Abstract
1. The dependence of force (F) on length (L) in smooth muscle remains uncertain since (i) it is influenced by changes in activation (myosin light chain phosphorylation), (ii) no anatomical reference length for the contractile unit is available, (iii) the length at which optimum force is generated (L(o)) exhibits a broad, flat optimum, and (iv) the presence of an extensive connective tissue network makes it difficult to stretch tissues without damage. 2. A swine carotid medial ring preparation prepared by removal of the adventitia and endothelium could be stretched to 1.8 L(o) without decreasing active force generation on return to shorter lengths. 3. A highly reproducible mechanically defined reference length, L(o), was obtained by fitting force-length data between 0.3 and 1.6 L(o) with a third-order polynomial where L = L(o) when dF/dL = 0. 4. Activation as assessed by myosin regulatory light chain (MRLC) phosphorylation increased with length in 100 microM histamine-stimulated tissues from 0.6 to 1.8 L(o). 5. Activation was constant in K(+)-depolarized and field-stimulated tissues from 1.0 to 1.8 L(o) allowing determination of the descending limb of the force-length relation to be assessed independently of activation. 6. The slope of the descending limb of the force-length relation was linear except at very long lengths, which often produced tissue damage. The slope was not statistically different from that estimated for sarcomeres in vertebrate skeletal muscle. 7. The medial ring preparation and the procedures used to define the reference length provide advantages for the measurement of length-dependent variables.
Full text
PDF

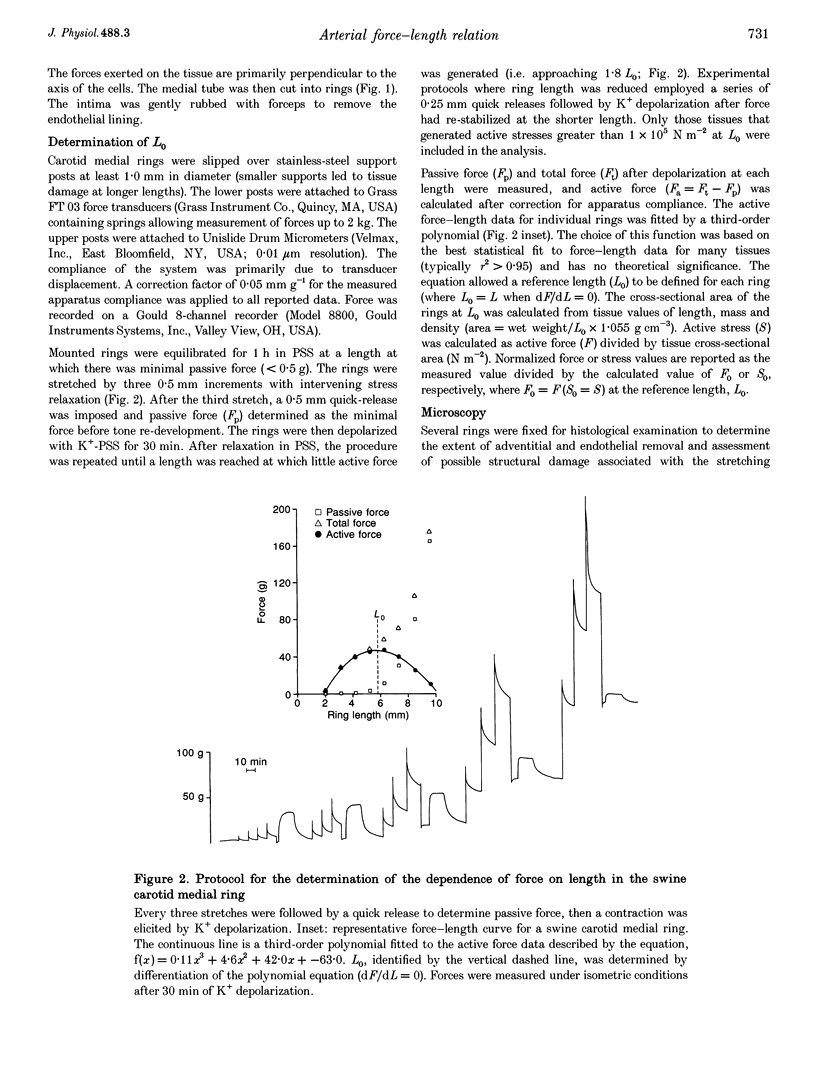








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis M. J., Gore R. W. Length-tension relationship of vascular smooth muscle in single arterioles. Am J Physiol. 1989 Mar;256(3 Pt 2):H630–H640. doi: 10.1152/ajpheart.1989.256.3.H630. [DOI] [PubMed] [Google Scholar]
- Di Blasi P., Van Riper D., Kaiser R., Rembold C. M., Murphy R. A. Steady-state dependence of stress on cross-bridge phosphorylation in the swine carotid media. Am J Physiol. 1992 Jun;262(6 Pt 1):C1388–C1391. doi: 10.1152/ajpcell.1992.262.6.C1388. [DOI] [PubMed] [Google Scholar]
- Driska S. P., Aksoy M. O., Murphy R. A. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol. 1981 May;240(5):C222–C233. doi: 10.1152/ajpcell.1981.240.5.C222. [DOI] [PubMed] [Google Scholar]
- Gaylinn B. D., Eddinger T. J., Martino P. A., Monical P. L., Hunt D. F., Murphy R. A. Expression of nonmuscle myosin heavy and light chains in smooth muscle. Am J Physiol. 1989 Nov;257(5 Pt 1):C997–1004. doi: 10.1152/ajpcell.1989.257.5.C997. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. R., Siegman M. J. Mechanical properties of smooth muscle. I. Length-tension and force-velocity relations. Am J Physiol. 1971 Nov;221(5):1243–1249. doi: 10.1152/ajplegacy.1971.221.5.1243. [DOI] [PubMed] [Google Scholar]
- Gunst S. J. Effect of length history on contractile behavior of canine tracheal smooth muscle. Am J Physiol. 1986 Jan;250(1 Pt 1):C146–C154. doi: 10.1152/ajpcell.1986.250.1.C146. [DOI] [PubMed] [Google Scholar]
- Gunst S. J. Effects of muscle length and load on intracellular Ca2+ in tracheal smooth muscle. Am J Physiol. 1989 Apr;256(4 Pt 1):C807–C812. doi: 10.1152/ajpcell.1989.256.4.C807. [DOI] [PubMed] [Google Scholar]
- Hai C. M. Length-dependent myosin phosphorylation and contraction of arterial smooth muscle. Pflugers Arch. 1991 Jul;418(6):564–571. doi: 10.1007/BF00370572. [DOI] [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Force-velocity and series elastic characteristics of smooth muscle from the hog carotid artery. Circ Res. 1974 Apr;34(4):461–466. doi: 10.1161/01.res.34.4.461. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973 Sep;33(3):275–283. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Murphy R. A. Velocity and myosin phosphorylation transients in arterial smooth muscle: effects of agonist diffusion. Experientia. 1985 Aug 15;41(8):1010–1017. doi: 10.1007/BF01952123. [DOI] [PubMed] [Google Scholar]
- Komuro T., Desaki J., Uehara Y. Three-dimensional organization of smooth muscle cells in blood vessels of laboratory rodents. Cell Tissue Res. 1982;227(2):429–437. doi: 10.1007/BF00210897. [DOI] [PubMed] [Google Scholar]
- Lowy J., Small J. V. The organization of myosin and actin in vertebrate smooth muscle. Nature. 1970 Jul 4;227(5253):46–51. doi: 10.1038/227046a0. [DOI] [PubMed] [Google Scholar]
- Malmqvist U., Arner A., Uvelius B. Mechanics and Ca(2+)-sensitivity of human detrusor muscle bundles studied in vitro. Acta Physiol Scand. 1991 Dec;143(4):373–380. doi: 10.1111/j.1748-1716.1991.tb09248.x. [DOI] [PubMed] [Google Scholar]
- Martyn D. A., Coby R., Huntsman L. L., Gordon A. M. Force-calcium relations in skinned twitch and slow-tonic frog muscle fibres have similar sarcomere length dependencies. J Muscle Res Cell Motil. 1993 Feb;14(1):65–75. doi: 10.1007/BF00132181. [DOI] [PubMed] [Google Scholar]
- Meiss R. A. Limits to shortening in smooth muscle tissues. J Muscle Res Cell Motil. 1992 Apr;13(2):190–198. doi: 10.1007/BF01874156. [DOI] [PubMed] [Google Scholar]
- Moreland R. S., Moreland S., Murphy R. A. Dependence of stress on length, Ca2+, and myosin phosphorylation in skinned smooth muscle. Am J Physiol. 1988 Oct;255(4 Pt 1):C473–C478. doi: 10.1152/ajpcell.1988.255.4.C473. [DOI] [PubMed] [Google Scholar]
- Mulvany M. J., Warshaw D. M. The active tension-length curve of vascular smooth muscle related to its cellular components. J Gen Physiol. 1979 Jul;74(1):85–104. doi: 10.1085/jgp.74.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. A. What is special about smooth muscle? The significance of covalent crossbridge regulation. FASEB J. 1994 Mar 1;8(3):311–318. doi: 10.1096/fasebj.8.3.8143937. [DOI] [PubMed] [Google Scholar]
- Obara K., Kunimoto M., Ito Y., Yabu H. Relationship between length-tension relation and 20,000 dalton myosin light chain phosphorylation in guinea-pig taenia caeci. Comp Biochem Physiol A Comp Physiol. 1987;87(2):503–508. doi: 10.1016/0300-9629(87)90158-7. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Paul R. J. Effects of initial length and active shortening on vascular smooth muscle contractility. Am J Physiol. 1974 Nov;227(5):1019–1024. doi: 10.1152/ajplegacy.1974.227.5.1019. [DOI] [PubMed] [Google Scholar]
- Price J. M., Davis D. L., Knauss E. B. Length-dependent sensitivity at lengths greater than Lmax in vascular smooth muscle. Am J Physiol. 1983 Sep;245(3):H379–H384. doi: 10.1152/ajpheart.1983.245.3.H379. [DOI] [PubMed] [Google Scholar]
- Price J. M., Davis D. L., Knauss E. B. Length-dependent sensitivity in vascular smooth muscle. Am J Physiol. 1981 Oct;241(4):H557–H563. doi: 10.1152/ajpheart.1981.241.4.H557. [DOI] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Muscle length, shortening, myoplasmic [Ca2+], and activation of arterial smooth muscle. Circ Res. 1990 May;66(5):1354–1361. doi: 10.1161/01.res.66.5.1354. [DOI] [PubMed] [Google Scholar]
- Rice R. V., Moses J. A., McManus G. M., Brady A. C., Blasik L. M. The organization of contractile filaments in a mammalian smooth muscle. J Cell Biol. 1970 Oct;47(1):183–196. doi: 10.1083/jcb.47.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegman M. J., Butler T. M., Mooers S. U., Davies R. E. Calcium-dependent resistance to stretch and stress relaxation in resting smooth muscles. Am J Physiol. 1976 Nov;231(5 Pt 1):1501–1508. doi: 10.1152/ajplegacy.1976.231.5.1501. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R. Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Muscle Res Cell Motil. 1984 Jun;5(3):243–272. doi: 10.1007/BF00713107. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Gallagher P. J., Herring B. P., Kamm K. E. Vascular smooth muscle contractile elements. Cellular regulation. Hypertension. 1991 Jun;17(6 Pt 1):723–732. doi: 10.1161/01.hyp.17.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw D. M., Fay F. S. Cross-bridge elasticity in single smooth muscle cells. J Gen Physiol. 1983 Aug;82(2):157–199. doi: 10.1085/jgp.82.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabau R. J., Wang M. B., Dorst C. L., Ryan J. P. Effect of muscle length on isometric stress and myosin light chain phosphorylation in gallbladder smooth muscle. Am J Physiol. 1991 Jun;260(6 Pt 1):G920–G924. doi: 10.1152/ajpgi.1991.260.6.G920. [DOI] [PubMed] [Google Scholar]
- Wingard C. J., Paul R. J., Murphy R. A. Dependence of ATP consumption on cross-bridge phosphorylation in swine carotid smooth muscle. J Physiol. 1994 Nov 15;481(Pt 1):111–117. doi: 10.1113/jphysiol.1994.sp020422. [DOI] [PMC free article] [PubMed] [Google Scholar]