation of ventricular repolarization to electrocardiographic T wave-form and arrhythmia vulnerability. Am J Physiol. 1979 Mar;236(3):H391–H402. doi: 10.1152/ajpheart.1979.236.3.H391. [DOI] [PubMed] [Google Scholar]
- Bénitah J. P., Gomez A. M., Bailly P., Da Ponte J. P., Berson G., Delgado C., Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. J Physiol. 1993 Sep;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Qu Y., Rasmusson R. L., Strauss H. C. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. II. Closed state reverse use-dependent block by 4-aminopyridine. J Gen Physiol. 1993 Apr;101(4):603–626. doi: 10.1085/jgp.101.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Mechanisms and control of repolarization. Eur Heart J. 1993 Nov;14 (Suppl H):3–13. doi: 10.1093/eurheartj/14.suppl_h.3. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Bouchard R. A., Salinas-Stefanon E., Sanchez-Chapula J., Giles W. R. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res. 1993 Oct;27(10):1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Giles W. R., Imaizumi Y. Properties of the transient outward current in rabbit atrial cells. J Physiol. 1988 Nov;405:147–168. doi: 10.1113/jphysiol.1988.sp017326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Giles W., Noble D. Cellular basis for the T wave of the electrocardiogram. Nature. 1976 Aug 19;262(5570):657–661. doi: 10.1038/262657a0. [DOI] [PubMed] [Google Scholar]
- El-Sherif N., Hope R. R., Scherlag B. J., Lazzara R. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 2. Patterns of initiation and termination of re-entry. Circulation. 1977 May;55(5):702–719. doi: 10.1161/01.cir.55.5.702. [DOI] [PubMed] [Google Scholar]
- Fedida D., Giles W. R. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J Physiol. 1991 Oct;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermini B., Wang Z., Duan D., Nattel S. Differences in rate dependence of transient outward current in rabbit and human atrium. Am J Physiol. 1992 Dec;263(6 Pt 2):H1747–H1754. doi: 10.1152/ajpheart.1992.263.6.H1747. [DOI] [PubMed] [Google Scholar]
- Franz M. R., Bargheer K., Rafflenbeul W., Haverich A., Lichtlen P. R. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 1987 Feb;75(2):379–386. doi: 10.1161/01.cir.75.2.379. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Kimura S., Furukawa N., Bassett A. L., Myerburg R. J. Potassium rectifier currents differ in myocytes of endocardial and epicardial origin. Circ Res. 1992 Jan;70(1):91–103. doi: 10.1161/01.res.70.1.91. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Myerburg R. J., Furukawa N., Bassett A. L., Kimura S. Differences in transient outward currents of feline endocardial and epicardial myocytes. Circ Res. 1990 Nov;67(5):1287–1291. doi: 10.1161/01.res.67.5.1287. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Burke R. P., Castle N. A. Characterisation of transient outward current in young human atrial myocytes. Cardiovasc Res. 1995 Jan;29(1):112–117. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jeck C. D., Boyden P. A. Age-related appearance of outward currents may contribute to developmental differences in ventricular repolarization. Circ Res. 1992 Dec;71(6):1390–1403. doi: 10.1161/01.res.71.6.1390. [DOI] [PubMed] [Google Scholar]
- Jourdon P., Feuvray D. Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol. 1993 Oct;470:411–429. doi: 10.1113/jphysiol.1993.sp019866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Bassett A. L., Kohya T., Kozlovskis P. L., Myerburg R. J. Simultaneous recording of action potentials from endocardium and epicardium during ischemia in the isolated cat ventricle: relation of temporal electrophysiologic heterogeneities to arrhythmias. Circulation. 1986 Aug;74(2):401–409. doi: 10.1161/01.cir.74.2.401. [DOI] [PubMed] [Google Scholar]
- Kuo C. S., Munakata K., Reddy C. P., Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983 Jun;67(6):1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988 Jan;62(1):116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Scherlag B. J., Kabell G., Harrison L., Lazzara R. Mechanisms of bradycardia-induced ventricular arrhythmias in myocardial ischemia and infarction. Circulation. 1982 Jun;65(7):1429–1434. doi: 10.1161/01.cir.65.7.1429. [DOI] [PubMed] [Google Scholar]
- Shibata E. F., Drury T., Refsum H., Aldrete V., Giles W. Contributions of a transient outward current to repolarization in human atrium. Am J Physiol. 1989 Dec;257(6 Pt 2):H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Banno H., Clark R. B. Hyperthyroidism selectively modified a transient potassium current in rabbit ventricular and atrial myocytes. J Physiol. 1992 Nov;457:369–389. doi: 10.1113/jphysiol.1992.sp019383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Firek L., Severson D., Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994 Apr;74(4):620–628. doi: 10.1161/01.res.74.4.620. [DOI] [PubMed] [Google Scholar]
- Surawicz B. Role of potassium channels in cycle length dependent regulation of action potential duration in mammalian cardiac Purkinje and ventricular muscle fibres. Cardiovasc Res. 1992 Nov;26(11):1021–1029. doi: 10.1093/cvr/26.11.1021. [DOI] [PubMed] [Google Scholar]
- Thierfelder S., Hirche H., Benndorf K. Anoxia decreases the transient K+ outward current in isolated ventricular heart cells of the mouse. Pflugers Arch. 1994 Jul;427(5-6):547–549. doi: 10.1007/BF00374273. [DOI] [PubMed] [Google Scholar]
- Tomita F., Bassett A. L., Myerburg R. J., Kimura S. Diminished transient outward currents in rat hypertrophied ventricular myocytes. Circ Res. 1994 Aug;75(2):296–303. doi: 10.1161/01.res.75.2.296. [DOI] [PubMed] [Google Scholar]
- Wang Z. G., Fermini B., Nattel S. Repolarization differences between guinea pig atrial endocardium and epicardium: evidence for a role of Ito. Am J Physiol. 1991 May;260(5 Pt 2):H1501–H1506. doi: 10.1152/ajpheart.1991.260.5.H1501. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Rautaharju P. M., McDonald T. F. Ventricular action potentials, ventricular extracellular potentials, and the ECG of guinea pig. Circ Res. 1985 Sep;57(3):362–373. doi: 10.1161/01.res.57.3.362. [DOI] [PubMed] [Google Scholar]
- Wettwer E., Amos G. J., Posival H., Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994 Sep;75(3):473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Xu X. P., Best P. M. Decreased transient outward K+ current in ventricular myocytes from acromegalic rats. Am J Physiol. 1991 Mar;260(3 Pt 2):H935–H942. doi: 10.1152/ajpheart.1991.260.3.H935. [DOI] [PubMed] [Google Scholar]
- von Olshausen K., Bischoff S., Kahaly G., Mohr-Kahaly S., Erbel R., Beyer J., Meyer J. Cardiac arrhythmias and heart rate in hyperthyroidism. Am J Cardiol. 1989 Apr 15;63(13):930–933. doi: 10.1016/0002-9149(89)90142-2. [DOI] [PubMed] [Google Scholar]



