Abstract
Background:
Optic disc edema is a feature of many ophthalmic and neurologic conditions. It remains an underappreciated feature of birdshot chorioretinitis (BSCR), leading to delay in diagnosis and treatment. The purpose of our study was to identify clinical features that are concomitant with optic disc edema and suggest a diagnosis of BSCR.
Methods:
Retrospective multicenter case series of 29 patients who were referred to a neuro-ophthalmologist or uveitis specialist for evaluation of disc edema and were ultimately diagnosed with BSCR.
Results:
Fifty-four eyes of 30 patients, from the practices of 15 uveitis specialists, met the eligibility criteria. In addition to disc edema, concomitant features in all patients included vitritis, chorioretinal lesions, and retinal vasculitis. Visual recovery to 20/40 or better occurred in 26 of 29 patients. Visual acuity remained 20/100 or worse in 2 patients previously diagnosed with idiopathic intracranial hypertension, 1 patient previously diagnosed with optic neuritis, and 1 patient for whom treatment was delayed for years, leading to optic disc atrophy.
Conclusions:
Optic disc edema is a presenting feature in some cases of BSCR. A diagnosis of BSCR should be considered when disc edema occurs with vitritis, chorioretinal inflammation, and retinal vasculitis. Patients should be referred to a uveitis specialist for treatment.
Optic disc edema is a presenting feature of multiple ocular, systemic, and neurologic diseases. Of these, optic disc edema is known to occur in a variety of ocular inflammatory diseases.2,3 Although historically, birdshot chorioretinitis (BSCR) has not been among these diseases, this has been observed clinically by the authors.
One such ocular inflammatory disease is BSCR, a chronic, bilateral posterior uveitis with characteristic choroidal lesions and a strong association with the HLA-A29 allele.4,5 We therefore sought to determine whether optic disc edema was a presenting feature of BSCR and identify concomitant clinical features that suggest the correct diagnosis. In this article, we present a series of 54 eyes in 29 patients in which optic disc edema was a prominent initial finding and BSCR was the final diagnosis. This series highlights the importance of considering uveitis entities such as BSCR in the differential diagnosis for optic disc edema and illustrates how a comprehensive ophthalmic examination and utilization of multimodal imaging can facilitate the diagnosis of BSCR in the setting of optic disc edema.
METHODS
This is a multicenter retrospective case series of patients who presented with optic disc edema and visual symptoms and ultimately diagnosed with BSCR. This study was approved by the institutional review board of all participating institutions and conformed to the tenets of the Declaration of Helsinki. The study was approved by the Washington University in St. Louis Institutional Review Board, and all data were collected and deidentified under local IRB approval as required by each institution. Patient permission for retinal imaging was obtained.
First, an email survey was sent to the members of the American Uveitis Society to determine whether patients presenting with optic disc edema and a final diagnosis of BSCR could be identified. Fifteen uveitis specialists responded with between 1 and 5 such cases each and were asked to submit deidentified clinical data on each case, as well as an estimate of how many total patients with BSCR they were currently following. A total of 31 potential cases were identified.
Patients were included in this study if they had unilateral or bilateral optic disc edema on clinical examination or angiography at the time of presentation and were eventually diagnosed with BSCR between 2017 and 2022. The diagnosis of BSCR was supported by clinical examination findings and laboratory analysis including, at a minimum, a positive HLA-A29 and negative tuberculosis and syphilis testing. Patients were excluded if clinical examination and further laboratory testing suggested an alternative diagnosis of autoimmune or infectious chorioretinitis, such as sarcoidosis, syphilis, or tuberculosis. One respondent submitted 2 cases that presented only with optic disc atrophy suggestive of previous optic disc edema and therefore were not included in this series. Ultimately 29 cases from 15 respondents were included in this series. One case was previously published by Lee et al.1 Age, gender, race, ethnicity, initial presumptive diagnosis, symptoms at presentation, color vision, and examination findings including anterior chamber cell, vitreous cell, optic disc findings, retinal vasculitis, and choroidal lesions, as well as HLA-A29 laboratory testing and electroretinogram (ERG) findings were collected from the patient's electronic medical records. Ancillary studies including fundus photography, fundus autofluorescence, fluorescein angiogram (FA), and optical coherence tomography (OCT) were also provided for some patients. Two representative cases were selected for narrative and imaging presentation. The estimated proportion of patients of BSCR presenting with significant optic disc edema was calculated by dividing the BSCR cases presenting with disc edema by the total BSCR cases per respondent. Each case was presented individually, and no statistical software was used for data analysis.
RESULTS
Case Presentations
Case 1 (Patient 10)
A 61-year-old woman presented with complaints of transient visual obscurations and a “gauze curtain” in her left eye and her visual acuity was 20/20 in the right eye and 20/25 in the left eye. The patient reported a history of Grave disease status after radioactive ablation and complained of headaches, weight gain, tinnitus, and joint pain. Examination showed disc edema in the left eye, the left automated visual field showed an enlarged blind spot, and MRI of the brain and orbit demonstrated increased fluid along the optic sheaths bilaterally with subtle perineural enhancement. A tentative diagnosis of IIH was made, but she was referred to a uveitis specialist for further evaluation.
Fundus examination revealed subtle yellow chorioretinal lesions in both eyes (Fig. 1A). FA showed retinal vasculitis and optic nerve leakage in the left eye (Fig. 1B). ICG revealed choroidal lesions in both eyes (Fig. 1C), confirming the presence of multifocal chorioretinitis. Laboratory evaluation was negative for tuberculosis and syphilis, but positive for the HLA-A29 allele. The patient was diagnosed with BSCR and started on immunomodulatory therapy that resulted in resolution of her symptoms, APD, disc edema, vitritis, and retinal vasculitis.
FIG. 1.
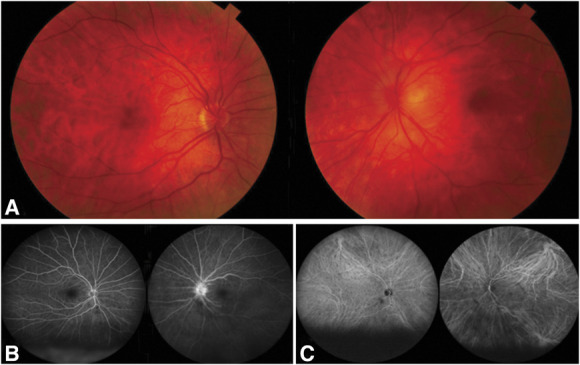
Case 1. A. Fundus photographs showing normal right optic disc and prominent left disc edema, images mildly obscured because of vitritis. B. Fluorescein angiography (FA) showing left disc hyperfluorescence and leakage. C. Indocyanine green angiography (ICGA) showing hypofluorescent choroidal spots more numerous in the right eye.
Case 2 (Patient 1)
A 39-year-old woman with a history of left ductal carcinoma status after bilateral mastectomy, chemotherapy, and radiation and a history of migraines presented to neuro-ophthalmology clinic for evaluation of vision loss in the right eye. She described a scotoma in the right temporal field that progressed to loss of vision described as a “dimmer switch” getting progressively darker for 4–6 weeks. She also endorsed a constant nonpositional pressure sensation behind her right eye, ringing in her ears, flashing lights in right temporal visual field, trouble seeing at night, and floaters. Her ophthalmic examination was notable for bilateral disc edema right greater than left with no APD or decreased color plates, and vitreous cells in the right eye. Automated visual field showed temporal defect in the right eye. MRI did not show enhancement of her optic nerves, parenchymal lesions, or meningeal enhancements. Her lumbar puncture had normal opening pressure and no inflammatory or malignant cells. She was therefore referred to a uveitis specialist for evaluation of posterior uveitis of right eye.
Her examination demonstrated bilateral multifocal choroiditis, periphlebitis, and vitritis in addition to optic disc edema in both eyes (Fig. 2A). Fundus autofluorescence showed hyperautofluorescent lesions nasal to the optic disc (Fig. 2B). OCT showed significant disruption of the retinal pigment epithelium with granular hyperreflective accumulations in the right eye (Fig. 2C). FA showed disc and venous leakage (Fig. 2D), and ICG showed many hypofluorescent spots in both eyes (Fig. 2E). Laboratory and radiologic evaluations were negative for syphilis, tuberculosis, sarcoidosis, and malignancy. HLA-A29 was positive and her ERG showed severely reduced rod function, mildly reduced cone function, and mildly delayed 30-Hz flicker in the right eye. She was diagnosed with BSCR, treated with immunomodulatory therapy, and achieved disease quiescence, including resolution of the disc edema and retinal vasculitis, as well as normalization of the OCT, HVF, and ERG.
FIG. 2.
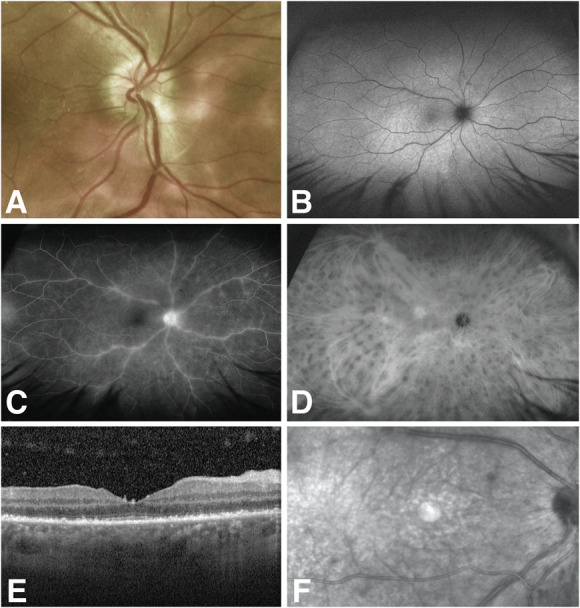
Case 2: A. Fundus photograph showing multifocal choroiditis with 360-degree disc edema and obscuration of nasal vessels, image mildly obscured because of vitritis. B. Fundus autofluorescence of the right eye with hyperautofluorescent spots. C. Fluorescein angiography of the right eye with disc and venous leakage. D. Indocyanine green angiography of the right eye with hypofluorescent spots. E and F. Optical coherence tomography of the right eye with granular hyperreflective accumulation at the level of the retinal pigment epithelium.
Proportion of Patients With Clinical Features of Birdshot Chorioretinitis With Optic Disc Edema
Fifteen survey respondents identified between 1 and 5 cases each, for a total of 29 cases with unilateral or bilateral disc edema. The estimated proportion of patients with BSCR presenting with disc edema in our clinics was between 1.82% and 33.33% of total BSCR cases (See Supplemental Digital Content, Table 1, http://links.lww.com/WNO/A810).
The demographics, presenting symptoms and diagnoses, and clinical examination findings of each case are presented in Tables 1 and 2. All patients in this case series were Caucasian, with 1 patient (#9) also of Hispanic ethnicity. Sixty-nine percent were female and the average age was 50 years, in range with published data.7 Most patients presented with blurred vision, floaters, photopsias, and nyctalopia while only 3 complained of ocular pain. Six patients (21%) had reduced color vision on presentation. Several patients were originally diagnosed with another ophthalmic condition before receiving the final diagnosis of BSCR, including IIH (5 patients), optic neuritis (1 patient), ocular migraine, and toxoplasmosis-associated optic neuritis, the latter of which was later deemed unlikely due to the absence of characteristic chorioretinal lesions.
TABLE 1.
Patient demographics and presenting symptoms and signs
| Patient | Age | Sex | Photopsias | Floaters | Nyctalopia | Pain | Bilateral Disc Edema | AC Cell | Vitritis | Choroidal Lesions | Retinal Vasculitis |
| 1 | 39 | F | + | + | + | + | + | + | + | + | + |
| 2 | 48 | M | + | + | + | + | + | − | + | + | + |
| 3 | 53 | F | − | + | − | − | + | − | + | + | + |
| 4 | 55 | F | + | + | − | − | + | − | + | + | + |
| 5 | 61 | F | + | + | − | − | + | + | + | + | + |
| 6 | 66 | F | − | − | − | − | + | − | + | + | + |
| 7 | 34 | F | + | + | − | + | + | + | + | + | + |
| 8 | 27 | F | − | + | − | − | + | − | + | + | + |
| 9 | 45 | F | + | + | − | − | + | − | + | + | + |
| 10 | 61 | F | + | + | − | − | + | − | + | + | + |
| 11 | 52 | F | − | + | − | − | + | − | + | + | + |
| 12 | 39 | M | − | + | − | − | + | − | + | + | + |
| 13 | 46 | M | − | + | − | − | + | − | + | + | + |
| 14 | 64 | M | + | + | − | − | + | − | + | + | + |
| 15 | 17 | F | + | + | + | − | + | − | + | + | + |
| 16 | 41 | F | + | + | − | − | + | − | + | + | + |
| 17 | 50 | M | + | + | − | − | + | − | + | + | + |
| 18 | 55 | M | − | + | + | − | + | − | + | + | + |
| 19 | 28 | F | + | + | − | − | + | − | + | + | + |
| 20 | 53 | F | − | + | − | − | + | + | + | − | + |
| 21 | 42 | M | + | + | − | − | + | + | + | + | + |
| 22 | 42 | F | + | + | − | − | − | − | + | + | + |
| 23 | 56 | F | + | − | − | − | + | + | + | + | + |
| 24 | 50 | F | + | + | − | − | + | − | + | + | + |
| 25 | 61 | F | − | + | − | − | + | + | + | + | + |
| 26 | 70 | M | − | + | − | − | + | − | + | + | + |
| 27 | 54 | F | − | + | − | − | − | − | + | + | + |
| 28 | 70 | M | − | + | − | − | + | − | + | + | + |
| 29 | 63 | F | + | + | − | − | − | + | + | + | + |
Visual acuity after treatment.
CF, counting fingers; F, female; M, male; IIH, idiopathic intracranial hypertension.
TABLE 2.
Patient clinical history
| Patient | Presenting Diagnosis and Testing | APD | Reduced Color Vision | ERG Changes | Posttreatment Result | Final Visual Acuity | |
| OD | OS | ||||||
| 1 | − | − | + | ERG normalized | 20/15 | 20/30 | |
| 2 | IIH with bilateral sequential disc edema for >1 year, MRI: no optic neuritis | OD | − | − | Poor visual recovery despite normal ERG and resolution of disc edema and intraocular inflammation | 20/400 | 20/300 |
| 3 | − | − | + | ERG normalized | 20/20 | J1+ | |
| 4 | Ocular migraine | − | − | + | ERG normalized | 20/25 | 20/25 |
| 5 | − | + | + | ERG normalized | 20/20 | 20/20 | |
| 6 | − | − | − | ERG normalized | 20/20 | 20/20 | |
| 7 | IIH | − | − | + | Blind spot enlargement on HVF resolved | 20/20 | 20/20 |
| 8 | Presumed IIH with bilateral disc edema and unremarkable MRI brain | − | − | N/A | Central scotoma, vision declined from 20/60 OD and 20/40 OS despite resolution of disc edema and intraocular inflammation | 20/100 | 20/80 |
| 9 | Optic disc edema | − | + | N/A | 20/25+2 | 20/30-2 | |
| 10 | MRI showed fluid along the optic sheaths and subtle perineal enhancement | OS | − | − | 20/25 | 20/20 | |
| 11 | − | + | + | 20/20 | 20/20 | ||
| 12 | OS | + | + | 20/20 | 20/20 | ||
| 13 | OS | − | − | 20/25 | 20/25 | ||
| 14 | Presumed optic neuritis due to toxoplasmosis | − | − | + | ERG normalized | 20/20 | 20/20 |
| 15 | IIH | − | − | + | 20/20 | 20/15 | |
| 16 | − | − | N/A | 20/20 | 20/20 | ||
| 17 | Disc edema | − | − | N/A | 20/30-2 | 20/20 | |
| 18 | Intermediate uveitis | − | − | − | 20/32 | 20/32 | |
| 19 | Optic disc edema | − | − | + | 20/20 | 20/20 | |
| 20 | − | − | N/A | 20/25 | 20/25 | ||
| 21 | IIH | − | − | N/A | 20/20 | 20/25 | |
| 22 | Presumed optic neuritis, no MRI was performed | OS | − | N/A | Poor visual recovery despite resolution of disc edema and intraocular inflammation | 20/40 | 20/150 |
| 23 | − | − | + | 20/20 | 20/20 | ||
| 24 | − | − | − | 20/20 | 20/25 | ||
| 25 | − | − | − | 20/20 | 20/20 | ||
| 26 | − | − | − | 20/20 | 20/20 | ||
| 27 | Long-standing optic atrophy | OD | + | − | Poor visual recovery despite resolution of disc edema and intraocular inflammation | CF | 20/20 |
| 28 | − | − | − | 20/30 | 20/20 | ||
| 29 | − | − | + | ERG normalized | 20/25 | 20/40 | |
IIH, idiopathic intracranial hypertension; ERG, electroretinogram; OD, right eye; OS, left eye; N/A, not available.
Ninety percent of patients with BSCR presenting with optic disc edema (26/29) had bilateral disc edema and 3 patients had unilateral disc edema. Concomitant examination findings included vitritis and retinal vasculitis in all patients, as well as chorioretinal lesions visible on fundus examination in all but 1 patient. All patients had chorioretinal lesions apparent on ICG. Anterior chamber inflammation was less common, present only in 21% or 6 of 29 patients. ERG abnormalities were found in 12 of 15 patients (80%) for whom testing was available and ranged from mild reduction in blue wave amplitudes to reduction of all waveforms, with most showing prolonged 30-Hz flicker implicit time. HLA-A29 was a requirement for diagnosis and was present in all patients.
After treatment, visual recovery to 20/40 or better occurred in 25 of 29 patients, whereas visual acuity remained 20/100 or worse in at least 1 eye in 4 patients in this series (Table 2). Patient 2 had been previously diagnosed with IIH and initially had mild ERG changes. His initial MRI showed narrowing of the transverse sinus and partially empty sella consistent with the initial diagnosis of IIH, but no evidence of optic neuritis. Treatment of his uveitis resolved the ERG abnormality, but did not significantly improve his vision because of persistent optic neuropathy. Patient 8 had bilateral disc edema and suffered progressive visual decline with optic atrophy despite treatment of her intraocular inflammation. She had a previous diagnosis of IIH based on elevated opening pressure, but an MRI without evidence of optic neuritis. Patient 22 had presumed left optic neuritis and her visual acuity did not improve beyond 20/150 despite adequate treatment of her intraocular inflammation. Patient 27 presented with disc edema in the left eye and preexisting right optic atrophy of unknown etiology but had never undergone MRI. Like the previous cases, her vision in the right eye did not improve despite resolution of disc edema, vitritis, and retinal vasculitis with adequate treatment of her uveitis.
DISCUSSION
Clinical Features of Birdshot Chorioretinitis Presenting With Optic Disc Edema
In summary, we present 29 cases of BSCR that presented with clinically apparent bilateral or unilateral optic disc edema. The patients in this series were most commonly female, which is typical for BSCR. Their ages were between 17 and 70 years, illustrating the extremes of range typical for BSCR, for which the mean age is 50 years.7 In addition to blurred vision and floaters, most patients experienced photopsias and/or nyctalopia, 2 key symptoms that indicate perturbation of the photoreceptors.5 Although transient visual obscurations can accompany disc edema, photopsias generally distinguish BSCR from other potential causes of disc edema. By contrast, pain and impaired color vision were uncommon in our series. While reviewable MRIs for 5 patients did not show optic neuritis, although 1 had subtle perineural fluid, we cannot exclude a concomitant inflammatory optic neuritis in other patients.
In addition to optic disc edema, the patients in our series had both vitritis and hypopigmented chorioretinal lesions radiating from the optic disc, typical of BSCR.6
BSCR can be distinguished from other causes of optic disc edema using multimodal imaging and ophthalmic testing. FA may show retinal vasculitis particularly in the proximal veins and disc leakage. FAF may demonstrate hyperautofluorescence and/or hypoautofluorescence.8 ICG testing reveals characteristic hypofluorescent spots that may predate the visible choroidal lesions.9 OCT may demonstrate cystoid macular edema, epiretinal membranes, and outer retinal loss and accumulation of choroidal fluid.10,11 OCT angiography may demonstrate choroidal flow deficit that correspond with hypofluorescent spots visible on ICG.12 ERG typically demonstrates reduced scotopic b-wave amplitudes and a delay and decrease in amplitude in 30-Hz flicker.13 Finally, key laboratory testing includes evaluation to rule out syphilis and tuberculosis, as well as positive testing for HLA-A29.
Concomitant Optic Disc Edema and Retinal Vasculitis in Birdshot Chorioretinitis
All patients in our series had retinal vasculitis demonstrable by FA. Retinal vasculitis is a common, but not a universal feature of BSCR, and the presence of this finding in all patients in our series in association with optic disc edema may suggest a pathophysiologic link between the proximal retinal phlebitis in BSCR and optic disc edema. One interpretation may be that the cases in our series represent more severe or prolonged inflammation. Future studies could compare the level of vitreous haze, extent of vasculitis, and thickening of the optic disc using validated tools including the SUN criteria and OCT.14 In addition to clinically apparent inflammation of the optic disc in some patients with BSCR, there is histologic evidence for lymphocytic infiltration of the optic nerve in BSCR,15 suggesting that the optic nerve could be a primary site of inflammation in at least some cases of BSCR.
Management and Prognosis of Birdshot Chorioretinitis With Optic Disc Edema
BSCR is initially treated with systemic or local steroids with many patients requiring the early introduction of systemic immunosuppressive therapy for long-term inflammatory control and preservation of global retinal function.16,17 Early diagnosis and treatment improves the vision significantly,18 whereas undertreated disease can lead to poor long-term visual outcomes.19 In our series, most patents experienced good visual recovery after treatment; however, 2 of 4 patients initially diagnosed with IIH, as well as 1 patient initially diagnosed with optic neuritis and another with optic atrophy of unknown etiology, did not experience good visual recovery despite OCT evidence of restoration of the outer retinal layers. We were unable to ascertain whether poor visual recovery was related to preexisting pathology, treatment delay, or whether optic disc edema may be a risk factor for permanent visual impairment in BSCR. Taken together, our cases suggest that a subset of patients presenting with disc edema as a feature of BSCR may develop optic atrophy. This previously underrecognized feature of BSCR warrants additional research to determine whether it may be a pathologic variant or the result of treatment delay.
Study Limitations and Conclusions
Our study was limited by its retrospective nature and the lack of true case controls. Retrospectively, it was subject to selection and ascertainment biases. Our case series focused on cases that may have been initially misdiagnosed because of prominent disc edema, so the variable numbers may reflect regional variations in referral and early treatment patterns as well as survey respondents' memory of clinical cases. In this small case series, it was not possible to ascertain whether the concomitant optic nerve pathology was part of the same disease process as the chorioretinitis or was a confounding second diagnosis (such as IIH) nor whether significant optic disc edema was a risk factor for poor visual recovery.
As demonstrated by our case series, it is critical to maintain a broad differential diagnosis for optic nerve edema to make the proper diagnosis. If undiagnosed, BSCR has the potential to result in long-term irrecoverable damage to the retina and choroid that can lead to significant visual impairment.19 A careful dilated examination to evaluate vitreous cells, subtle choroidal lesions, and retinal vasculitis should be performed in all patients with new-onset optic disc edema to evaluate for uveitic entities, such as BSCR, that can present with optic nerve edema secondary to inflammation. Considering BSCR in patients with optic disc edema and other signs of inflammation and promptly referring them to uveitis specialists may help prevent misdiagnosis and allow for early initiation of sight-preserving therapies.
STATEMENT OF AUTHORSHIP
Conception and design: S. Sabapathypillai, G. Van Stavern, L. M. Hassman; Acquisition of data: S. Sabapathypillai, V. J. Miller, A. Shakoor, A. G. Palestine, J. E. Thorne, D. A. Goldstein, P. A. Gaudio, N. Goldberg, A. Vitale, A. Schlaen, A. Thomas, P. T. Merrill, V. Raiji, P. Lin, A. L. Oliver, R. S. Moorthy, G. Chandra, E. Carreno, W. M. Smith, G. Van Stavern, L. M. Hassman; Analysis and interpretation of data: S. Sabapathypillai, L. M. Hassman. Drafting the manuscript: S. Sabapathypillai, V. J. Miller, G. Van Stavern, L. M. Hassman; Revising the manuscript for intellectual content: S. Sabapathypillai, V. J. Miller, A. Shakoor, A. G. Palestine, J. E. Thorne, D. A. Goldstein, P. A. Gaudio, N. Goldberg, A. Vitale, A. Schlaen, A. Thomas, P. T. Merrill, V. Raiji, P. Lin, A. L. Oliver, R. S. Moorthy, G. Chandra, E. Carreno, W. M. Smith, G. Van Stavern, L. M. Hassman. Final approval of the completed manuscript: S. Sabapathypillai, V. J. Miller, A. Shakoor, A. G. Palestine, J. E. Thorne, D. A. Goldstein, P. A. Gaudio, N. Goldberg, A. Vitale, A. Schlaen, A. Thomas, P. T. Merrill, V. Raiji, P. Lin, A. L. Oliver, R. S. Moorthy, G. Chandra, E. Carreno, W. M. Smith, G. Van Stavern, L. M. Hassman.
Supplementary Material
Footnotes
Supported by an unrestricted grant from Research to Prevent Blindness award to the Department of Ophthalmology and Visual Sciences at Washington University in St. Louis, Missouri. L. M. Hassman is supported by unrestricted grants from Research to Prevent Blindness and the Birdshot Society of North America. J. E. Thorne is supported by the Meredith Cross Birdshot Research Fund at Johns Hopkins University School of Medicine, Baltimore, Maryland.
This material was submitted for presentation at the Women in Ophthalmology Conference at Marco Island, Florida, August 24–27, 2023.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www.jneuro-ophthalmology.com).
S. Sabapathypillai and V. J. Miller contributed equally to the work.
Contributor Information
Sharon Sabapathypillai, Email: s.l.sabapathypillai@wustl.edu.
Victoria J. Miller, Email: tori.js.miller@gmail.com.
Akbar Shakoor, Email: akbar.shakoor@hsc.utah.edu.
Alan G. Palestine, Email: alan.palestine@cuanschutz.edu.
Jennifer E. Thorne, Email: jthorne@jhmi.edu.
Debra A. Goldstein, Email: debra.goldstein61@gmail.com.
Paul A. Gaudio, Email: paul.gaudio@yale.edu.
Naomi Goldberg, Email: naomigw7@gmail.com.
Albert Vitale, Email: albert.vitale@hsc.utah.edu.
Ariel Schlaen, Email: ariel.schlaen@gmail.com.
Akshay Thomas, Email: akshaysthomas@gmail.com.
Pauline T. Merrill, Email: pauline.merrill@gmail.com.
Veena Raiji, Email: veena.raiji@gmail.com.
Phoebe Lin, Email: linp3@ccf.org.
Armando L. Oliver, Email: armandoolivermd@gmail.com.
Ramana S. Moorthy, Email: rsmoorthy46032@yahoo.com.
Gaurav Chandra, Email: gmchandra@gmail.com.
Ester Carreno, Email: carregnito@gmail.com.
Wendy M. Smith, Email: smith.wendy1@mayo.edu.
Gregory Van Stavern, Email: vanstaverng@wustl.edu.
REFERENCES
- 1.Lee J, Smith WM, Goldstein DA. Birdshot chorioretinopathy presenting in a teenager. Am J Ophthalmol Case Rep. 2020;19:100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichani P, Christakis PG, Micieli JA. Headache and bilateral optic disc edema as the initial manifestation of Vogt-Koyanagi-Harada disease. J Neuroophthalmol. 2021;41:e128-e130. [DOI] [PubMed] [Google Scholar]
- 3.Nagashima T, Ishihara M, Shibuya E, Nakamura S, Mizuki N. Three cases of tubulointerstitial nephritis and uveitis syndrome with different clinical manifestations. Int Ophthalmol. 2017;37:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;89:31–45. [DOI] [PubMed] [Google Scholar]
- 5.Shah KH, Levinson RD, Yu F, et al. Birdshot chorioretinopathy. Surv Ophthalmol. 2005;50:519–541. [DOI] [PubMed] [Google Scholar]
- 6.Levinson RD, Brezin A, Rothova A, Accorinti M, Holland GN. Research criteria for the diagnosis of birdshot chorioretinopathy: results of an international consensus conference. Am J Ophthalmol. 2006;141:185–187. [DOI] [PubMed] [Google Scholar]
- 7.Faia LJ. Gender differences in birdshot chorioretinopathy and the white dot syndromes: do they exist? J Ophthalmol. 2014;2014:146768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böni C, Thorne JE, Spaide RF, et al. Fundus autofluorescence findings in eyes with birdshot chorioretinitis. Invest Ophthalmol Vis Sci. 2017;58:4015–4025. [DOI] [PubMed] [Google Scholar]
- 9.Reddy AK, Gonzalez MA, Henry CR, Yeh S, Sobrin L, Albini TA. Diagnostic sensitivity of indocyanine green angiography for birdshot chorioretinopathy. JAMA Ophthalmol. 2015;133:840–843. [DOI] [PubMed] [Google Scholar]
- 10.Keane PA, Allie M, Turner SJ, et al. Characterization of birdshot chorioretinopathy using extramacular enhanced depth optical coherence tomography. JAMA Ophthalmol. 2013;131:341–350. [DOI] [PubMed] [Google Scholar]
- 11.Kopplin LJ, Munk M, Baynham J, et al. Association of fundus autofluorescence findings and outer retinal lesions on optical coherence tomography with visual acuity in birdshot chorioretinopathy. J VitreoRetinal Dis. 2019;3:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepple KL, Chu Z, Weinstein J, Munk MR, Van Gelder RN, Wang RK. Use of en face swept-source optical coherence tomography angiography in identifying choroidal flow voids in 3 patients with birdshot chorioretinopathy. JAMA Ophthalmol. 2018;136:1288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holder GE, Robson AG, Pavesio C, Graham EM. Electrophysiological characterisation and monitoring in the management of birdshot chorioretinopathy. Br J Ophthalmol. 2005;89:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudio PA, Kaye DB, Crawford JB. Histopathology of birdshot retinochoroidopathy. Br J Ophthalmol. 2002;86:1439–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajwa A, Peck T, Reddy AK, Netland PA, Shildkrot Y. Dexamethasone implantation in birdshot chorioretinopathy—long-term outcome. Int Med Case Rep J. 2018;11:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss S, Ahmed M, Letko E, Foster CS. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmol. 2005;112:1066–1071. [DOI] [PubMed] [Google Scholar]
- 18.Knecht PB, Papadia M, Herbort CP, Jr. Early and sustained treatment modifies the phenotype of birdshot retinochoroiditis. Int Ophthalmol. 2014;34:563–574. [DOI] [PubMed] [Google Scholar]
- 19.Rothova A, Berendschot TT, Probst K, van Kooij B, Baarsma GS. Birdshot chorioretinopathy: long-term manifestations and visual prognosis. Ophthalmol. 2004;111:954–959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


