Abstract
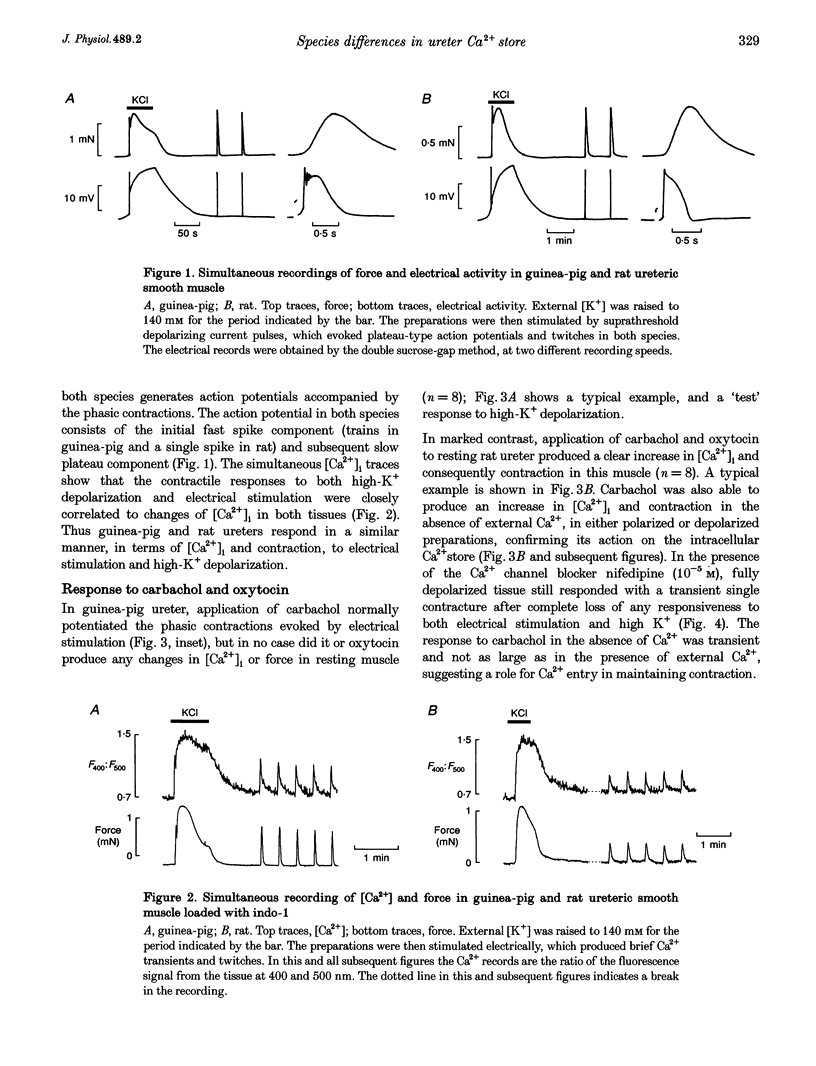
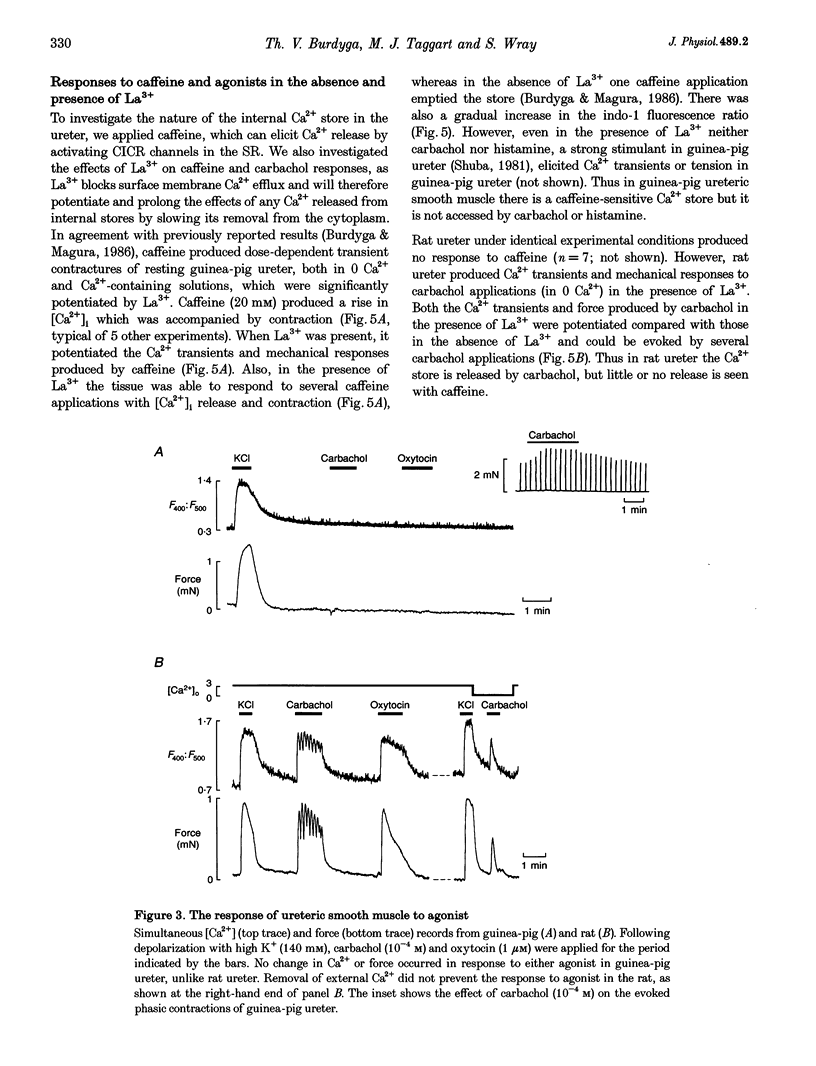
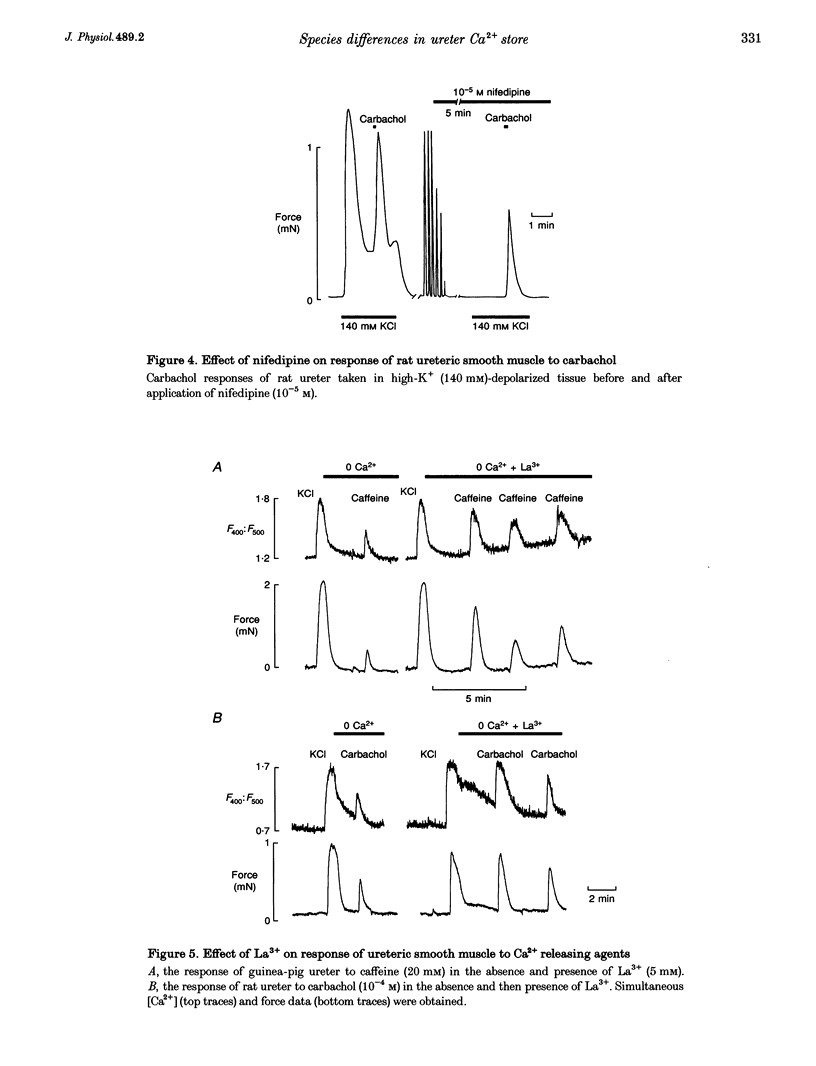
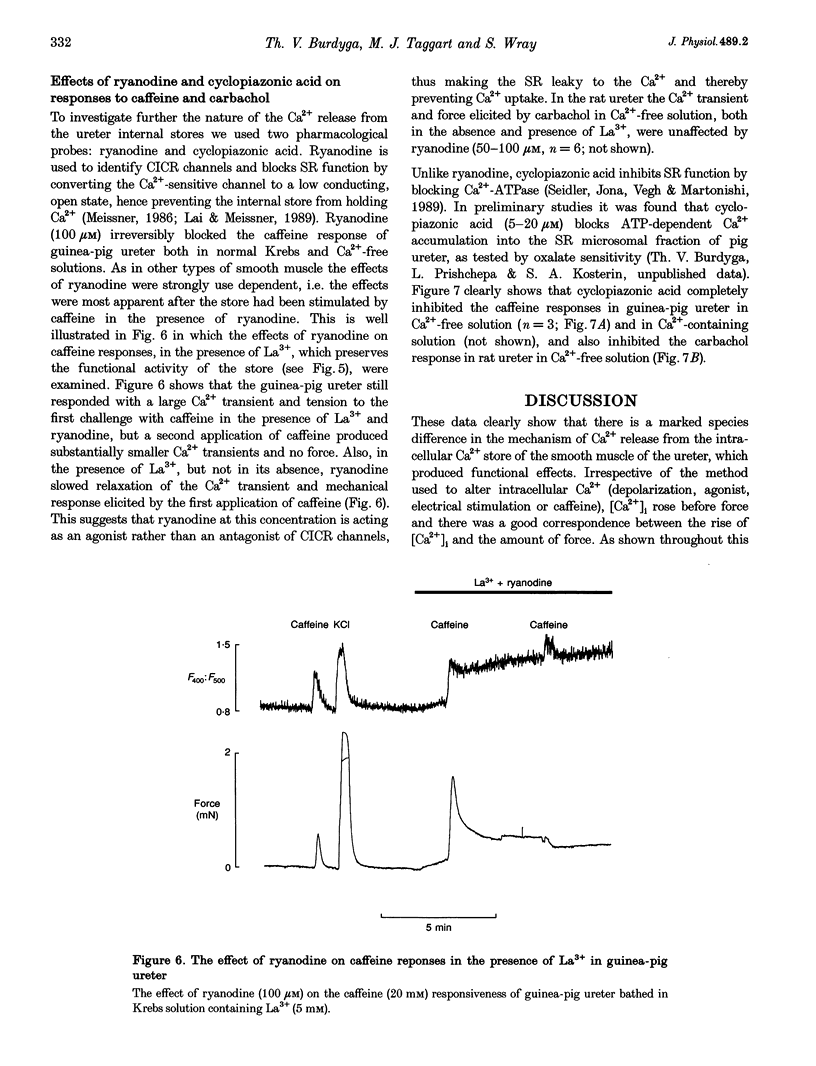
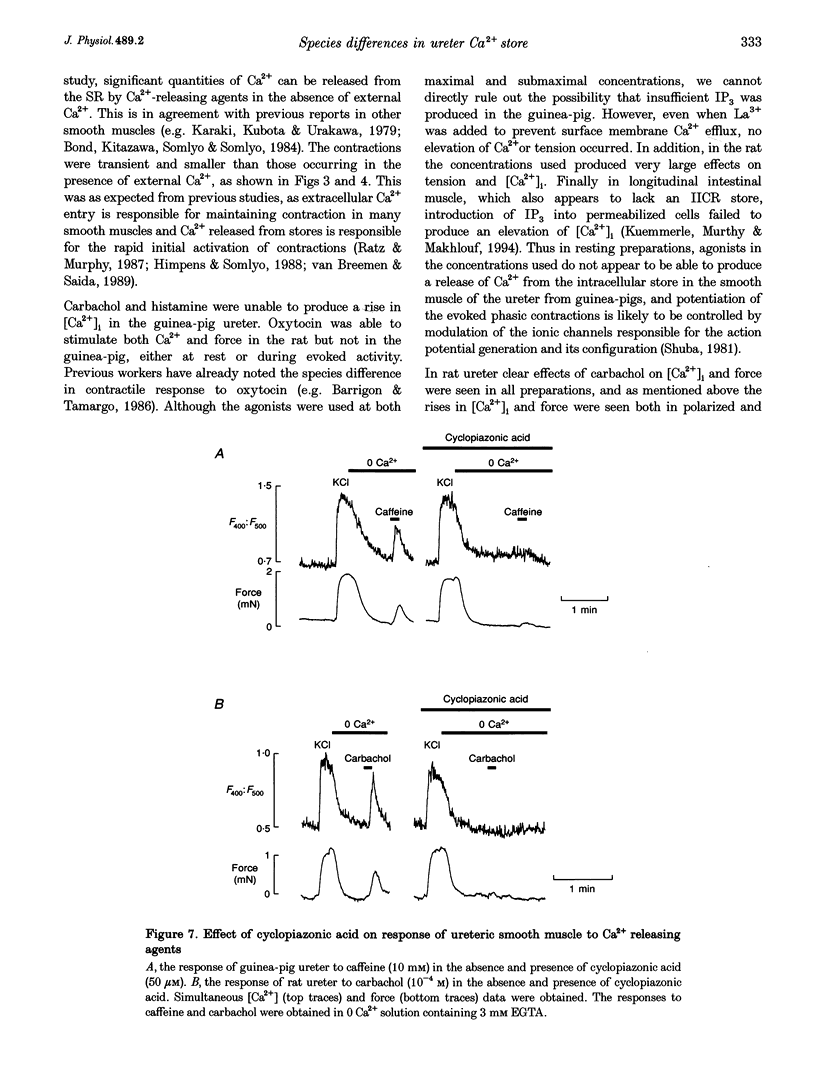
1. We have investigated the internal Ca2+ store and its ability to affect contraction by simultaneously measuring force and Ca2+ in the ureter from guinea-pig and rat. Both species responded in a similar manner to electrical stimulation and depolarization with high-K+, generating plateau-type action potentials and increasing intracellular calcium ([Ca2+]i) and force. 2. In the guinea-pig, carbachol had no effect on [Ca2+]i and force in the resting ureter. In contrast, resting rat ureter always responded with a large [Ca2+]i rise and maintained force to carbachol in Ca(2+)-containing solution, and in Ca(2+)-free solution it showed a transient increase in [Ca2+]i and force. This Ca2+ release and force development was also present in both polarized and high-K(+)-depolarized preparations and was insensitive to nifedipine, suggesting the presence of a receptor-coupled pathway of Ca2+ release in rat ureter. 3. Caffeine was able to produce a release of Ca2+ from the internal store of guinea-pig ureter and elicit contraction. However, rat ureter failed to respond to caffeine. In the presence of La3+, the caffeine response in the guinea-pig ureter and carbachol response in the rat ureter, elicited in Ca(2+)-free solutions, were always increased and prolonged and could be repeatedly evoked, suggesting similarity in Ca2+ uptake behaviour of the store in both species. 4. Ryanodine blocked the caffeine responses of the guinea-pig ureter elicited both in Ca(2+)-containing and Ca(2+)-free solutions, both in the absence and presence of La3+. However, ryanodine failed to prevent the rat ureter responding to carbachol, suggesting that carbachol was releasing Ca2+ from a ryanodine-insensitive channel in the sarcoplasmic reticulum (SR). 5. Cyclopiazonic acid, which inhibits the SR Ca(2+)-ATPase, abolished the effects of both caffeine and carbachol in Ca(2+)-free solutions in guinea-pig and rat, respectively. 6. We conclude that there is a major difference in the mechanisms of Ca2+ release in the internal Ca2+ store of smooth muscle from guinea-pig and rat ureter. The data suggest that the guinea-pig store is purely a calcium-induced calcium release (CICR)-type store and that the rat store is a pure receptor-operated Ca2+ store.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Ito K. Time- and use-dependent inhibition by ryanodine of caffeine-induced contraction of guinea-pig aortic smooth muscle. Biochem Biophys Res Commun. 1988 Jul 15;154(1):219–226. doi: 10.1016/0006-291x(88)90673-0. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Barrigón S., Tamargo J. The effect of oxytocin on contractile responses and 45Ca movements in rat isolated aortic strips. Br J Pharmacol. 1986 Apr;87(4):763–770. doi: 10.1111/j.1476-5381.1986.tb14595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga T. V., Magura I. S. Effects of caffeine on the electrical and mechanical activity of guinea-pig ureter smooth muscle. Gen Physiol Biophys. 1986 Dec;5(6):581–591. [PubMed] [Google Scholar]
- Burdyga T. V., Magura I. S. Effects of temperature and Na0+ on the relaxation of phasic and tonic tension of guinea-pig ureter muscle. Gen Physiol Biophys. 1988 Feb;7(1):3–15. [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. The effects of caffeine on the contraction of the frog heart. J Physiol. 1974 Nov;242(3):589–613. doi: 10.1113/jphysiol.1974.sp010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., van Breemen C. Function of smooth muscle sarcoplasmic reticulum. Adv Second Messenger Phosphoprotein Res. 1992;26:335–350. [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium release in skinned cardiac cells: variations with species, tissues, and development. Fed Proc. 1982 May;41(7):2238–2244. [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T., Dorner J. W., Cole R. J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988 Mar 1;37(5):978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., Somlyo A. P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J Physiol. 1988 Jan;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Iino M., Endo M. Caffeine inhibits Ca(2+)-mediated potentiation of inositol 1,4,5-trisphosphate-induced Ca2+ release in permeabilized vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Jul 30;194(2):726–732. doi: 10.1006/bbrc.1993.1882. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol. 1990 Dec;54(4):345–354. doi: 10.1254/jjp.54.345. [DOI] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Casteels R. Properties of intracellular calcium stores in pregnant rat myometrium. Br J Pharmacol. 1988 Sep;95(1):284–290. doi: 10.1111/j.1476-5381.1988.tb16575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Kuemmerle J. F., Murthy K. S., Makhlouf G. M. Agonist-activated, ryanodine-sensitive, IP3-insensitive Ca2+ release channels in longitudinal muscle of intestine. Am J Physiol. 1994 May;266(5 Pt 1):C1421–C1431. doi: 10.1152/ajpcell.1994.266.5.C1421. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Meissner G. The muscle ryanodine receptor and its intrinsic Ca2+ channel activity. J Bioenerg Biomembr. 1989 Apr;21(2):227–246. doi: 10.1007/BF00812070. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Munro D. D., Wendt I. R. Effects of cyclopiazonic acid on [Ca2+]i and contraction in rat urinary bladder smooth muscle. Cell Calcium. 1994 May;15(5):369–380. doi: 10.1016/0143-4160(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Murphy R. A. Contributions of intracellular and extracellular Ca2+ pools to activation of myosin phosphorylation and stress in swine carotid media. Circ Res. 1987 Mar;60(3):410–421. doi: 10.1161/01.res.60.3.410. [DOI] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Multiple effects of caffeine on contraction and cytosolic free Ca2+ levels in vascular smooth muscle of rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1988 Oct;338(4):443–448. doi: 10.1007/BF00172125. [DOI] [PubMed] [Google Scholar]
- Savineau J. P., Mironneau J. Caffeine acting on pregnant rat myometrium: analysis of its relaxant action and its failure to release Ca2+ from intracellular stores. Br J Pharmacol. 1990 Feb;99(2):261–266. doi: 10.1111/j.1476-5381.1990.tb14691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Shima H., Blaustein M. P. Modulation of evoked contractions in rat arteries by ryanodine, thapsigargin, and cyclopiazonic acid. Circ Res. 1992 May;70(5):968–977. doi: 10.1161/01.res.70.5.968. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Flash photolysis studies of excitation-contraction coupling, regulation, and contraction in smooth muscle. Annu Rev Physiol. 1990;52:857–874. doi: 10.1146/annurev.ph.52.030190.004233. [DOI] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992 May;106(1):208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Mann C., Hu Q., Sturek M. Multiple effects of ryanodine on intracellular free Ca2+ in smooth muscle cells from bovine and porcine coronary artery: modulation of sarcoplasmic reticulum function. Br J Pharmacol. 1992 Apr;105(4):903–911. doi: 10.1111/j.1476-5381.1992.tb09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J., Karibe A., Horiguchi S., Keitoku M., Satoh S., Takishima T., Shirato K. Modification of myogenic intrinsic tone and [Ca2+]i of rat isolated arterioles by ryanodine and cyclopiazonic acid. Circ Res. 1993 Sep;73(3):465–472. doi: 10.1161/01.res.73.3.465. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


