Abstract
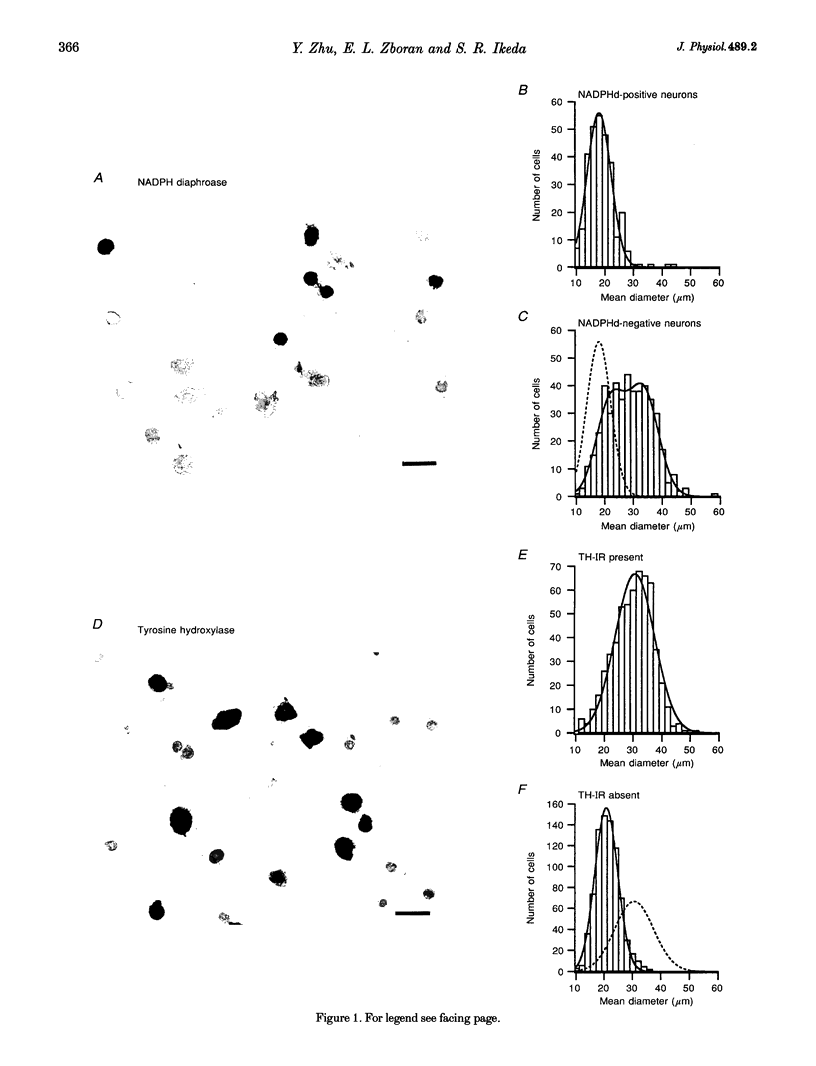
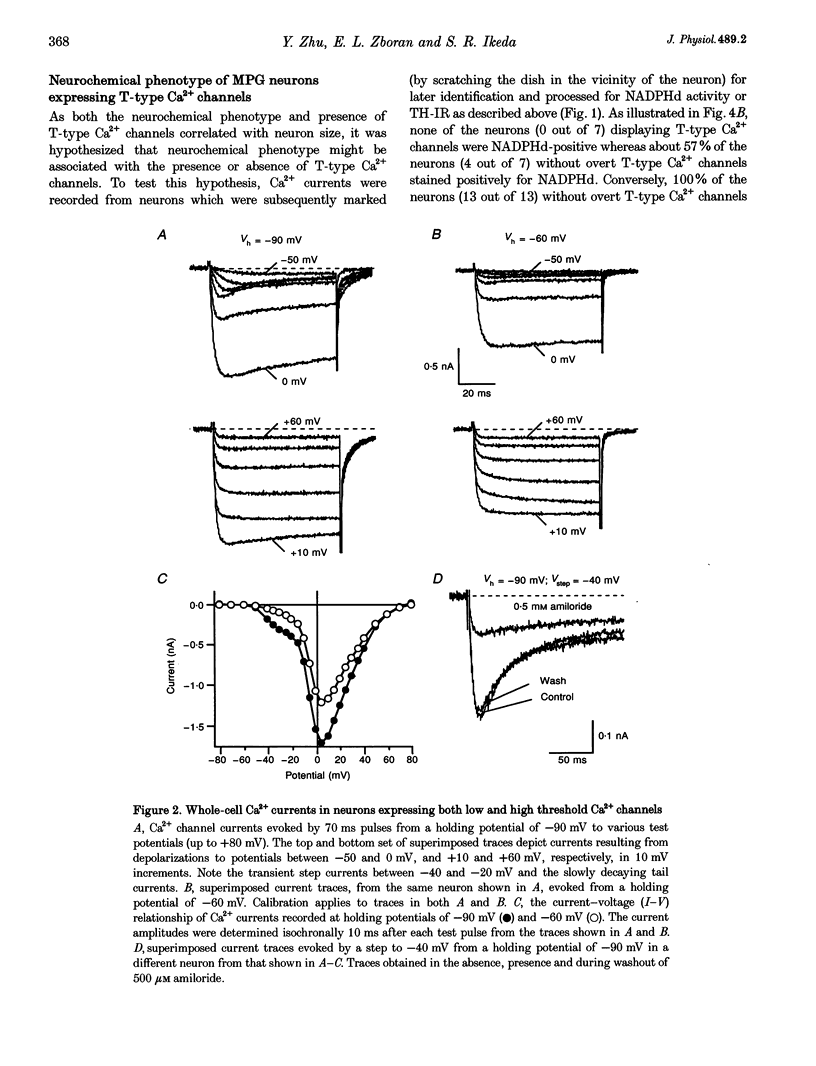
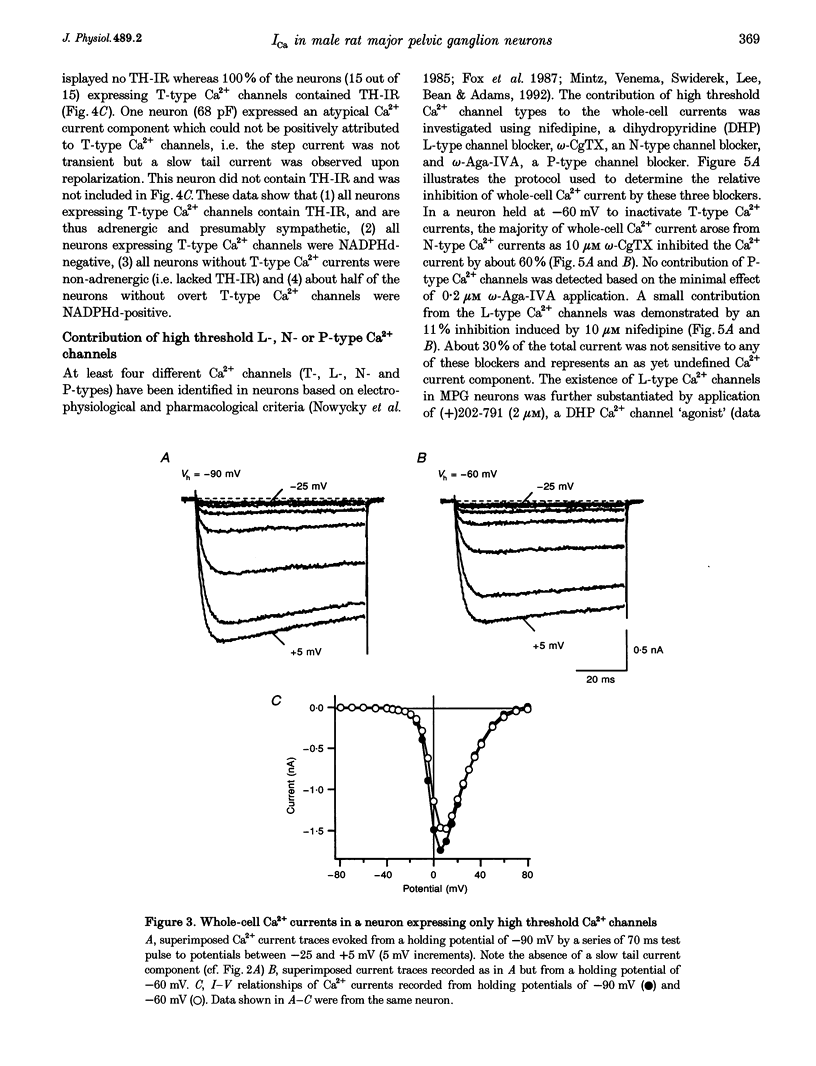
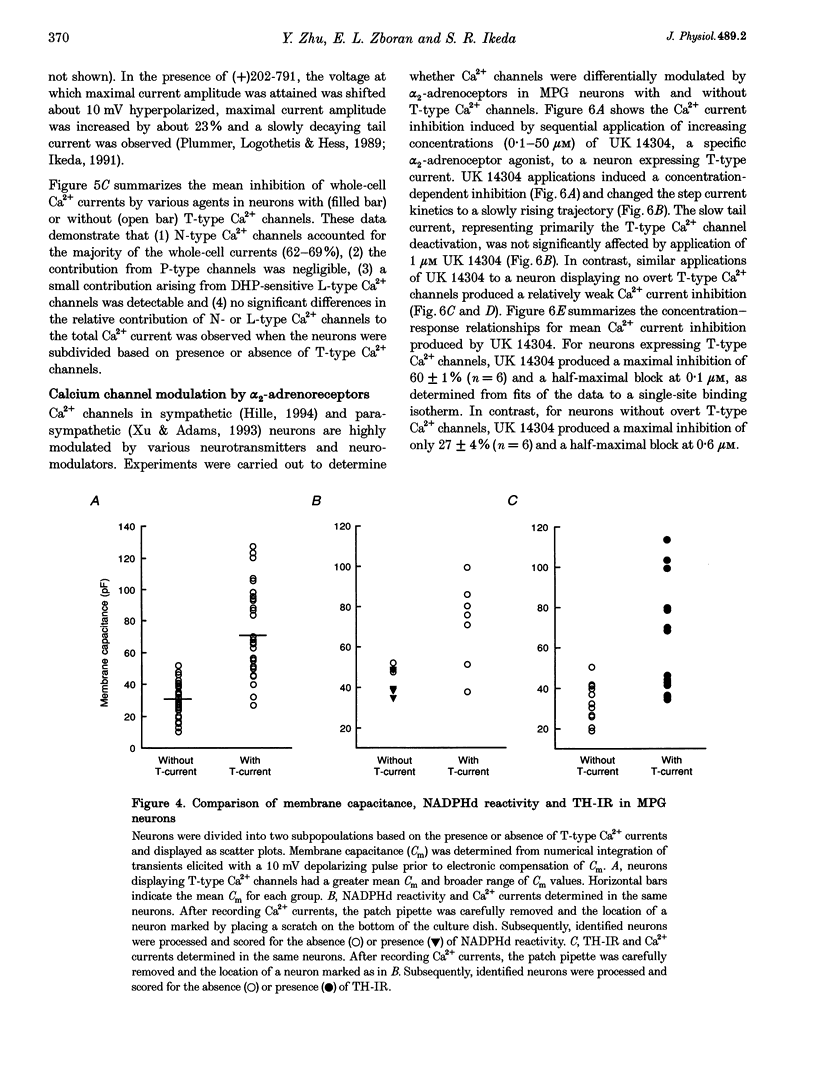
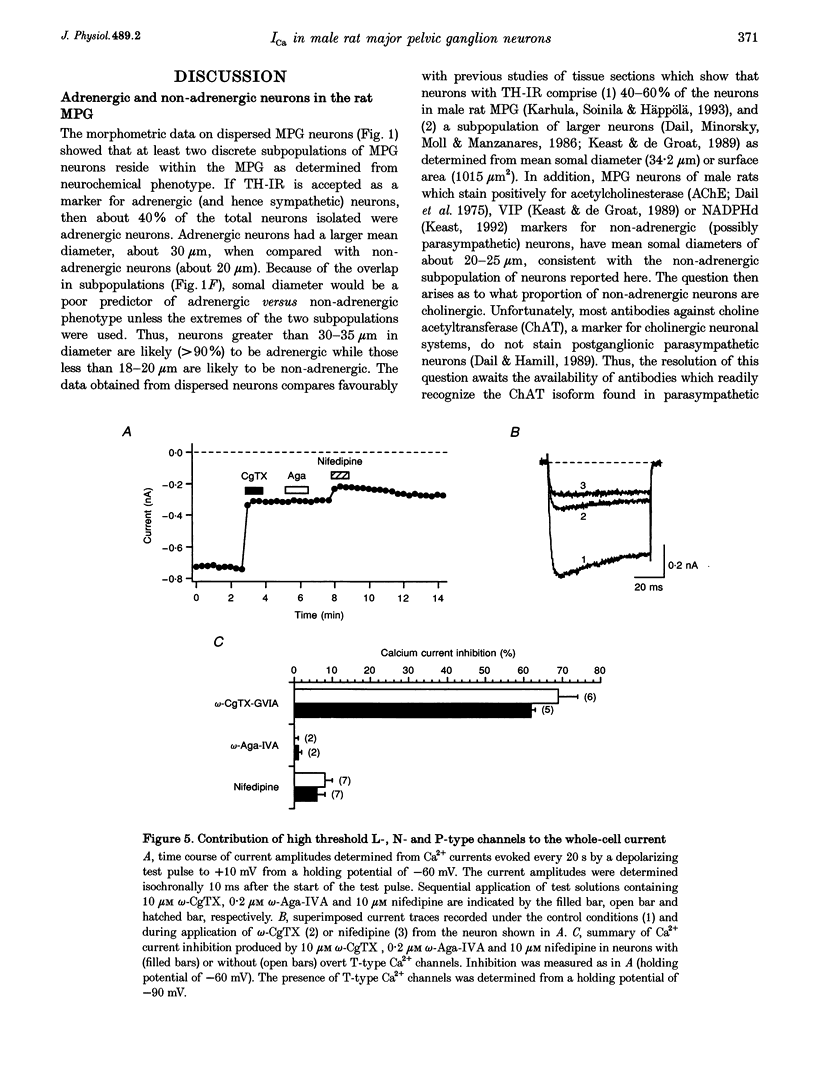
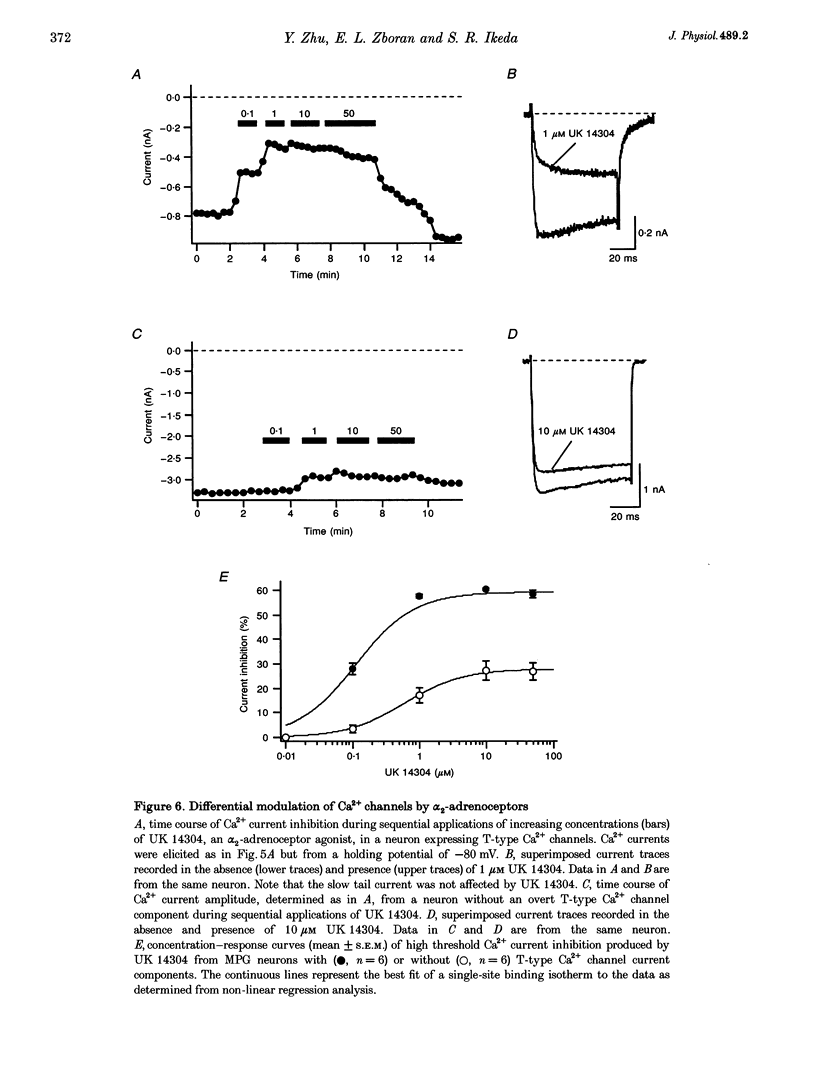
1. Neurons from the major pelvic ganglia (MPG) of adult male rats were enzymatically dissociated and the neurochemical phenotype and Ca2+ current properties examined. 2. Neurons were divided into two subpopulations based on the presence or absence of low threshold T-type Ca2+ channels. The subpopulation of neurons expressing T-type Ca2+ channels was characterized by a mean diameter of 34 microns, a mean membrane capacitance (Cm) of 72 pF, tyrosine hydroxylase immunoreactivity (TH-IR), a lack of NADPH diaphorase (NADPHd) reactivity and a high degree of alpha 2-adrenoceptor-mediated Ca2+ current inhibition (60%). 3. The subpopulation of neurons without overt T-type Ca2+ channels had a mean diameter of 23 microns, a mean Cm of 30 pF, a lack of TH-IR and a moderate degree of alpha 2-adrenoceptor-mediated Ca2+ current inhibition (27%). About 50% of this subpopulation stained positively for NADPHd. 4. The contribution of high threshold N-type Ca2+ channels (60-70%), as determined from omega-conotoxin GVIA inhibition, and L-type Ca2+ channels (< 10%), as determined from nifedipine inhibition, to the whole-cell Ca2+ current was similar for both subpopulations of neurons. 5. These data indicate that the MPG contain at least two subpopulations of postganglionic neurons, i.e. adrenergic and non-adrenergic, with distinct electrophysiological and neurochemical properties. Furthermore, we propose that the presence or absence of T-type Ca2+ channels provides an electrophysiological means of identifying adrenergic and non-adrenergic phenotype, respectively, in neurons of the male rat MPG.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aibara K., Ebihara S., Akaike N. Voltage-dependent ionic currents in dissociated paratracheal ganglion cells of the rat. J Physiol. 1992 Nov;457:591–610. doi: 10.1113/jphysiol.1992.sp019396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Tsurusaki M., Tokimasa T. Reduction of the N-type calcium current by noradrenaline in neurones of rabbit vesical parasympathetic ganglia. J Physiol. 1990 Jul;426:439–452. doi: 10.1113/jphysiol.1990.sp018148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier G. O., Ikeda S. R. TTX-sensitive Na+ channels and Ca2+ channels of the L- and N-type underlie the inward current in acutely dispersed coeliac-mesenteric ganglia neurons of adult rats. Pflugers Arch. 1992 May;421(1):7–16. doi: 10.1007/BF00374726. [DOI] [PubMed] [Google Scholar]
- Chen C., Schofield G. G. Differential neuromodulation of calcium currents by norepinephrine in rat sympathetic neurons. J Neurophysiol. 1993 Oct;70(4):1440–1450. doi: 10.1152/jn.1993.70.4.1440. [DOI] [PubMed] [Google Scholar]
- Dail W. G., Evan A. P., Eason H. R. The major ganglion in the pelvic plexus of the male rat: a histochemical and ultrastructural study. Cell Tissue Res. 1975 May 27;159(1):49–62. doi: 10.1007/BF00231994. [DOI] [PubMed] [Google Scholar]
- Dail W. G., Hamill R. W. Parasympathetic nerves in penile erectile tissue of the rat contain choline acetyltransferase. Brain Res. 1989 May 15;487(1):165–170. doi: 10.1016/0006-8993(89)90953-0. [DOI] [PubMed] [Google Scholar]
- Dail W. G., Minorsky N., Moll M. A., Manzanares K. The hypogastric nerve pathway to penile erectile tissue: histochemical evidence supporting a vasodilator role. J Auton Nerv Syst. 1986 Apr;15(4):341–349. doi: 10.1016/0165-1838(86)90019-6. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano F., Rampin O., Schirar A., Jardin A., Rousseau J. P. Autonomic control of penile erection: modulation by testosterone in the rat. J Neuroendocrinol. 1993 Dec;5(6):677–683. doi: 10.1111/j.1365-2826.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992 Dec;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R., Schofield G. G. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol. 1989 Feb;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva L., Lietzén R., Teräväinen H. Catecholamines and cholinesterases in the paracervical (Frankenhäuser) ganglion of normal and pregnant rats. Acta Physiol Scand. 1972 Oct;86(2):271–277. doi: 10.1111/j.1748-1716.1972.tb05332.x. [DOI] [PubMed] [Google Scholar]
- Karhula T., Soinila S., Häppäolä O. Comparison of immunohistochemical localization of [Met5] enkephalin-Arg6-Gly7-Leu8, vasoactive intestinal polypeptide and tyrosine hydroxylase in the major pelvic ganglion of the rat. Neuroscience. 1993 May;54(1):253–261. doi: 10.1016/0306-4522(93)90397-x. [DOI] [PubMed] [Google Scholar]
- Keast J. R. A possible neural source of nitric oxide in the rat penis. Neurosci Lett. 1992 Aug 31;143(1-2):69–73. doi: 10.1016/0304-3940(92)90235-y. [DOI] [PubMed] [Google Scholar]
- Keast J. R., de Groat W. C. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. J Comp Neurol. 1989 Oct 15;288(3):387–400. doi: 10.1002/cne.902880303. [DOI] [PubMed] [Google Scholar]
- LANGWORTHY O. R. INNERVATION OF THE PELVIC ORGANS OF THE RAT. Invest Urol. 1965 Mar;2:491–511. [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Brown D. A. Membrane currents in adult rat superior cervical ganglia in dissociated tissue culture. Neurosci Lett. 1987 Jun 1;77(1):55–60. doi: 10.1016/0304-3940(87)90606-9. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills T. M., Wiedmeier V. T., Stopper V. S. Androgen maintenance of erectile function in the rat penis. Biol Reprod. 1992 Mar;46(3):342–348. doi: 10.1095/biolreprod46.3.342. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Schofield G. G., Ikeda S. R. Sodium and calcium currents of acutely isolated adult rat superior cervical ganglion neurons. Pflugers Arch. 1988 May;411(5):481–490. doi: 10.1007/BF00582368. [DOI] [PubMed] [Google Scholar]
- THOMAS E., PEARSE A. G. THE SOLITARY ACTIVE CELLS. HISTOCHEMICAL DEMONSTRATION OF DAMAGE-RESISTANT NERVE CELLS WITH A TPN-DIAPHORASE REACTION. Acta Neuropathol. 1964 Jan 2;3:238–249. doi: 10.1007/BF00684399. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Toth P. T., Bindokas V. P., Bleakman D., Colmers W. F., Miller R. J. Mechanism of presynaptic inhibition by neuropeptide Y at sympathetic nerve terminals. Nature. 1993 Aug 12;364(6438):635–639. doi: 10.1038/364635a0. [DOI] [PubMed] [Google Scholar]
- Vizzard M. A., Erdman S. L., Förstermann U., de Groat W. C. Differential distribution of nitric oxide synthase in neural pathways to the urogenital organs (urethra, penis, urinary bladder) of the rat. Brain Res. 1994 May 23;646(2):279–291. doi: 10.1016/0006-8993(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Xu Z. J., Adams D. J. Alpha-adrenergic modulation of ionic currents in cultured parasympathetic neurons from rat intracardiac ganglia. J Neurophysiol. 1993 Apr;69(4):1060–1070. doi: 10.1152/jn.1993.69.4.1060. [DOI] [PubMed] [Google Scholar]
- Xu Z. J., Adams D. J. Voltage-dependent sodium and calcium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol. 1992 Oct;456:425–441. doi: 10.1113/jphysiol.1992.sp019344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Ikeda S. R. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol. 1993 Aug;70(2):610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]