Abstract
Despite the rising prevalence of nonalcoholic fatty liver disease (NAFLD), the underlying disease pathophysiology remains unclear. There is a great need for an efficient and reliable “human” in vitro model to study NAFLD and the progression to nonalcoholic steatohepatitis (NASH), which will soon become the leading indication for liver transplantation. Here, we review the recent developments in the use of three-dimensional (3D) liver organoids as a model to study NAFLD and NASH pathophysiology and possible treatments. Various techniques that are currently used to make liver organoids are discussed, such as the use of induced pluripotent stem cells versus primary cell lines and human versus murine cells. Moreover, methods for inducing lipid droplet accumulation and fibrosis to model NAFLD are explored. Finally, the limitations specific to the 3D organoid model for NAFLD/NASH are reviewed, highlighting the need for further development of multilineage models to include hepatic nonparenchymal cells and immune cells. The ultimate goal is to be able to accurately recapitulate the complex liver microenvironment in which NAFLD develops and progresses to NASH.
Keywords: NAFLD, NASH, human liver organoids, 3D cell culture
Graphical Abstract
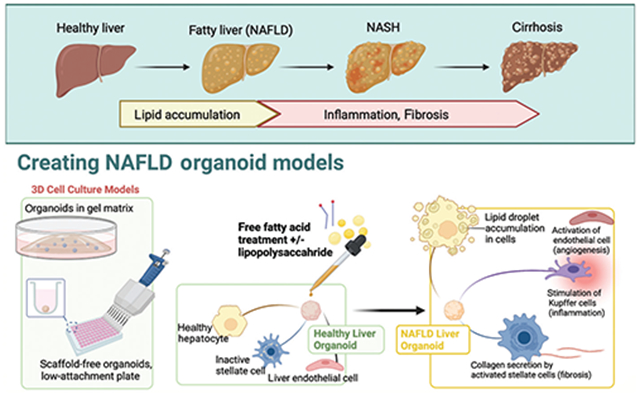
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the Western world with its rising prevalence and lack of available treatments.1,2 NAFLD encompasses a wide range of disease states, from simple steatosis to nonalcoholic steatohepatitis (NASH), with the latter defined by the presence of inflammation in addition to steatosis and necrosis, and potential for progression to cirrhosis with increased risk of hepatocellular carcinoma.3 NASH is soon expected to become the leading indication for liver transplantation.2
Although the growing clinical and economic burden of NAFLD has been recognized, the underlying mechanism by which NASH develops, unfortunately, remains unclear.4 The traditional “two-hit hypothesis” for the development of steatohepatitis postulated that in the presence of steatosis, a second “hit,” such as oxidative stress or inflammation could result in fibrosis and development of NASH.5,6 However, this explanation paints an incomplete picture without accounting for numerous other potential contributing factors, such as the direct effect of lipotoxicity on hepatocytes, as well as on other liver cells.7 A multihit hypothesis may be more appropriate to account for genetic and epigenetic factors that predispose to NAFLD/NASH.7
It is currently believed that NASH develops when the liver’s ability to metabolize carbohydrates and lipids is overwhelmed, resulting in the accumulation of lipotoxic metabolites that activate the cellular injury response.8 Other risk factors for NASH include insulin-resistant diabetes mellitus and/or a high fructose diet. The underlying mechanism is purported to be the result of increased de novo lipogenesis in the liver, which is a major contributor to hepatic steatosis, though the exact mechanism is unknown.9 Although Western (high fat and high fructose) diet-induced NAFLD and NASH murine models have been broadly used to delineate the underlying mechanism for the pathogenesis of NAFLD/NASH, the discrepancy between human and small rodent metabolisms makes it difficult to interpret the data generated in these models. Therefore, there is a great need for an efficient and reliable “human” in vitro model for NAFLD and NASH, which (i) further elucidates the pathways involved and identifies therapeutic targets, and (ii) tests potential treatments to help reduce the progression to an end-stage liver disease requiring transplantation. In this review, we focus on recent developments in the use of three-dimensional (3D) liver organoids as a model to study NAFLD and NASH pathophysiology and potential treatments.
The 3D Organoid Model
Brief History
Publications describing 3D cell culture date back to the 1960s, but there has been a steady growth in organoid research over the past 20 years as the importance of 3D structure on functional differentiation and cell signaling has become broadly accepted.10 Though the term “organoids” has been defined slightly differently in various fields of study,11 they have commonly been described as in vitro 3D constructs made of cells that self-organize and demonstrate organ functionality.12 One of the earliest hepatic organoid models was described by Soto-Gutierrez et al in 2010, in which primary mouse hepatocytes were co-cultured in a gel matrix with human liver non-parenchymal cell lines (i.e., hepatic stellate cells, HSCs, liver endothelial cells, LECs, and cholangiocytes). These cells were found to self-aggregate into sinusoid-like structures and supported long-term hepatic function.13 In 2013, Takebe et al were the first to create hepatic organoids derived from induced pluripotent stem cells (iPSCs). These tri-lineage organoids (iPSC-hepatic endoderm cells, human umbilical vein endothelial cells [HUVECs], and human mesenchymal stem cells [MSCs]), were self-organizing and had significantly increased expressions of hepatic marker genes, such as alpha-fetoprotein (AFP), retinol-binding protein 4 (RBP4), transthyretin (TTR), and albumin (ALB).14 These early in vitro studies suggested a close relationship between structure and preservation of function, and that the interactions between hepatocytes and nonparenchymal cells of the liver may play an important role in establishing a niche signaling environment to stabilize mature hepatocyte function in culture.
Characteristics and Key Benefits
In recent years, the key advantages of 3D cell culture over traditional 2D cell culture have become widely accepted, with particular emphasis on the importance of the cell microenvironment and cell-to-cell interactions. The liver is a complex organ composed of not only hepatocytes, which perform its primary functions, but also cholangiocytes, LECs, Kupffer cells, and HSCs. Furthermore, there are numerous immune cells including the liver myeloid cell population (dendritic cell population), liver lymphoid immune cell population (NK cells, NKT cells, B-cells, and T-cells), and immune-regulating liver nonhematopoietic cell population (Kupffer cells and LECs), which play an important role in maintaining immunological activity and homeostasis.15 Unfortunately, 2D co-cultures are challenging to maintain due to (i) difficulty establishing optimal culture conditions,16 and (ii) vital cell-to-cell interactions that depend on spatial structural organization and the in vivo microenvironment. The latter cannot be overlooked when attempting to determine the pathophysiology behind disease processes or response to medications.17,18
Furthermore, freshly isolated primary human hepatocytes have been noted to rapidly lose their function and ability to differentiate after only a few days in standard 2D monolayer culture, making it challenging to study long-term effects on liver function.19 In contrast, it has been well-demonstrated that primary hepatocytes in organoids can maintain their function for at least 5 weeks, with proteomic analysis revealing that in vivo phenotypes are maintained in 3D organoids, while the 2D monolayer proteome underwent striking changes after only 24 hours.20,21 Xiang et al have recently demonstrated that mature primary human hepatocyte function may be maintained even in monolayer culture for up to 4 weeks using enhanced media that modulates cyclic adenosine monophosphate, transforming growth factor beta (TGF-β), notch, bone morphogenic protein, and Wnt signaling pathways.22 While useful and cost-efficient, it is clear that homogenous hepatocyte cell culture is limited in its ability to model more complex features such as hepatic zonation, an important determinant in pathophysiological features of the liver, and the in vivo cellular microenvironment.19
The significant differences between standard cell culture of primary human hepatocytes and in vivo physiology are perhaps best demonstrated by the fact that in the past three decades, 14 drugs have been discontinued in the postmarketing stage after numerous reports of clinically significant acute liver failure, sometimes even resulting in death.23,24 One such drug was troglitazone, first approved in 1997 for use in diabetes and withdrawn after 3 years due to liver toxicity that was not flagged in initial in vitro or animal studies.25 In 2020, Ramli et al used a pluripotent stem cell-derived 3D liver organoid model to demonstrate cholestatic hepatotoxic changes following troglitazone exposure, further supporting that 3D culture allows for more reliable drug toxicity testing compared to the standard monolayer.26 These findings (i) support the notion that conventional 2D cell culture is limited in testing liver disease progression due to a lack of interaction between different relevant cell types, and (ii) highlight the differences in cell signaling in 3D versus 2D culture, which ultimately result in inconsistent drug screening outcomes between in vitro and in vivo settings.27,28
Modeling NAFLD/NASH with 3D Organoids
As discussed above, there is a great need for a reliable in vitro model for NAFLD and NASH that can support long-term hepatocyte function and recapitulate the human in vivo microenvironment, and 3D organoids have been a very promising approach thus far. The various methods for creating organoids to date have been summarized in detail elsewhere,10,11 but, in short, organoids can be derived from tissue-resident progenitors, commercially available and primary hepatic cell lines, embryonic stem cells (ESCs), and/or iPSCs, or even tissue fragments (►Fig. 1).
Fig. 1.
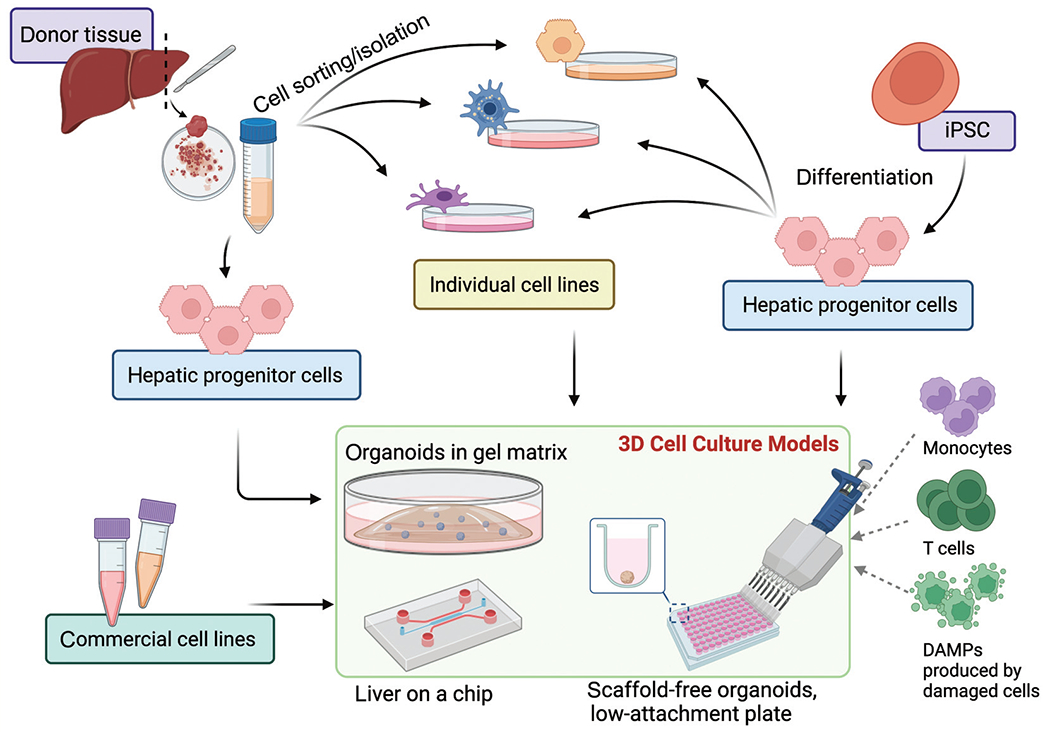
Cell origins for 3D cell culture models. A depiction of various three-dimensional cell culture models and the cells which they can be derived from. (i) Donor tissue can be digested, and isolated hepatic progenitor cells can be seeded directly, or alternatively, (ii) the cell mixture can be carefully differentiated and sorted into individual cell types to be characterized and recombined before seeding. (iii) Induced pluripotent stem cells (iPSCs) can be differentiated into hepatic progenitor cells, which can be directly seeded or further differentiated (e.g., hepatocyte-like, hepatic stellate cell-like cells) before seeding. (iv) Finally, carefully selected commercial cell lines can be aggregated to create organoids. These cell lines and organoids can be supported by (a) Matrigel or (b) low-attachment plates as a scaffold-free fashion or (c) on a liver-on-a-chip model. Immune cells, such as monocytes, T cells, and activated or damaged cells that release damage-associate molecular patterns (DAMPs) or other immune cells could be added to the organoid culture systems.
Organoids Derived from Cell Lines or Hepatic Lineage Cells
One clear benefit of using individual cell lines to make organoids is the ability to control the characteristics of each component and to decide upon an exact ratio of cell types to include in the organoid. Interactions between hepatocytes and nonparenchymal cells of the liver are key in the development of fibrosis,29 and inclusion of a variety of cell types is therefore ideal in organoids modeling NAFLD. Specifically, liver damage results in the activation of HSCs and their subsequent differentiation into myofibroblasts with the hallmark expression of a-smooth muscle actin, as well as an increase in the production of extracellular matrix proteins, such as type 1 collagen. The resulting change in liver architecture can ultimately progress to cirrhosis.30
Pingitore et al recently developed a multilineage NAFLD organoid model comprised of established commercially available immortalized cell lines HepG2 (human hepatocellular carcinoma hepatocytes) and LX-2 (HSCs).31,32 HepG2 cells were specifically selected because they are homozygous for the patatin-like phospholipase domain-containing 3 I148M sequence variant, which is one of the strongest genetic determinants of NAFLD in humans.32 They successfully demonstrated that incubating these organoids with free fatty acids (FFAs) resulted in fat accumulation and increased collagen secretion and that this phenotype could be rescued by the administration of antisteatotic and antifibrotic drugs that are currently in clinical trials.31 Potential limitations to the use of the established HepG2 cell line are (i) its low metabolic capacity compared to primary hepatocytes, which may hinder the ability to replicate the complex metabolic interactions that occur in the NAFLD microenvironment, and (ii) its origin as a hepatocellular carcinoma cell line with high proliferation rates and resistance to cytotoxicity compared to primary human hepatocytes.33,34
Organoids can also be developed from hepatic progenitor cells that have been isolated from donor tissue, as demonstrated by McCarron et al, who developed a bipotent ductal organoid model using tissue from diseased NASH livers,35 based on a design for bipotent ductal organoids previously described by the Clevers group.36 The bipotent ductal organoids were further hepatically differentiated by supplementation with a special differentiation medium for 11 to 14 days (►Table 1). Compared to healthy liver organoids, NASH liver organoids exhibited reduced regenerative ability and liver function, and a detailed transcriptomic analysis revealed upregulation of proinflammatory and fibrosis markers, such as aldo-keto reductase family 1 member B10.35 This study also supports the feasibility of deriving organoids directly from diseased tissue, which can be expected to more accurately model the disease and the associated microenvironment, as opposed to starting with organoids from healthy liver tissue and attempting to recreate a diseased state.35
Table 1.
Summary of existing NAFLD/NASH 3D organoid models
| Author (y) | Model | Use of NAFLD/NASH cells | Cell types | #Cells seeded | Max duration in culture | Types of analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte function | Lipid accumulation | Inflammation | Fibrosis | ||||||
| Primary cells from minced tissue | |||||||||
| Elbadawy et al (2020)51 | Organoidsa in gel matrix | Yes | Mixture of cells from liver tissue | 200,000 per drop of Matrigel | Not specified (7–14 d formation) | IF, RT-qPCR | Oil Red O stain, RT-qPCR | H&E stain, cytokine expression RT-qPCR | Masson’s Trichrome, IF, RT-qPCR |
| McCarron et al (2021)35 | Organoids in gel matrix | Yes | Bipotent hepatic ductal cells—HC and CHOL-like | N/A | wk (2 wk + 5 d expansion, 13 d differentiation) | Albumin secretion, ammonia degradation, CYP3A4 activity, reduced passage/growth capacity | LipidTox, caspase3/7 detection, transcriptomic analysis | Transcriptomic analysis | Transcriptomic analysis |
| Commercial cell lines | |||||||||
| Gori et al (2016)60 | Quasi-3D cells on a chip | No | HC only (HepG2) | 40,000 per chip | 8 d | Cell viability/cytotoxicity | AdipoRed assay, carboxy-H2DCFDA | N/A | N/A |
| Pingitore et al (2019)31 | Organoids in low attachment plate | No | HC (HepG2), HSC (LX-2)—1:1, 24:1 | 2,000 per organoid | 4 d (including 2 days for formation) | APOB secretion, cell viability | Oil Red O stain, AdipoRed assay | N/A | IF |
| Prill et al (2019)37 | Organoids in low attachment plate | No | HC only (BiolVT primary HC) | 2,000 per organoid | 17 d (including 7 days for formation) | APOB secretion, cell viability | Nile Red stain, AdipoRed assay, RT-qPCR | N/A | N/A |
| Induced pluripotent stem cells | |||||||||
| Gurevich et al (2020)43 | Organoids in low attachment plate | Yes | HC-like, MSC-like, KC-like, LEC-like | 0.5 × 106 cells/mL | 10 d | Albumin secretion, CYP3A4, RT-qPCR | BODIPY | N/A | N/A |
| Ouchi et al (2019)44 | Organoids in gel matrix | Yes | HC-like, HSC-like, KC-like | 200,000 per drop of Matrigel | 33 d | RNAseq, scRNAseq | Triglyceride ELISA, RNAseq, scRNAseq | FC/FACS, IL-6 ELISA, monocyte migration, RNAseq, scRNAseq | AFM, Trichrome stain, IF, P3NP ELISA, RNAseq, scRNAseq |
| Wang et al (2020)58 | Organoids on a chip | No | HC-like, CHOL-like | 2 × 106 on micro-pillar plate | 32 d | Albumin secretion, RT-qPCR | Oil Red O stain, triglyceride assay | RT-qPCR | IHC staining, RT-qPCR |
Abbreviations: AFM, atomic force microscopy stiffness measurement; APOB, apolipoprotein B; CHOL, cholangiocyte; ELISA, enzyme-linked immunoassay; FACS, fluorescence-activated cell sorting; FC, flow cytometry; H&E hematoxylin and eosin; HC, hepatocyte; HSC, hepatic stellate cell; IF, immunofluorescence staining; IL-6, interleukin-6; KC, Kupffer cell; LEC, liver endothelial cell; MSC, mesenchymal stem cell; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; scRNAseq single-cell RNA sequence.
Made from mouse liver cells (all other models used human cells).
In contrast, organoids can also be derived from primary cell lines which are directly isolated from donor liver tissue.37,38 Prill et al37 were able to develop a NAFLD model using primary human hepatocytes from different donors, which demonstrated that there was reproducible inter-donor variability in response to FFA treatment in terms of the degree of resulting steatosis. In their study, the organoid model was used to explore the underlying mechanisms for human genetic variants at higher risk for developing NAFLD, such as the transmembrane 6 superfamily member 2 (TM6SF2) E167K mutation. In fact, the hepatic TM6SF2 E167K organoids were noted to have increased expression of metabolic genes associated with cholesterol synthesis (FDPS, HMGCS1, FDFT1, DHCR7, and SC5D), de novo lipogenesis (FASN and ACSS2), and phospholipid dephosphorylation (PLPP3) when compared with wild type. These findings highlight both the strength and limitation of organoids made from cell lines isolated from individual donors. Although interdonor variability could potentially limit the broad generalizability of findings based on a smaller sample size of donors, the reproducibility of donor variability in vitro can potentially be harnessed to identify specific genetic variants associated with a higher risk for the development of NAFLD. Finally, the organoids in this model used hepatocytes alone, and the lack of other liver cell types limits the ability to recreate the cell-to-cell interactions that contribute to the NAFLD microenvironment.37
One of the ongoing challenges in 3D organoid culture, particularly in models using primary cells, has been incorporating additional liver cell types besides hepatocytes and HSCs, such as LECs, cholangiocytes, and Kupffer cells, to more thoroughly model inflammatory processes associated with NAFLD and more closely approximate the in vivo microenvironment. In our lab, we have developed a scaffold-free human 3D liver organoid model which incorporates up to 5 liver cell lineages (hepatocytes, HSC, LEC, cholangiocytes, Kupffer cells), derived from primary cells that we isolated from donor liver tissue, characterized, and then reaggregated into 3D organoids in a 96-well ultra-low-attachment plate.39,40 These organoids maintained mature hepatocyte function (albumin secretion, urea synthesis, and bile acid synthesis) after being held in culture for 30 days.40 Organoids using hepatocytes from healthy donors can be further challenged with treatments to model specific disease processes (such as FFA loading for NAFLD, which will be discussed in a subsequent section).
Induced Pluripotent Stem Cell and Embryonic Stem Cell-Derived Organoids
The lack of access to fresh donor liver tissue can be a barrier to using primary cell lines for organoids. Therefore, iPSCs have emerged as a reasonable alternative. It is important to note that iPSCs must go through multiple differentiation steps over the course of a couple of weeks before they can become hepatic progenitor cells, which can then be used to make organoids. Furthermore, the cell types that are derived from iPSCs are typically not fully differentiated and are therefore termed as “hepatocyte-like,” “HSC-like,” and “cholangiocyte-like” cells.41
Hepatocyte-like cells (HLCs) can be produced from iPSCs via a stepwise differentiation protocol developed by several research groups. This process involves the differentiation of iPSCs to endodermal cells and then further to HLCs.42 The iPSCs are still able to successfully differentiate into HLCs after being cryopreserved during the early differentiation process and retain the genetic background of the donor patient. Interestingly, Gurevich et al found that HLCs generated from NASH donor iPSCs displayed lipid accumulation even in the absence of fatty acid supplementation43 (►Table 1). This group also generated organoids using the HLCs from NASH donors in addition to HSC-like cells and Kupffer cell-like cells, but these remained intact for only 10 days. Other research groups such as Akbari et al have been able to generate functional hepatic organoids from healthy donor iPSCs which were successfully maintained for as long as 16 months without loss of differentiation. They initially differentiated the iPSCs into epithelial cell adhesion molecule positive hepatic progenitor cells, from which they subsequently derived their organoids.41
Furthermore, Ouchi et al developed a multilineage organoid model that included hepatocyte-like, HSC-like, and Kupffer cell-like cells, all derived from iPSCs or ESCs, which were treated with fatty acid and demonstrated phenotypes associated with steatohepatitis.40 Interestingly, organoids derived from iPSCs of patients with a baseline lysosomal enzyme deficiency developed more severe features of steatohepatitis, again reflecting the potential impact that 3D organoids could have on the personalized study of specific disease mechanisms and the development of treatments. While iPSC-derived organoids were found to have similar transcriptomic profiling related to lipid metabolism when compared to primary hepatic cells, their functional activity was undetermined and requires further study to identify potential differences when compared with primary cell functionality.44 Moreover, iPSC-derived organoid models are limited by the inability to control the exact ratio of other liver cell types in their composition, as done in primary liver cell-derived organoids.
Inducing and Measuring Steatosis and Fibrosis
The detailed mechanisms for the development of NASH may still require further elucidation, but in order to model hepatic steatosis, liver organoids are often treated with FFAs, which results in lipid droplet accumulation within the organoid and upregulation in markers of fibrosis, such as type 1 collagen (e.g., COL1A1).31 Alternatively, instead of treating healthy liver organoids with FFAs, NAFLD/NASH organoids can also be created directly from tissue specimens taken from patients with known diseases.35,37
Pingitore et al31 measured the accumulation of fat in their multilineage (HepG2 with HSC) organoids by staining with Oil Red O and further quantified this with an intracellular lipid droplet bioassay. The organoids were additionally incubated with TGF-β due to their potency as a fibrogenic cytokine,45 which resulted in increased collagen levels (►Table 2). This model was the first to demonstrate that incubating liver organoids with fatty acids leads to lipid droplet accumulation and fibrosis, which was consistent with previous findings in 2D models.31,46,47
Table 2.
Comparison of FFA loading methods
| Author | Year | FFA treatment concentration | Carrier molecule | Use of inflammatory stimulator | FFA treatment duration | Molecular compounds tested for rescue |
|---|---|---|---|---|---|---|
| Primary cells from minced tissue | ||||||
| McCarron et al 35 | 2021 | PA—1.2 mM, 2 mM or OA 2 mM | BSA | No | PA 6 h or OA 24 h | No |
| Commercial cell lines | ||||||
| Gori et al60 | 2016 | 2:1 OA:PA (OA 0.66 mM, PA 0.33 mM) | Methanol | No | 24 or 48 h | No |
| Pingitore et al31 | 2019 | 2:1 OA:PA—500 μM | BSA | TGF-β, PDGF | 24 or 48 h | Liraglutide, elafibranor, obeticholic acid, vitamin E |
| Prill et al37 | 2019 | 2:1 OA:PA—107 μM/53 μM, 213/107 μM | BSA | No | 10 d | No |
| Induced pluripotent stem cells | ||||||
| Gurevich et al43 | 2020 | OA and linoleic acid mix—100 μM or 300 μM | BSA | No | 24 h | No |
| Ouchi et al44 | 2019 | OA—200 μM, 400 μM, 800 μM | Not specified | LPS | 7 d | FGF19 |
| Wang et al58 | 2020 | 2:1 OA:PA—600 μM | BSA | No | 7 d | No |
Abbreviations: BSA, bovine serum albumin; FFA, free fatty acid; FGF19, fibroblast growth factor 19; LPS, lipopolysaccharide; μM, micromolar; mM, millimolar; OA, oleic acid; PA, palmitic acid; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor beta.
In our lab, we have treated our multilineage (5-cell–hepatocytes, LECs, HSCs, cholangiocytes, and Kupffer cells) 3D liver organoid models with FFAs and lipopolysaccharide for several days to induce steatosis, fibrosis, and inflammation in order to appropriately model NAFLD (►Fig. 2). Different types of staining were used for lipid accumulation and fibrosis.39
Fig. 2.
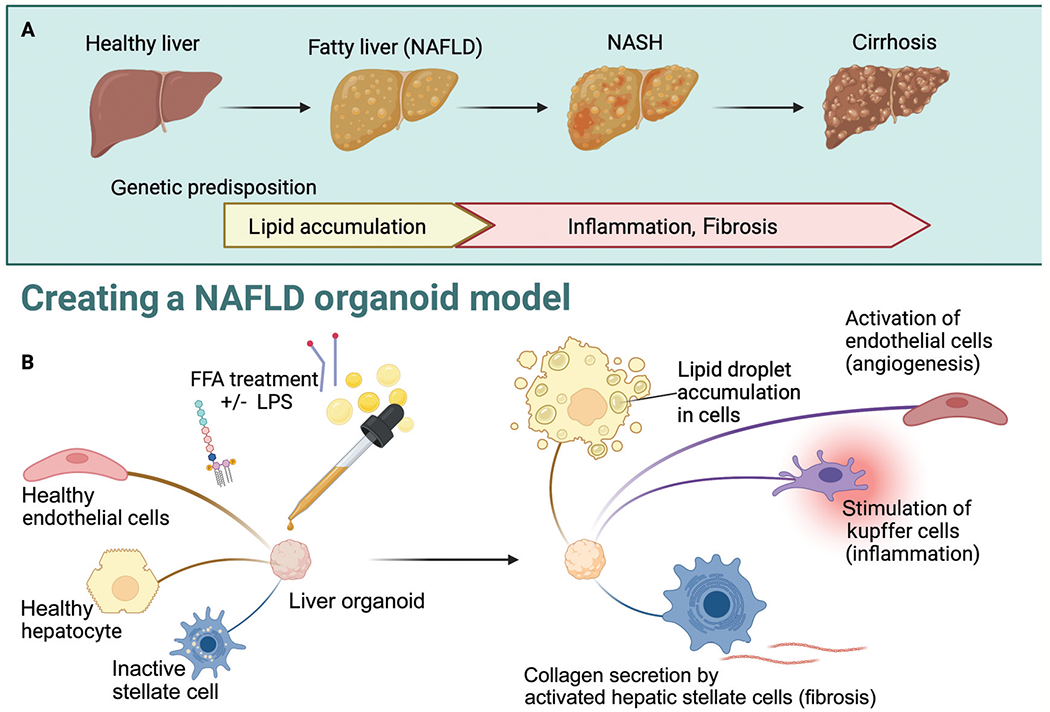
Free fatty acid (FFA) loading for nonalcoholic fatty liver disease (NAFLD) organoid model. (A) Progression of disease at the organ level with contributing factors. (B) Depiction of how treating liver organoids with FFA with or without costimulation with lipopolysaccharide (LPS) or TGF-beta can model the NAFLD disease process at the individual cell level with NAFLD phenotypes in fibrosis, angiogenesis, and inflammation. NASH, nonalcoholic steatohepatitis.
Organoids for Screening and Development of NAFLD/NASH Treatment
In addition to their utility as a model to further elucidate the NAFLD/NASH disease mechanism, 3D liver organoids, especially when they represent the complete liver microenvironment, also have significant potential for application in NAFLD drug development. Molecular compounds that are currently under FDA (Food and Drug Administration of the United States) evaluation for NAFLD treatment, such as liraglutide and elafibranor, have been incorporated into 3D organoid experiments, which demonstrated that they decreased COL1A1 expression levels and prevented lipid droplet accumulation in human liver organoids.31 Ouchi et al also demonstrated that the severe steatohepatitis resulting from FFA loading of their iPSC-derived multilineage (HLC, HSC-like cells) organoids could be rescued by treatment with the farnesoid x receptor agonist, obeticholic acid.44
Murine Organoid Models
Various murine models have been established to model the NASH phenotype, such as those based on feeding mice a high fat and high fructose diet.48 A key advantage of murine models is that genetically engineered mouse cell lines can be used to interrogate the contribution of specific genetic pathways to disease progression. Different genetic strains of mice result in overeating, such as the ob/ob strain which results in leptin deficiency in mice, db/db strain that results in a defect in the leptin receptor, or MC4R (melanocortin 4 receptor) deficient mice that results in late onset of obesity, hyperphagia, hyperinsulinemia, and hyperglycemia.49 C57BL mice are the most commonly used mice strain to mimic experimental NASH due to being more prone to develop diet-induced necroinflammation and fibrosis.50
Elbadawy et al notably designed mouse liver organoids (isolated hepatocytes in Matrigel) that were derived from methionine- and choline-deficient diet-induced NASH model mice categorized by disease severity, with findings such as markedly upregulated alpha-smooth muscle actin and type I Collagen in the organoids derived from mice with more advanced disease.51 This was consistent with the existing knowledge that activated HSCs deposit collagen in the setting of advanced liver disease.29
On the other hand, a clear limitation of murine models is that NASH in humans is likely the result of a series of genetic and environmental factors that may or may not be reproducible in mice. Murine models replicate only parts of the disease process, making it hard to determine the interaction between the different pathologic features of NASH.52 Overall, murine models have a less severe NASH pathology than what is found in humans due to the different metabolic and immune response profile in mice, and the inability to replicate the complex interactions that occur in the human liver microenvironment.50 Furthermore, murine liver organoids may have cell markers specific to mice that may be a barrier to generalizing experimental results to humans.53 Although these models may be used to determine treatment strategies for early stages of NASH in humans, they are much less reliable for studying late-stage disease or developing therapeutic targets.54 This notion is supported by the fact that approximately 90% of pharmaceutical drugs that are shown to be safe and effective in small animal models ultimately fail in human clinical trials due to lack of efficacy and toxicity.55
Other Types of 3D Liver Models
Liver-on-a-Chip
Other models that are being developed to study NAFLD include a “liver-on-a-chip” model that are dynamic 3D models that recreate the liver tissue on a microscopic scale. These models have been created to overcome some of the limitations of animal and in vitro models.56 The “liver-on-a-chip” model is designed to recapitulate in vivo liver architecture by allowing the seeded hepatocytes to form a structure that imitates the hepatic lobule and allows for the active flow of nutrients to and removal of waste from the cells.57,58 A simple version of the chip contains only hepatocytes and sometimes HSCs to create the hepatic layer, with the option to add endothelial cells and Kupffer cells on the vascular layer to introduce more complexity to the system. These cultures are then embedded into a biochip which maintains fluid perfusion to allow for nutrient supply.57 The biochip is exposed to FFAs to induce NAFLD characteristics and the models are then analyzed for triglyceride uptake and production of reactive oxygen species as a measure of oxidative stress.59 It has been found that the chip allows for increased cell viability compared to 2D culture and gradual lipid accumulation thus mimicking the chronic condition of hepatic steatosis.60 Additionally, the “liver-on-a-chip” model has been used to study the interactions of the liver and other organs such as the colon in the pathogenesis of NAFLD, and one of its benefits is that it can be used to study the multiorgan involvement in the pathogenesis of liver disease.57 That said, the “liver-on-a-chip” model is not currently able to support the long-term culture of primary hepatocytes or maintain an appropriate microenvironment within the device for multiple liver cell lines concurrently, which therefore limits its applications.38 Once it has been optimized to appropriately model the in vivo liver microenvironment, the “liver-on-a-chip” model holds great potential for application in the high-throughput screening of drugs.61,62
Limitations of 3D liver models
One of the primary limitations in all 3D liver organoids cultured in plates is the reliance on passive diffusion for oxygen, nutrients, and waste exchange, which can result in central necrosis, especially when organoids are bigger than 200 μm due to limited oxygen diffusion capacity. There have been attempts to address this issue via the “liver-on-a-chip” model or the use of a perfusion bioreactor, but further research is needed.38 Other limitations include (i) the use of commercial tumoral and immortalized cell lines, which may differ in gene expression or function compared to primary cell lines; and (ii) single cell-derived organoids (e.g., hepatocyte-derived or cholangiocyte-derived with trans-differentiation), which are limited in their ability to recapitulate a complete liver microenvironment.63
Furthermore, some organoid models use an extracellular matrix, such as Matrigel, which is a reconstituted basement membrane derived from extracts of Engelbreth-Holm-Swarm mouse chondrosarcoma.55 Since the specific components of Matrigel are not clearly defined and the safety of Matrigel-based materials is difficult to predict in the human body or in transcriptomics and genomics studies due to its presence as another biomaterial, it is difficult to obtain approval from the FDA for its use in clinical trials or any clinically-related tests.56 However, Matrigel can be used to support liver cells for NAFLD/NASH modeling purposes.
Finally, limitations specific to using the 3D organoid model for NAFLD/NASH include (i) the need for further optimization of FFA loading conditions (e.g., ideal FFA concentration, additional agents to stimulate inflammation), (ii) the fact that current models do not incorporate important immune cells such as B- or T-cells which may play a role in NASH progression,64 and (iii) the lack of a model that addresses organ-organ interactions (i.e., between the gut, adipose tissue, and liver) that may also be an important factor in NASH pathogenesis.48,65
Conclusion
NAFLD/NASH is a disease process with rising prevalence and significant clinical and economic impact, for which the specific mechanism has yet to be determined and no definitive treatment exists. It has been demonstrated that 3D organoids can more closely recapitulate the in vivo microenvironment and allow cells to maintain their mature function for weeks. This has been particularly useful in modeling NAFLD/NASH because it allows time for the cells to be stimulated to develop states of steatosis, inflammation, angiogenesis, and fibrosis, as well as a longterm period to observe changes in gene expression or cellular function or even responses to potential drug therapies (►Fig. 3). Important next steps would be to further develop multicell type liver organoids and refine culturing and perfusion conditions to better model the complexity of the liver microenvironment,66 which would ideally include the incorporation of immune cells to mimic inflammation regulation and hyperactivation thought to be involved in the pathogenesis of NAFLD/NASH (►Fig. 1). The feasibility of accomplishing this in the liver organoid model in the near future is supported by recent studies demonstrating successful co-cultures of lymphocytes with intestinal organoids, in which intraepithelial lymphocytes were not only able to be maintained and expanded but also demonstrated the ability to migrate in and out of the organoid model.64,66
Fig. 3.
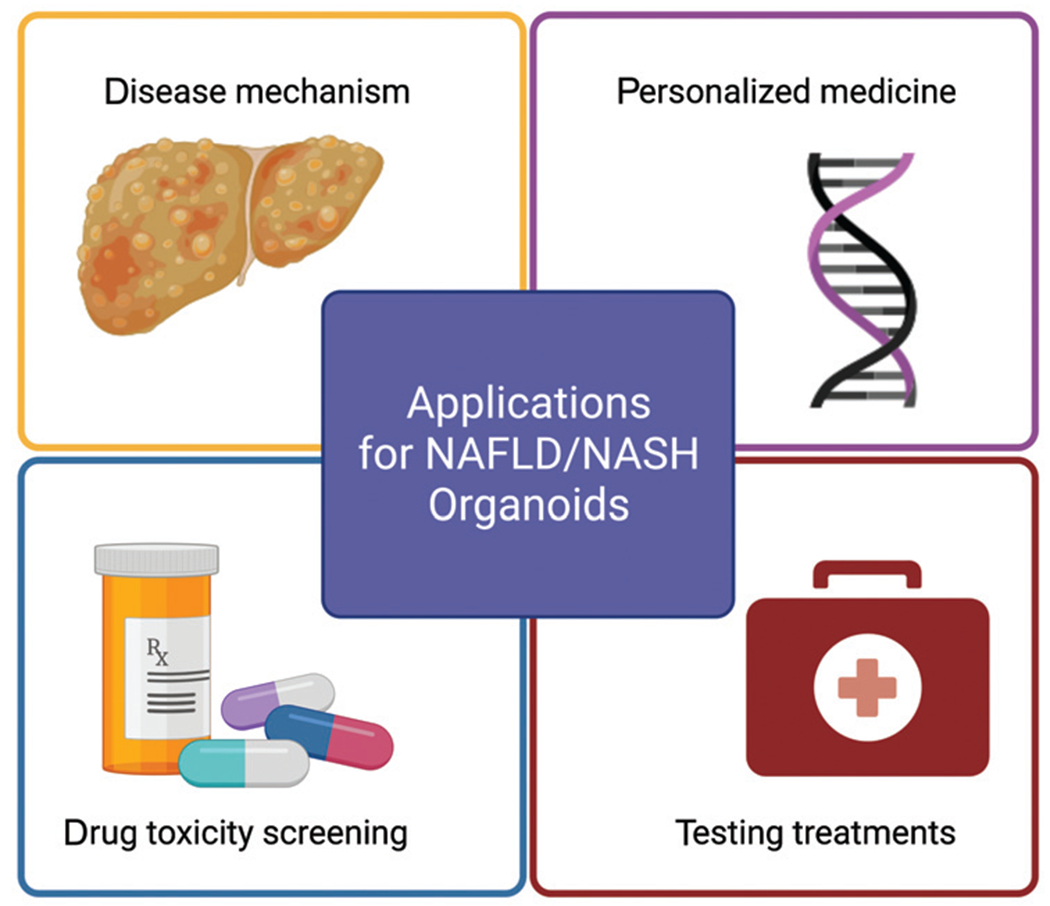
Applications for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) organoids. There are numerous potential applications for the NAFLD/NASH organoid model, including further elucidating disease mechanisms, testing treatments, high throughput drug toxicity screening, and even personalized medicine.
Acknowledgment
Figures were created using biorender.com.
Funding
This work was partially supported by ASTS Faculty Development Grant (BE), Indiana University Health Values Fund for Research Award (VFR-457-Ekser) (BE), and IU Health Foundation Jerome A. Josephs Fund for Transplant Innovation Grant (BE), the Hickam Endowed Chair, Gastroenterology, Medicine, Indiana University, the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative, the Senior Research Career Scientist Award (IK6 BX004601) and the VA Merit award (5I01BX000574) to GA and the Research Career Scientist Award (IK6BX005226) and the VA Merit award (1I01BX003031) to HF, and Career Development Award (1IK2BX004306) to LK from the United States Department of Veteran’s Affairs, Biomedical Laboratory Research and Development Service and NIH grants DK108959 and DK119421 (HF), DK054811, DK115184, DK076898, DK107310, DK110035, DK062975 and AA028711 (GA) and the PSC Partners Seeking a Cure (GA). Portions of these studies were supported by resources at the Richard L. Roudebush VA Medical Center, Indianapolis, IN. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- FFA
free fatty acid
- HLC
hepatocyte-like cell
- HSC
hepatic stellate cell
- HUVEC
human umbilical vein endothelial cell
- iPSC
induced pluripotent stem cell
- LEC
liver endothelial cell
- LPS
lipopolysaccharide
- MSC
mesenchymal stem cell
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Footnotes
Conflict of Interest
None declared.
References
- 1.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017;23(47):8263–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148(03):547–555 [DOI] [PubMed] [Google Scholar]
- 3.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43(08):617–649 [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64(05):1577–1586 [DOI] [PubMed] [Google Scholar]
- 5.Day CP. From fat to inflammation. Gastroenterology 2006;130(01):207–210 [DOI] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998;114(04):842–845 [DOI] [PubMed] [Google Scholar]
- 7.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65(08):1038–1048 [DOI] [PubMed] [Google Scholar]
- 8.Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med (Berl) 2009;87(07):679–695 [DOI] [PubMed] [Google Scholar]
- 9.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24(07):908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simian M, Bissell MJ. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol 2017;216(01):31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 2014;15(10):647–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsee A, Roos FJM, Verstegen MMA, et al. ; HPB Organoid Consortium. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 2021;28(05):816–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto-Gutierrez A, Navarro-Alvarez N, Yagi H, Nahmias Y, Yarmush ML, Kobayashi N. Engineering of an hepatic organoid to develop liver assist devices. Cell Transplant 2010;19(06):815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499(7459):481–484 [DOI] [PubMed] [Google Scholar]
- 15.Freitas-Lopes MA, Mafra K, David BA, Carvalho-Gontijo R, Menezes GB. Differential location and distribution of hepatic immune cells. Cells 2017;6(04):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller FA, Sturla SJ. Human in vitro models of nonalcoholic fatty liver disease. Curr Opin Toxicol 2019;16:9–16 [Google Scholar]
- 17.Kunst RF, Niemeijer M, vander Laan LJW, Spee B, van de Graaf SFJ. From fatty hepatocytes to impaired bile flow: matching model systems for liver biology and disease. Biochem Pharmacol 2020;180:114173. [DOI] [PubMed] [Google Scholar]
- 18.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol 2017;27(21):R1147–R1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissell DM, Tilles JG. Morphology and function of cells of human embryonic liver in monolayer culture. J Cell Biol 1971;50(01):222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell CC, Hendriks DF, Moro SM, et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep 2016;6:25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Gehart H, Artegiani B, et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 2018;175(06):1591–1606.e19 [DOI] [PubMed] [Google Scholar]
- 22.Xiang C, Du Y, Meng G, et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science 2019;364(6438):399–402 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Shen JX, Lauschke VM. Comprehensive evaluation of organotypic and microphysiological liver models for prediction of drug-induced liver injury. Front Pharmacol 2019;10:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinozawa T, Kimura M, Cai Y, et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology 2021;160(03):831–846.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Troglitazone. [Updated June 6, 2018]. Accessed on Sept 28, 2022, at: https://www.ncbi.nlm.nih.gov/books/NBK548142/ [Google Scholar]
- 26.Ramli MNB, Lim YS, Koe CT, et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology 2020;159(04):1471–1486.e12 [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A 1998;95(25):14821–14826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver VM, Lelièvre S, Lakins JN, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2002;2(03):205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves HL, Burt AD, Wood S, Day CP. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol 1996;25(05):677–683 [DOI] [PubMed] [Google Scholar]
- 30.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol 2007;22(Suppl 1):S73–S78 [DOI] [PubMed] [Google Scholar]
- 31.Pingitore P, Sasidharan K, Ekstrand M, Prill S, Lindén D, Romeo S. Human multilineage 3D spheroids as a model of liver steatosis and fibrosis. Int J Mol Sci 2019;20(07):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pingitore P, Romeo S. The role of PNPLA3 in health and disease. Biochim Biophys Acta Mol Cell Biol Lipids 2019;1864(06):900–906 [DOI] [PubMed] [Google Scholar]
- 33.Xu JJ, Diaz D, O’Brien PJ. Applications of cytotoxicity assays and pre-lethal mechanistic assays for assessment of human hepato-toxicity potential. Chem Biol Interact 2004;150(01):115–128 [DOI] [PubMed] [Google Scholar]
- 34.Gerets HH, Tilmant K, Gerin B, et al. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol 2012;28(02):69–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarron S, Bathon B, Conlon DM, et al. Functional characterization of organoids derived from irreversibly damaged liver of patients with NASH. Hepatology 2021;74(04):1825–1844 [DOI] [PubMed] [Google Scholar]
- 36.Broutier L, Andersson-Rolf A, Hindley CJ, et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 2016;11(09):1724–1743 [DOI] [PubMed] [Google Scholar]
- 37.Prill S, Caddeo A, Baselli G, et al. The TM6SF2 E167K genetic variant induces lipid biosynthesis and reduces apolipoprotein B secretion in human hepatic 3D spheroids. Sci Rep 2019;9(01):11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tostões RM, Leite SB, Serra M, et al. Human liver cell spheroids in extended perfusion bioreactor culture for repeated-dose drug testing. Hepatology 2012;55(04):1227–1236 [DOI] [PubMed] [Google Scholar]
- 39.Park Y, Thadasina D, Isidan K, et al. Optimization of human liver organoid model for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. [abstract] Am J Transplant 2022;22(s3):413 [Google Scholar]
- 40.Zhang W, Isidan A, Sato K, et al. Fr366 advanced 3D human liver organoids created by multiple-hepatic lineage cells for the study of liver diseases. [abstract] Gastroenterology 2021;160:S-798–S-799 [Google Scholar]
- 41.Akbari S, Sevinç GG, Ersoy N, et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Reports 2019;13(04):627–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuciforo S, Heim MH. Organoids to model liver disease. JHEP Rep 2020;3(01):100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurevich I, Burton SA, Munn C, et al. iPSC-derived hepatocytes generated from NASH donors provide a valuable platform for disease modeling and drug discovery. Biol Open 2020;9(12):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouchi R, Togo S, Kimura M, et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab 2019;30(02):374–384.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14(07):397–411 [DOI] [PubMed] [Google Scholar]
- 46.Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol 2012;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giraudi PJ, Becerra VJ, Marin V, Chavez-Tapia NC, Tiribelli C, Rosso N. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp Mol Pathol 2015;98(01):85–92 [DOI] [PubMed] [Google Scholar]
- 48.Stephenson K, Kennedy L, Hargrove L, et al. Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr 2018;18(01):5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrell G, Schattenberg JM, Leclercq I, et al. Mouse models of nonalcoholic steatohepatitis: toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology 2019;69(05):2241–2257 [DOI] [PubMed] [Google Scholar]
- 50.Hansen HH, Feigh M, Veidal SS, Rigbolt KT, Vrang N, Fosgerau K. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today 2017;22(11):1707–1718 [DOI] [PubMed] [Google Scholar]
- 51.Elbadawy M, Yamanaka M, Goto Y, et al. Efficacy of primary liver organoid culture from different stages of non-alcoholic steatohepatitis (NASH) mouse model. Biomaterials 2020;237:119823. [DOI] [PubMed] [Google Scholar]
- 52.Jiang M, Wu N, Chen X, et al. Pathogenesis of and major animal models used for nonalcoholic fatty liver disease. J Int Med Res 2019;47(04):1453–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut 2019;68(12):2228–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang W, Menke AL, Driessen A, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One 2014;9(12):e115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC Basic Transl Sci 2019;4(07):845–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lasli S, Kim HJ, Lee K, et al. A human liver-on-a-chip platform for modeling nonalcoholic fatty liver disease. Adv Biosyst 2019;3(08):e1900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soret PA, Magusto J, Housset C, Gautheron J. In vitro and in vivo models of non-alcoholic fatty liver disease: a critical appraisal. J Clin Med 2020;10(01):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Wang H, Deng P, et al. Modeling human nonalcoholic fatty liver disease (NAFLD) with an organoids-on-a-chip system. ACS Biomater Sci Eng 2020;6(10):5734–5743 [DOI] [PubMed] [Google Scholar]
- 59.Hassan S, Sebastian S, Maharjan S, et al. Liver-on-a-chip models of fatty liver disease. Hepatology 2020;71(02):733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gori M, Simonelli MC, Giannitelli SM, Businaro L, Trombetta M, Rainer A. Investigating nonalcoholic fatty liver disease in a liver-on-a-chip microfluidic device. PLoS One 2016;11(07):e0159729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura M, Hagi M, Ejiri Y, et al. Secretion of albumin and induction of CYP1A2 and CYP3A4 in novel three-dimensional culture system for human hepatocytes using micro-space plate. Drug Metab Pharmacokinet 2010;25(03):236–242 [DOI] [PubMed] [Google Scholar]
- 62.Goral VN, Hsieh YC, Petzold ON, Clark JS, Yuen PK, Faris RA. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip 2010;10(24):3380–3386 [DOI] [PubMed] [Google Scholar]
- 63.Kaur G, Dufour JM. Cell lines: valuable tools or useless artifacts. Spermatogenesis 2012;2(01):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nozaki K, Mochizuki W, Matsumoto Y, et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 2016;51(03):206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Wang H. Multiple organs involved in the pathogenesis of non-alcoholic fatty liver disease. Cell Biosci 2020;10(01):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogoz A, Reis BS, Karssemeijer RA, Mucida D. A 3-D enteroid-based model to study T-cell and epithelial cell interaction. J Immunol Methods 2015;421:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]


