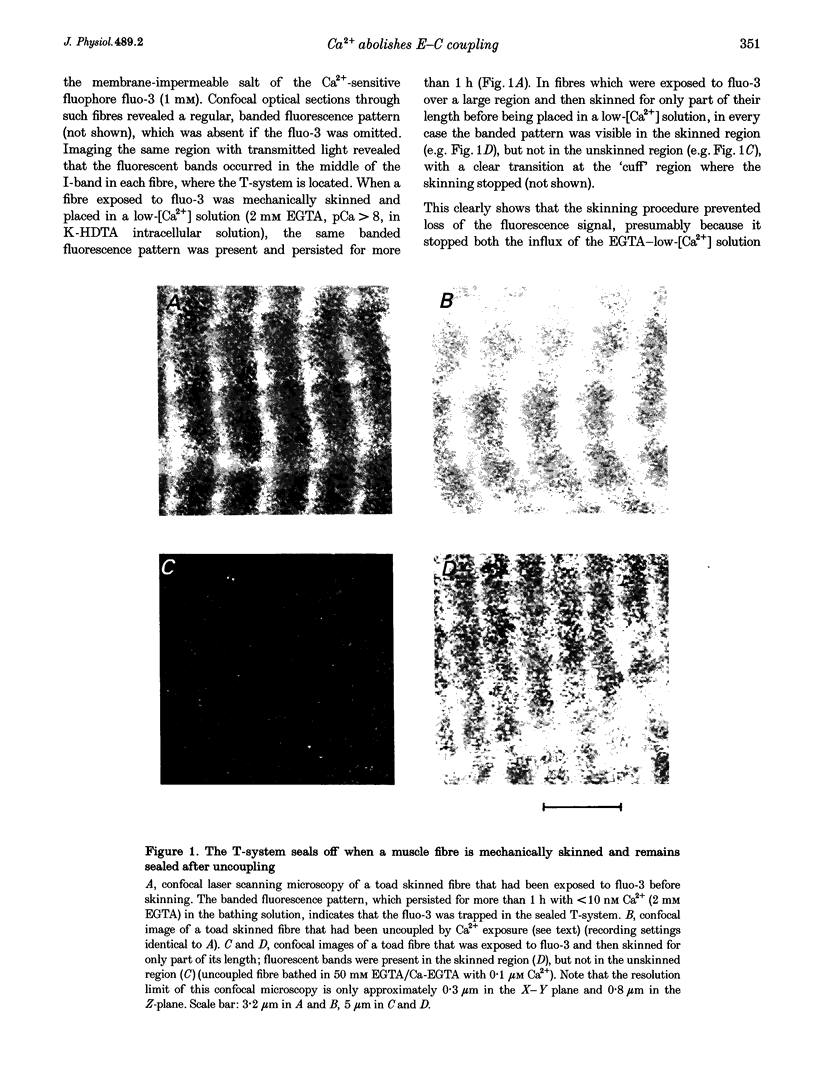
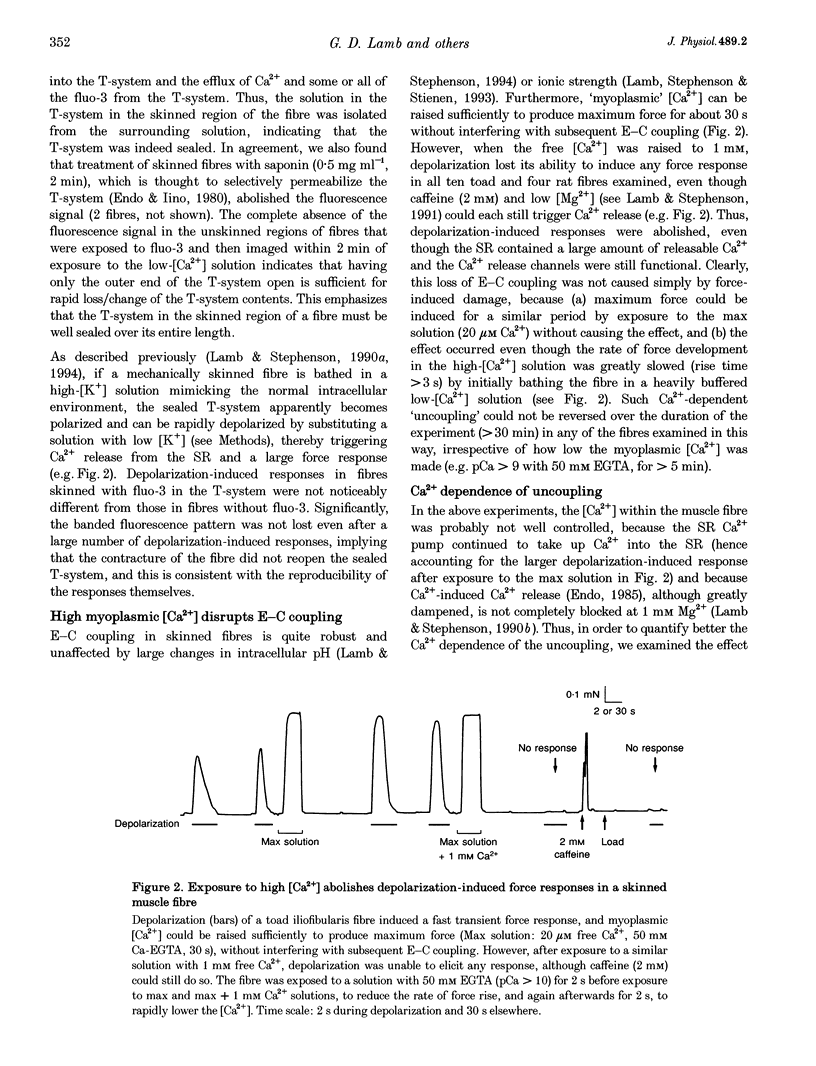
Abstract
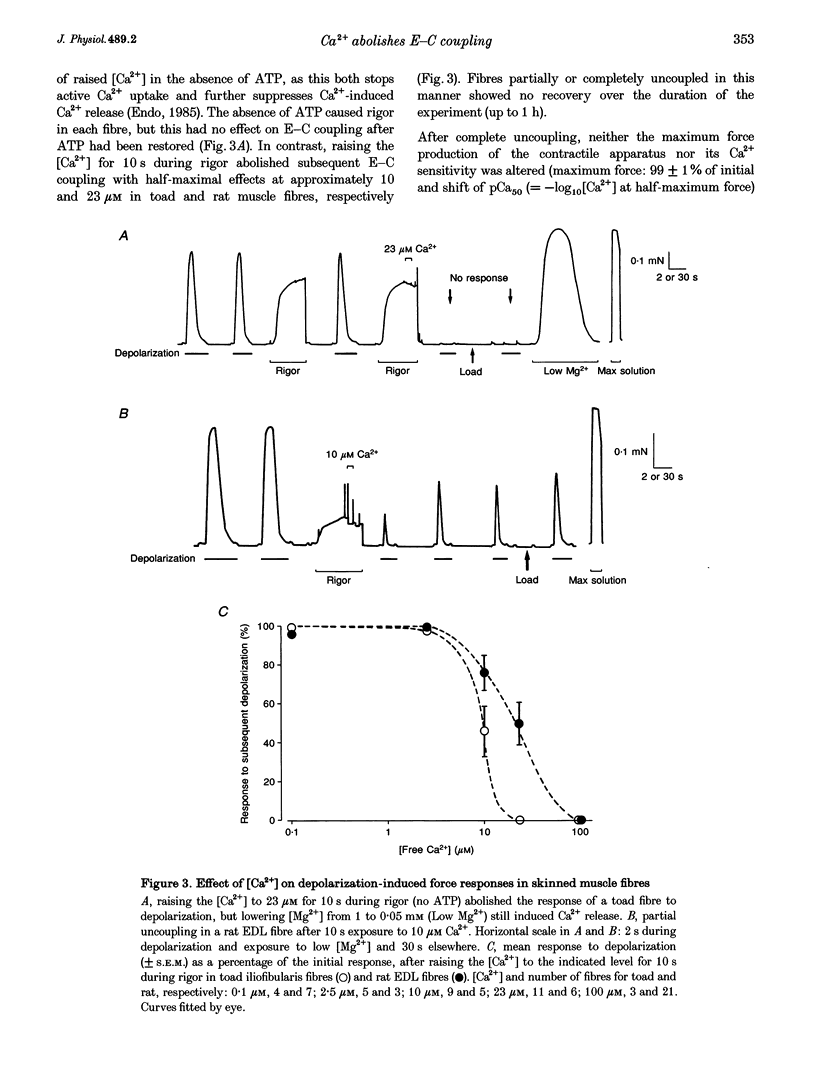
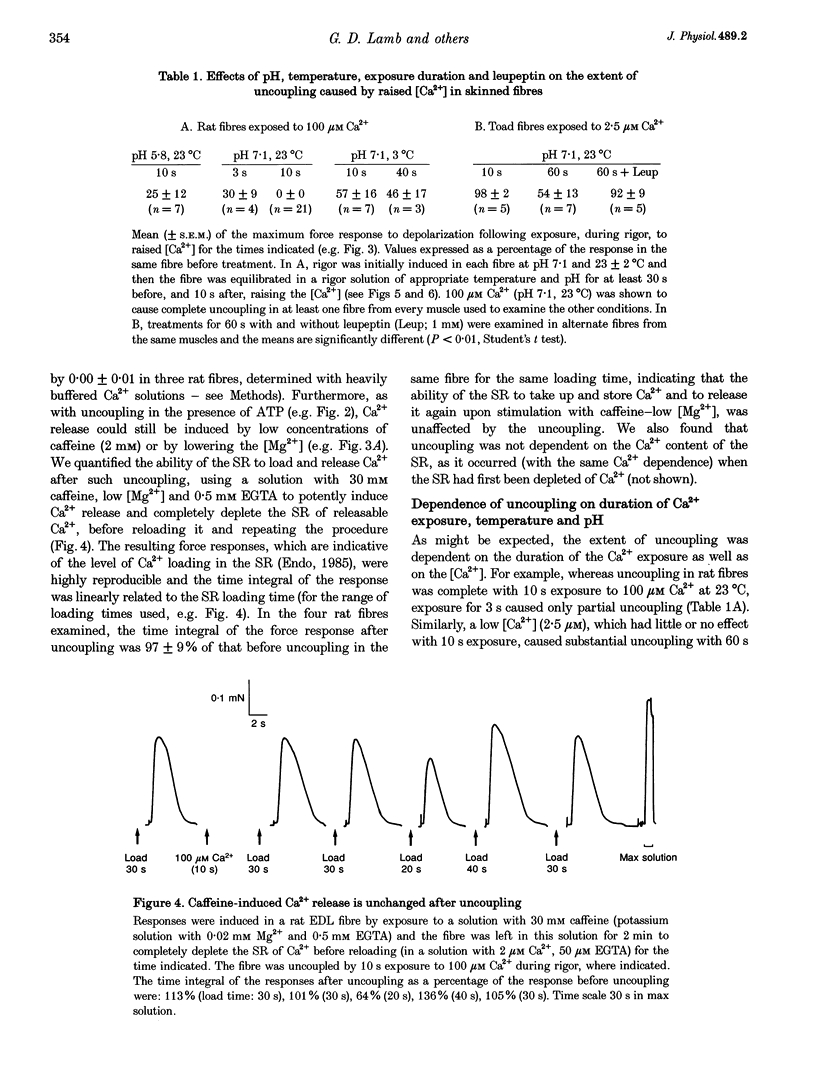
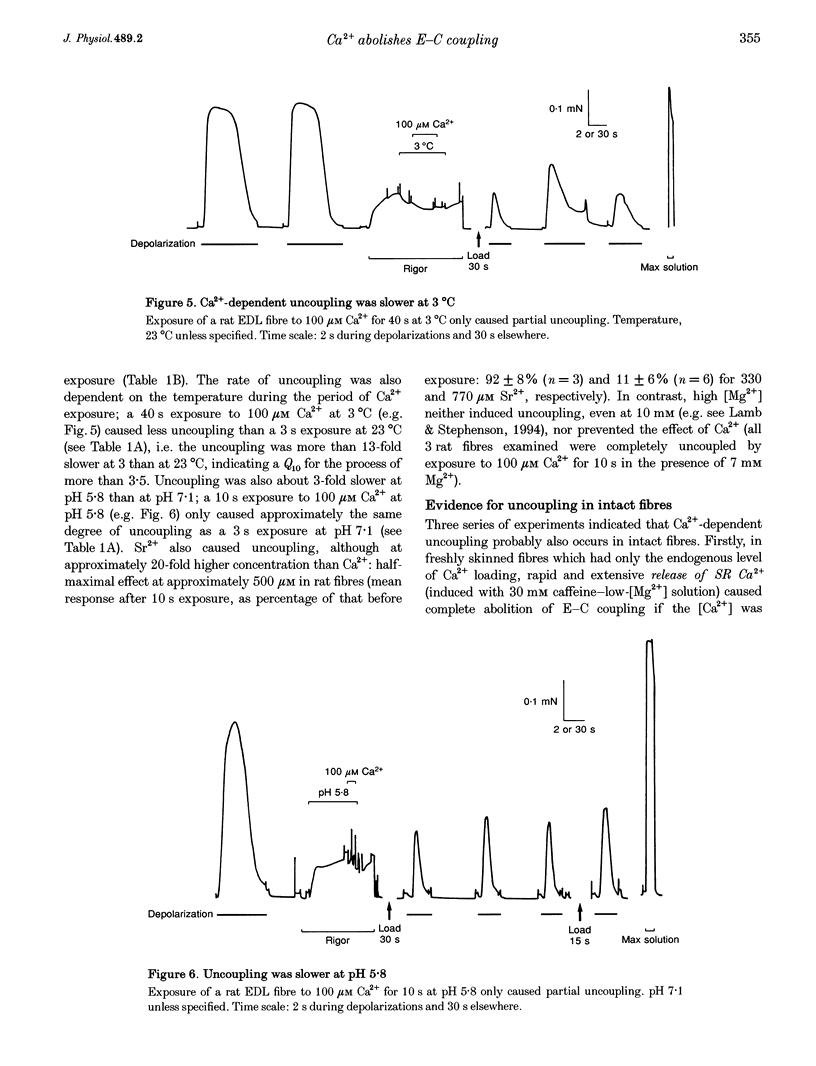
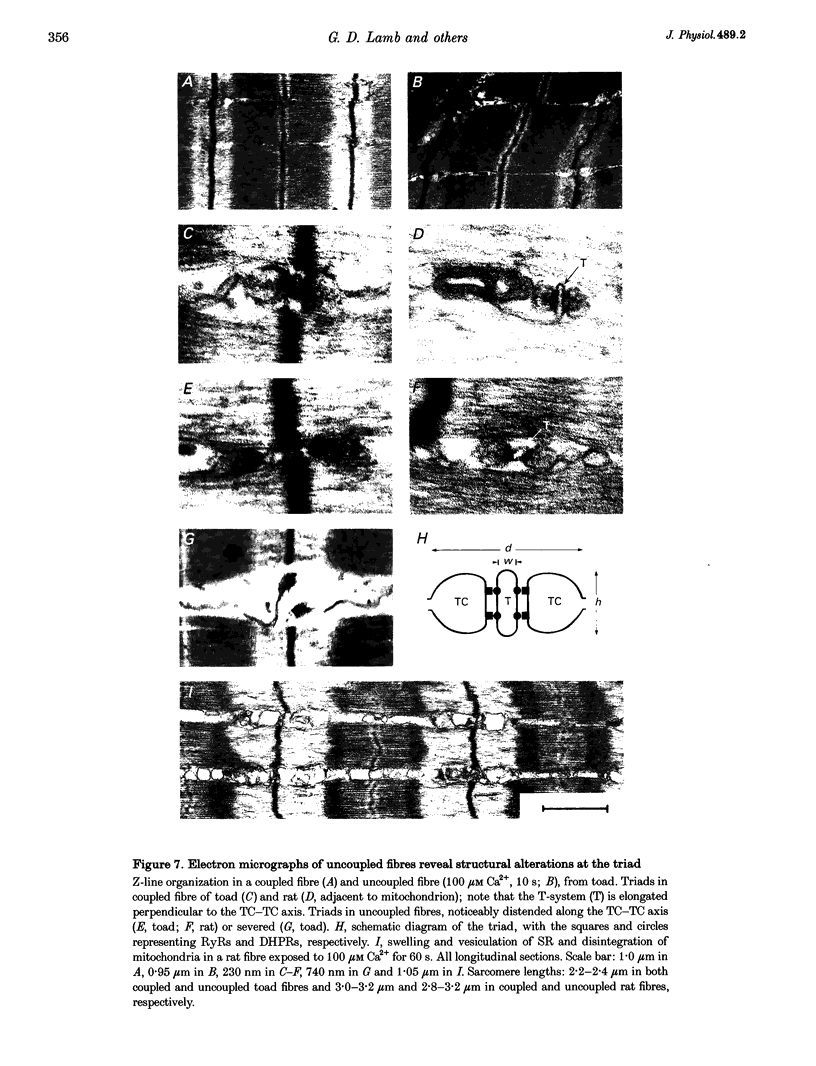
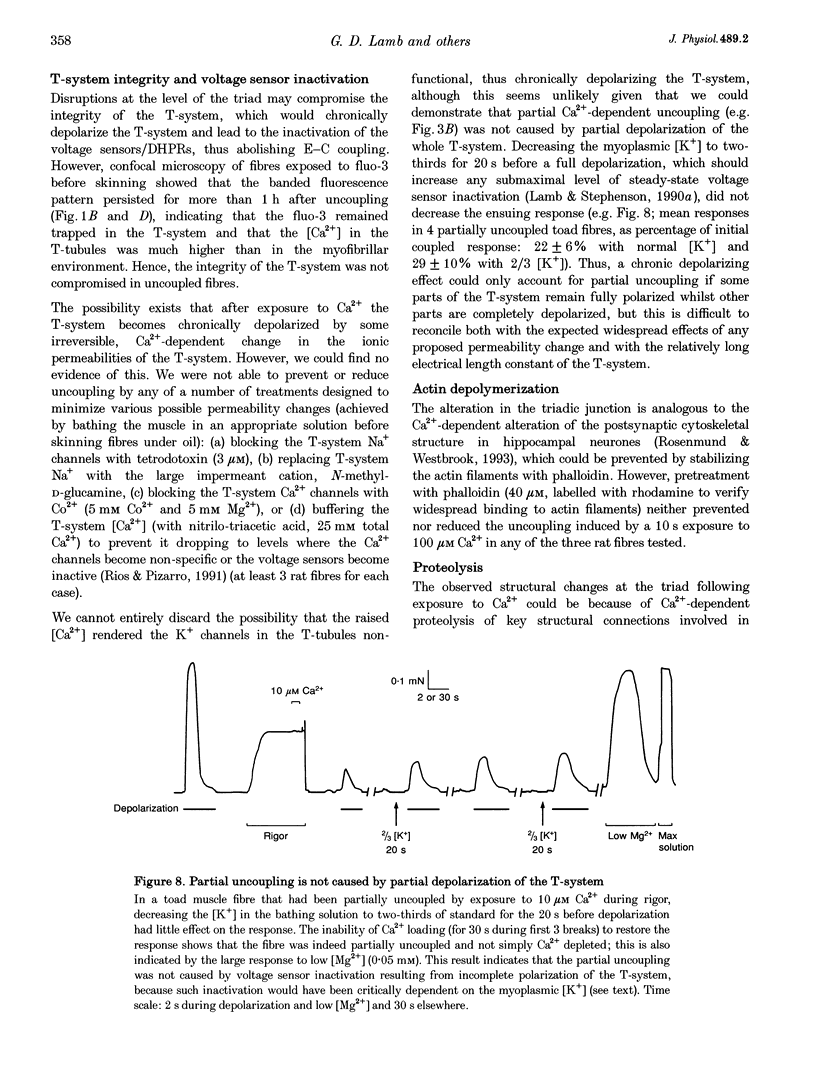
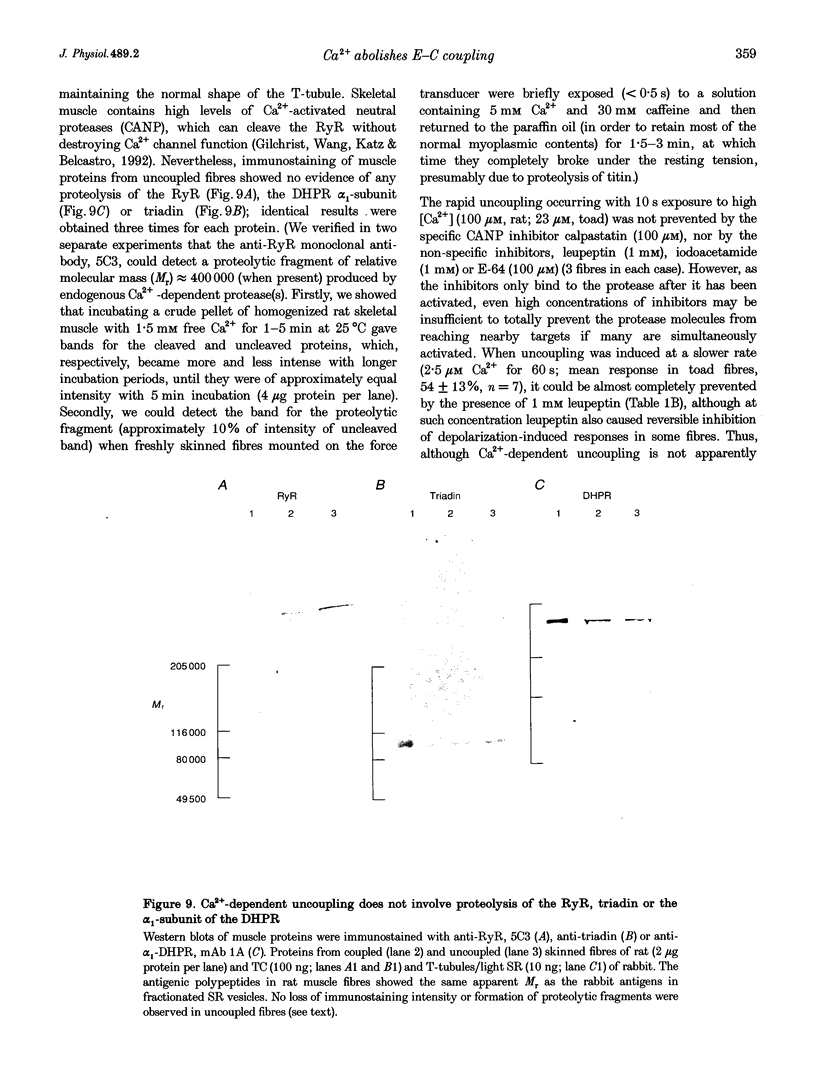
1. Raising the intracellular [Ca2+] for 10 s at 23 degrees C abolished depolarization-induced force responses in mechanically skinned muscle fibres of toad and rat (half-maximal effect at 10 and 23 microM, respectively), without affecting the ability of caffeine or low [Mg2+] to open the ryanodine receptor (RyR)/Ca2+ release channels. Thus, excitation-contraction coupling was lost, even though the Ca2+ release channels were still functional. Coupling could not be restored in the duration of an experiment (up to 1 h). 2. The Ca(2+)-dependent uncoupling had a Q10 > 3.5, and was three times slower at pH 5.8 than at pH 7.1. Sr2+ caused similar uncoupling at twenty times higher concentration, but Mg2+, even at 10 mM, was ineffective. Uncoupling was not noticeably affected by removal of ATP or application of protein kinase or phosphatase inhibitors. 3. Confocal laser scanning microscopy showed that the transverse tubular system was sealed in its entirety in mechanically skinned fibres and that its integrity was maintained in uncoupled fibres. Electron microscopy revealed distorted or severed triad junctions and Z-line aberrations in uncoupled fibres. 4. Only when uncoupling was induced at a relatively slow rate (e.g. over 60 s with 2.5 microM Ca2+) could it be prevented by the protease inhibitor leupeptin (1 mM). Immunostaining of Western blots showed no evidence of proteolysis of the RyR, the alpha 1-subunit of dihydropyridine receptor (DHPR) or triadin in uncoupled fibres. 5. Fibres which, whilst intact, were stimulated repeatedly by potassium depolarization with simultaneous application of 30 mM caffeine showed reduced responsiveness after skinning to depolarization but not to caffeine. Rapid release of endogenous Ca2+, or raised [Ca2+] under conditions which minimized the loss of endogenous diffusible myoplasmic molecules from the skinned fibre, caused complete uncoupling. Taken together, these results suggest that Ca(2+)-dependent uncoupling can also occur in intact fibres. 6. This Ca(2+)-dependent loss of depolarization-induced Ca2+ release may play an important feedback role in muscle by stopping Ca2+ release in localized areas where it is excessive and may be responsible for long-lasting muscle fatigue after severe exercise, as well as contributing to muscle weakness in various dystrophies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Mulligan I. P., Lea T. J. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991 Feb;24(1):1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Belcastro A. N., Parkhouse W., Dobson G., Gilchrist J. S. Influence of exercise on cardiac and skeletal muscle myofibrillar proteins. Mol Cell Biochem. 1988 Sep;83(1):27–36. doi: 10.1007/BF00223195. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brandt N. R., Caswell A. H., Brunschwig J. P., Kang J. J., Antoniu B., Ikemoto N. Effects of anti-triadin antibody on Ca2+ release from sarcoplasmic reticulum. FEBS Lett. 1992 Mar 24;299(1):57–59. doi: 10.1016/0014-5793(92)80100-u. [DOI] [PubMed] [Google Scholar]
- Chu A., Sumbilla C., Inesi G., Jay S. D., Campbell K. P. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry. 1990 Jun 26;29(25):5899–5905. doi: 10.1021/bi00477a003. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Hollingworth S., Marshall M. W. A comparative study of the transverse tubular system of the rat extensor digitorum longus and soleus muscles. J Anat. 1984 Mar;138(Pt 2):297–308. [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F. Heterogeneity of T-tubule geometry in vertebrate skeletal muscle fibres. J Muscle Res Cell Motil. 1984 Jun;5(3):333–347. doi: 10.1007/BF00713111. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F., Junankar P. R., Stanhope C. Extra-junctional ryanodine receptors in the terminal cisternae of mammalian skeletal muscle fibres. Proc Biol Sci. 1992 Jan 22;247(1318):69–75. doi: 10.1098/rspb.1992.0010. [DOI] [PubMed] [Google Scholar]
- Duncan C. J. Role of calcium in triggering rapid ultrastructural damage in muscle: a study with chemically skinned fibres. J Cell Sci. 1987 May;87(Pt 4):581–594. doi: 10.1242/jcs.87.4.581. [DOI] [PubMed] [Google Scholar]
- Duncan C. J., Smith J. L. Action of caffeine in initiating myofilament degradation and subdivision of mitochondria in mammalian skeletal muscle. Comp Biochem Physiol C. 1980;65(2):143–145. doi: 10.1016/0306-4492(80)90036-2. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A., Merton P. A. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977 Nov;272(3):769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Fill M. D., Best P. M. Contractile activation and recovery in skinned frog muscle stimulated by ionic substitution. Am J Physiol. 1988 Jan;254(1 Pt 1):C107–C114. doi: 10.1152/ajpcell.1988.254.1.C107. [DOI] [PubMed] [Google Scholar]
- Gilchrist J. S., Wang K. K., Katz S., Belcastro A. N. Calcium-activated neutral protease effects upon skeletal muscle sarcoplasmic reticulum protein structure and calcium release. J Biol Chem. 1992 Oct 15;267(29):20857–20865. [PubMed] [Google Scholar]
- Hain J., Nath S., Mayrleitner M., Fleischer S., Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from skeletal muscle. Biophys J. 1994 Nov;67(5):1823–1833. doi: 10.1016/S0006-3495(94)80664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junankar P. R., Dulhunty A. F. Monoclonal antibody to skeletal muscle ryanodine receptor detects a polypeptide in rat brain: comparison of immunogenic fragments after limited proteolysis. Biochem Mol Biol Int. 1994 Jan;32(1):29–37. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb G. D. DHP receptors and excitation-contraction coupling. J Muscle Res Cell Motil. 1992 Aug;13(4):394–405. doi: 10.1007/BF01738035. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol. 1990 Apr;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990 Apr;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991 Mar;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994 Jul 15;478(Pt 2):331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G., Stienen G. J. Effects of osmolality and ionic strength on the mechanism of Ca2+ release in skinned skeletal muscle fibres of the toad. J Physiol. 1993 May;464:629–648. doi: 10.1113/jphysiol.1993.sp019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. M., Dulhunty A. F., Junankar P. R., Stanhope C. Ultrastructure of sarcoballs on the surface of skinned amphibian skeletal muscle fibres. J Muscle Res Cell Motil. 1992 Dec;13(6):640–653. doi: 10.1007/BF01738254. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Mongini T., Ghigo D., Doriguzzi C., Bussolino F., Pescarmona G., Pollo B., Schiffer D., Bosia A. Free cytoplasmic Ca++ at rest and after cholinergic stimulus is increased in cultured muscle cells from Duchenne muscular dystrophy patients. Neurology. 1988 Mar;38(3):476–480. doi: 10.1212/wnl.38.3.476. [DOI] [PubMed] [Google Scholar]
- Morton M. E., Froehner S. C. Monoclonal antibody identifies a 200-kDa subunit of the dihydropyridine-sensitive calcium channel. J Biol Chem. 1987 Sep 5;262(25):11904–11907. [PubMed] [Google Scholar]
- Pape P. C., Jong D. S., Chandler W. K., Baylor S. M. Effect of fura-2 on action potential-stimulated calcium release in cut twitch fibers from frog muscle. J Gen Physiol. 1993 Aug;102(2):295–332. doi: 10.1085/jgp.102.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P., Palade P. Sarcoballs: direct access to sarcoplasmic reticulum Ca2+-channels in skinned frog muscle fibers. Biophys J. 1988 Aug;54(2):357–363. doi: 10.1016/S0006-3495(88)82967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E. W. Excitation of skinned muscle fibers by imposed ion gradients. I. Stimulation of 45Ca efflux at constant [K][Cl] product. J Gen Physiol. 1985 Dec;86(6):813–832. doi: 10.1085/jgp.86.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Duty S., Allen D. G. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol (1985) 1993 Jul;75(1):382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Head S. I., Bakker A. J., Stephenson D. G. Resting calcium concentrations in isolated skeletal muscle fibres of dystrophic mice. J Physiol. 1990 Sep;428:243–256. doi: 10.1113/jphysiol.1990.sp018210. [DOI] [PMC free article] [PubMed] [Google Scholar]