Abstract
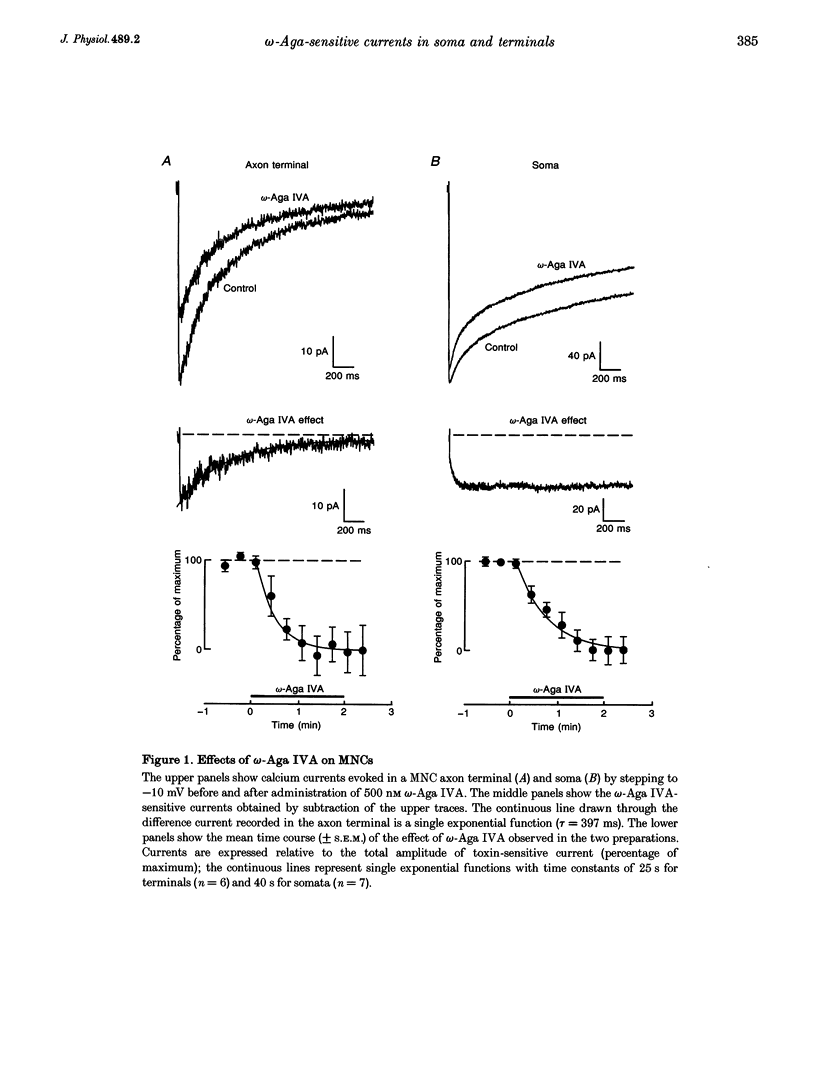
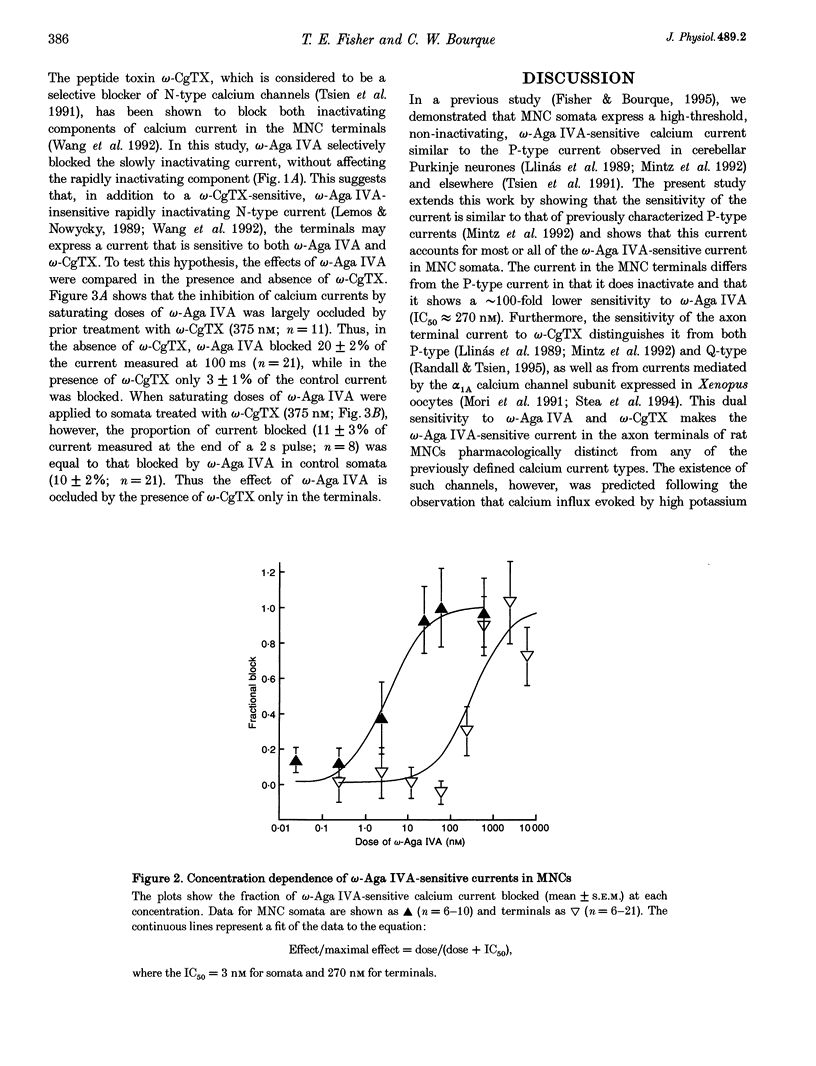
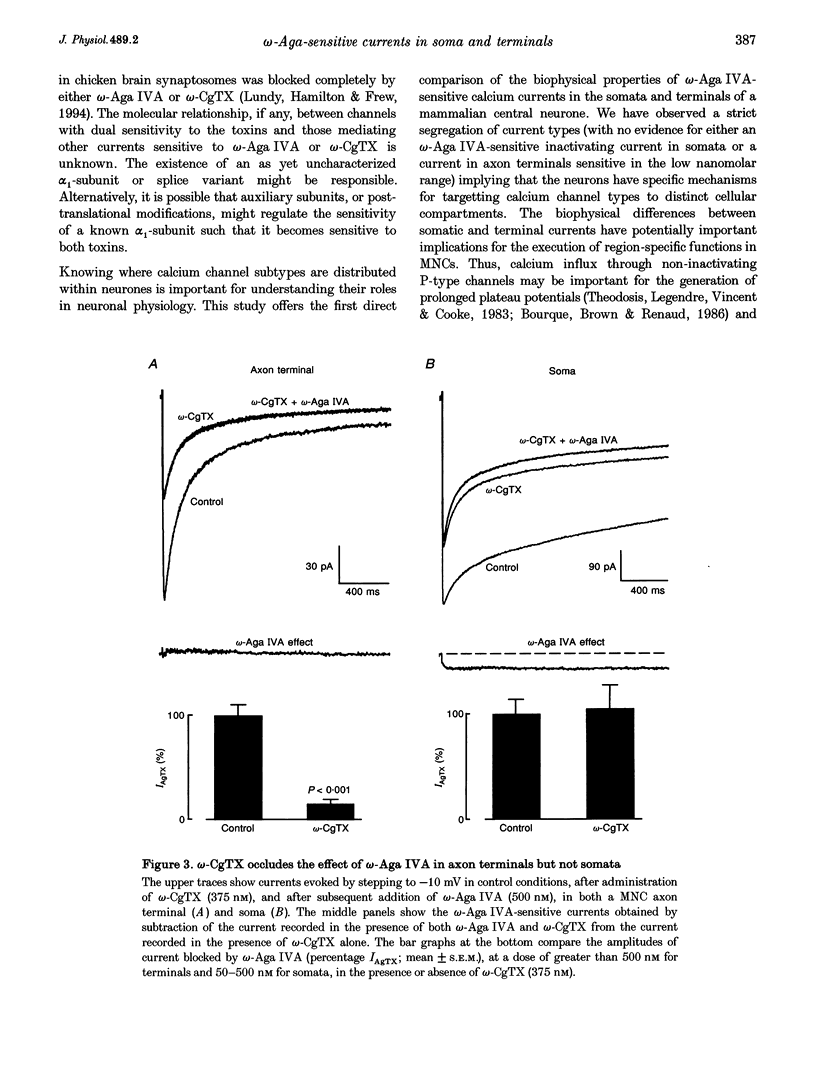
1. Voltage-dependent calcium currents were measured at room temperature using whole-cell patch clamp in acutely isolated somata and axon terminals of the magnocellular neurosecretory cells (MNCs) from the rat supraoptic nucleus. 2. Administration of omega-agatoxin IVA (omega-Aga IVA) blocked a high-threshold non-inactivating current. This current has an IC50 for omega-Aga IVA of 3 nM; no other types of currents were blocked at doses of up to 500 nM. 3. In the axon terminals omega-Aga IVA blocked a high-threshold current that inactivates markedly (tau approximately 448 ms), and has a much lower sensitivity to the toxin, with an IC50 of 270 nM. Unlike the somatic current, the effect of omega-Aga IVA in the terminals is largely prevented by omega-conotoxin GVIA (omega-CgTX). 4. These data suggest that MNC somata express a single type of omega-Aga IVA-sensitive calcium current similar to the P-type calcium current described in other cells. However, the omega-Aga IVA-sensitive current in axon terminals differs from both the P-type and the recently identified Q-type current in that it is also sensitive to omega-CgTX. The distinct biophysical properties of the currents in somata and axon terminals may have important physiological implications.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Bindokas V. P., Hasegawa L., Venema V. J. Omega-agatoxins: novel calcium channel antagonists of two subtypes from funnel web spider (Agelenopsis aperta) venom. J Biol Chem. 1990 Jan 15;265(2):861–867. [PubMed] [Google Scholar]
- Andrew R. D. Endogenous bursting by rat supraoptic neuroendocrine cells is calcium dependent. J Physiol. 1987 Mar;384:451–465. doi: 10.1113/jphysiol.1987.sp016463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988 Sep;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Brown D. A., Renaud L. P. Barium ions induce prolonged plateau depolarizations in neurosecretory neurones of the adult rat supraoptic nucleus. J Physiol. 1986 Jun;375:573–586. doi: 10.1113/jphysiol.1986.sp016134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986 Oct 8;70(2):204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Fisher T. E., Bourque C. W. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1995 Aug 1;486(Pt 3):571–580. doi: 10.1113/jphysiol.1995.sp020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy P. M., Hamilton M. G., Frew R. Pharmacological identification of a novel Ca2+ channel in chicken brain synaptosomes. Brain Res. 1994 Apr 18;643(1-2):204–210. doi: 10.1016/0006-8993(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C. Two high-threshold Ca2+ channels contribute Ca2+ for depolarization-secretion coupling in the mammalian neurohypophysis. Ann N Y Acad Sci. 1991;635:45–57. doi: 10.1111/j.1749-6632.1991.tb36480.x. [DOI] [PubMed] [Google Scholar]
- Randall A., Tsien R. W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995 Apr;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Tomlinson W. J., Soong T. W., Bourinet E., Dubel S. J., Vincent S. R., Snutch T. P. Localization and functional properties of a rat brain alpha 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993 Nov 11;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Legendre P., Vincent J. D., Cooke I. Immunocytochemically identified vasopressin neurons in culture show slow, calcium-dependent electrical responses. Science. 1983 Sep 9;221(4615):1052–1054. doi: 10.1126/science.6348947. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- Wang X., Treistman S. N., Lemos J. R. Two types of high-threshold calcium currents inhibited by omega-conotoxin in nerve terminals of rat neurohypophysis. J Physiol. 1992 Jan;445:181–199. doi: 10.1113/jphysiol.1992.sp018919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]


